Abstract
Membrane compartmentalization allows the spatial segregation of different functions, such as signal transduction and protein trafficking, and ensures their fidelity and efficiency. Eisosomes constitute nanoscale furrow-like invaginations of the plasma membrane where proteins and lipids segregate. The intense interest elicited by eisosomes over the last few years has led to the identification and molecular characterization of their key constituents. This review addresses eisosome structure, functions and its implications for the mechanistic understanding of curvature-induced membrane nanodomains formation and signaling compartmentalization in living cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The startling variety of membrane protein and lipid species of eukaryote cells and the propensity of some of them to self-associate in vitro have stimulated theoretical and experimental research supporting the existence of segregated domains in both model and biological membranes (Demel et al. 1977; Mouritsen and Bloom 1984; van Meer et al. 1987; Simons and Ikonen 1997). Extensive work on Madin–Darby canine kidney cells has demonstrated that segregation of the plasma membrane in well-defined compartments is essential to ascertain the functional identity of epithelial cells. Currently, the identities and mechanisms of formation of these micron-scale domains are well understood (Tanos and Rodriguez-Boulan 2008). However, the lack of conclusive microscopic evidence and the limitations of biochemical characterization techniques (e.g., detergent solubilization, sterol depletion) steered a long debate about the identity and even the existence of membrane domains below the micron level (Munro 2003; Hancock 2006). Recently, various novel microscopy techniques have provided conclusive evidence for the existence of plasma membrane nanodomains in living cells. Currently, it is accepted that essential functions such as cell signaling and endocytosis can be organized in nanoscale plasma membrane platforms. However, the debate concerning their identities and mechanisms of formation is still ongoing (Kusumi et al. 2011; Simons and Sampaio 2011; Mueller et al. 2012).
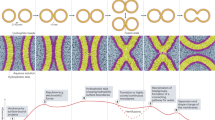
Membrane nanodomains span over a wide range of spatial and temporal scales, extending from tens to hundreds of nanometers and from milliseconds to highly stable and even immutable existence. This diversity contributes to disperse the efforts to build a unifying and simplistic model for plasma membrane nanodomain formation. The current picture involves a complex interplay between at least five different mechanisms (Fig. 1). Preferential association between lipids (e.g., sterols and sphingolipids) provides lipidic platforms, called lipid rafts, where certain protein–protein interactions are favored by selective sorting of proteins (Mouritsen and Bloom 1984; Kaiser et al. 2011; Simons and Sampaio 2011). Within the lipid bilayer, homotypic and heterotypic protein–protein interactions also drive lipid segregation by virtue of preferential protein-lipid interactions (McLaughlin and Murray 2005; Poveda et al. 2008). Dynamic protein–protein interactions of integral membrane proteins can be sufficient to organize domains (Douglass and Vale 2005). Lateral compartmentalization can be achieved by picket fences constituted by transmembrane proteins that are anchored to a submembrane cytoskeleton limiting free diffusion of membrane proteins (Kusumi et al. 2005). Finally, scaffolding of peripheral membrane proteins locally modifies the plasma membrane topography and/or composition by protein–protein and protein-lipid interactions (Johannes and Mayor 2010). As stated by these postulated mechanisms, nanodomain formation occurs throughout self-assembly of different components and consequently this process can be understood as an emergent property of biological membranes (Kusumi et al. 2011; Mueller et al. 2012).
Plasma membrane nanodomains are present in both eukaryotes and prokaryotes (de Bony et al. 1989; Fishov and Woldringh 1999; Matsumoto et al. 2006; Lopez and Kolter 2010). In the model eukaryote Saccharomyces cerevisiae, descriptions of plasma membrane nanodomains date from 1963 when electron microscopic (EM) views of frozen-etched cells showed hexagonal arrangements of 17 nm diameter particles coexisting with 300 nm long and 50 nm deep furrow-like invaginations (Moor and Muhlethaler 1963). Further studies reported the presence of similar domains in Schizosaccharomyces pombe and also indicated that in both, budding and fission yeast, furrow-like invaginations were enriched in quiescent parts of the plasma membrane and absent in sites of active growth (Streiblova 1968; Takeo 1984). Afterwards, yeast plasma membrane organization received little extra attention besides seminal work on endocytosis (Mulholland et al. 1994). A resurgence of interest in the past n years has resulted in a leap forward in our understanding of the identity, biogenesis and maintenance of yeast plasma membrane domains. In this review we will summarize these advances, focusing on eisosomes and the domains they form in budding yeast and other fungi as well.
Plasma membrane domains in S. cerevisiae
A widely used biochemical tool to define membrane domains is solubilization by non-ionic detergents such as Triton X-100. Membranes enriched in sterols and sphingolipids are tightly packed and resistant to Triton X-100 solubilization. This property earned these membranes the acronym DRMs for detergent-resistant membranes (Schroeder et al. 1998). Thus, membrane proteins that after detergent extraction remain associated with DRMs are categorized as lipid rafts components (London and Brown 2000). Prudence has been suggested on the use of this criterion since DRMs obtained with different detergents differ considerably in their protein and lipid content, and these variations are even more prominent between different cell types (Schuck et al. 2003). In S. cerevisiae, all plasma membrane proteins analyzed so far partition into DRMs (Bagnat et al. 2000; Malinska et al. 2003, 2004; Lauwers and Andre 2006). In remarkable contrast, fluorescence microscopy analysis of the same membrane revealed the existence of multiple domains (Malinska et al. 2003, 2004; Berchtold and Walther 2009; Spira et al. 2012). Thus, the DRMs criterion relies on a crude fractionation technique that cannot resolve yeast’s plasma membrane domains evidenced by fluorescence microscopy. Consequently, S. cerevisiae plasma membrane domains are better defined using cell biological evidence coming from different microscopy approaches. Based on this criterion, both dynamic and static domains have been observed. Dynamic domains include the polarized distribution of proteins and lipids that occurs at sites of cellular growth: buds in vegetative growing cells and “shmoo” projections in cells undergoing mating (Chen and Davis 2000; Bagnat and Simons 2002). These domains are above the micron scale and polarized secretion, differential endocytosis and septin-limited diffusion are postulated mechanisms that contribute to their formation and maintenance (Valdez-Taubas and Pelham 2003; Oh and Bi 2011). A recent survey of yeast plasma membrane organization using total internal reflection microscopy (TIRFM) and deconvolution revealed the existence of more than a dozen nanodomains with different dynamic behaviors and shapes ranging from discrete patches to roughly continuous networks (Spira et al. 2012). Clathrin-mediated endocytic patches are highly dynamic nanodomains (life span of 60–120 s) where cargo is first immobilized, surrounded by a cohort of cytosolic factors and then internalized in a process driven by actin polymerization (Kaksonen et al. 2006). Another example of highly dynamic nanodomains is the foci formed by the target of rapamycin complex 2 (TORC2). Each TORC2 focus is composed by less than ten complexes that assemble, move and disassemble at the plasma membrane within a timescale of a few minutes (Berchtold and Walther 2009).
Examples of static domains are MCCs (for Membrane Compartments occupied by the arginine H+-symporter Can1) and MCP (for Membrane Compartment occupied by Pma1) (Grossmann et al. 2007). MCCs are nanoscale patches that have a regular size and are evenly distributed along the plasma membrane. There are between 30 and 40 patches per cell, depending on the cell size, and they are occupied by members of the Sur7/PalI family (pfam06687) and several nutrient H+-symporters (Young et al. 2002; Malinska et al. 2003, 2004). Pma1, the major plasma membrane H+-ATPase, exports protons creating an electrochemical gradient that drives nutrient import. MCP is a nearly continuous network that partially overlaps with other nanodomains but not with MCCs (Malinska et al. 2003, 2004; Spira et al. 2012). On the cytoplasmic side of each MCC, thousands of units of the paralogous proteins Pil1 and Lsp1 constitute supramolecular membrane-associated complexes called eisosomes (Walther et al. 2006). The current list of static and dynamic domains is certainly incomplete as it is based on the observation of a large but still discrete set of proteins (Grossmann et al. 2008; Spira et al. 2012). Overall, our current view of yeast plasma membrane organization resembles a patchwork where a multitude of diverse domains coexist and, in some cases, partially overlap (Spira et al. 2012). As described below, eisosomes emerge as topographically distinct domains where lipids and a large number of different proteins are segregated.
Furrow-like invaginations, Mccs and eisosomes
Until recently, the identity of the plasma membrane furrow-like invaginations initially described more than forty years ago had remained mysterious. This knowledge gap has been closed by immuno-EM evidence showing that both the canonical MCC marker Sur7 and the eisosome core protein Pil1 localize at S. cerevisiae furrow-like invaginations (Stradalova et al. 2009). Moreover, budding yeast cells lacking Pil1 are deprived of such invaginations. Thus, eisosomes and MCCs are both part of the same subcellular structure: the plasma membrane furrow-like invagination. For simplicity, we will use the term eisosomes to describe furrow-like membrane invaginations and the proteins that partition in them (see Table 1; Fig. 2). Based on colocalization with the sterol-binding drug filipin, it has been proposed that eisosomes also concentrate sterols (Grossmann et al. 2007). Still, because of the propensity of filipin to form aggregates and pores in the plasma membrane of living cells, its use as a sterol marker has been criticized (Robinson and Karnovsky 1980; Valdez-Taubas and Pelham 2003). It has also been proposed that filipin binding is restricted to free but not to sphingolipid-associated sterols and, therefore, it is not an accurate marker for total sterol levels (Jin et al. 2008). Consequently, the usage of ergosterol-like fluorescent lipids such as dehydroergosterol may help to unambiguously determine whether sterols are concentrated at eisosomes (Georgiev et al. 2011). The number of furrow-like invaginations per membrane surface area seen by frozen-etch EM imaging is roughly twice the number of eisosomes observed by fluorescence microscopy. This apparent discrepancy is explained by the resolution limit of conventional light microscopy: two closely located 300 nm long invaginations can be mistaken for one when imaged using fluorescence microscopy (Stradalova et al. 2009). Accordingly, the number of eisosomes per cell seen by super-resolution microscopy is higher than previously calculated (Rankin et al. 2011). Thus, a Pil1-GFP (or Sur7-GFP) plasma membrane fluorescent punctum should not be interpreted as a single eisosome.
Saccharomyces cerevisiae’s plasma membrane resembles a patchwork where various domains ranging from discrete patches to roughly continuous networks coexist and partially overlap. Among them, eisosome core proteins Pil1 and Lsp1 (green) scaffold and sculpt the plasma membrane into furrow-like invaginations concentrating lipids; nutrient H+ -symporters (pink); tetra-spanning transmembrane proteins like Sur7 (dark blue) and Nce102 (lilac); and signaling proteins. Nce102 displays a dual partition that is sensitive to sphingolipid availability (see text for details). Pkh1 and Pkh2 (light blue) are transiently recruited to a subset of eisosomes where they phosphorylate Pil1 and Lsp1, and presumably other substrates. Pma1 ATPase (brown) is exclusively located at the network-like domain called MCP (brown), whereas TORC2 effectors Slm1 and Slm2 (red) are dynamically exchanged between membrane compartment occupied by TORC2 (MCTs) (yellow) and the invaginations
Eisosome composition in S. cerevisiae
The current list of eisosomal proteins spans almost two dozens and it is expected to increase over time (Table 1). Notably, few components have a molecular function assigned. Among them, proteins that structure eisosomes and proteins involved in nutrient uptake and signal processing have been described. Different genetic and phenotypic analyses provide clues about the function of less well-characterized components. Here, we will discuss major aspects of eisosomal proteins in budding yeast. Composition and organization of eisosomes in other fungi are summarized in “Box 1” and Table 2.
Structural proteins
Pil1 and Lsp1 constitute the core components of eisosomes. The cellular content of these two proteins is comparable to the amount of ribosomal proteins (roughly 2 × 105 molecules/cell) and is at least one order of magnitude higher than the next most abundant eisosomal protein (Ghaemmaghami et al. 2003; Walther et al. 2006). Fluorescence recovery after photobleaching (FRAP) analysis of Pil1 indicated that assemblies formed by this protein are highly stable (Walther et al. 2006). Crystallographic and in silico studies have shown that Lsp1 and Pil1 contain a bin/amphiphysin/rvs (BAR) domain encompassing 50 % of the proteins (Olivera-Couto et al. 2011; Ziolkowska et al. 2011). Like canonical BAR domain proteins, Pil1 and Lsp1 form banana-shape dimers that self-assemble into scaffolds which bind and bend liposomes forming tubular membranes (Karotki et al. 2011; Olivera-Couto et al. 2011). Accordingly, it has been proposed that Pil1 and Lsp1 are responsible for structuring the yeast plasma membrane into furrow-like invaginations (Stradalova et al. 2009; Karotki et al. 2011; Olivera-Couto et al. 2011; Ziolkowska et al. 2011). Based on the structure of Lsp1 dimers (Ziolkowska et al. 2011) and the cryo-EM reconstruction images of Pil1 and Lsp1 liposome-bound scaffolds (Karotki et al. 2011), it can be estimated that each furrow-like invagination contains approximately 1,000 molecules of both Pil1 and Lsp1, which is in good agreement with previous calculations (Walther et al. 2006). Pil1 and Lsp1 BAR domains preferentially bind to liposomes containing phosphatidylinositol 4,5 biphosphate (PI(4,5)P2). PI(4,5)P2 depletion causes detachment of Pil1-GFP from the plasma membrane and conversely, increased PI(4,5)P2 levels lead to formation of much larger Pil1-GFP scaffolds (Karotki et al. 2011). Thus, eisosome membranes might be reservoirs of this phosphoinositide.
Membrane-associated proteins
Pkh1 and Pkh2 kinases, the functional homologs of the mammalian phosphoinositide-dependent kinase 1 (PDK1), phosphorylate Pil1 and Lsp1 and regulate eisosome assembly (Zhang et al. 2004; Walther et al. 2007; Luo et al. 2008) (see below). Pkh kinases activity is regulated by long-chain bases (LCBs), which are precursors of sphingolipid biosynthesis (Sun et al. 2000; Friant et al. 2001; Zhang et al. 2004). LCBs are positive regulators of the phosphorylation of several Pkh kinases targets, including Lsp1. However, LCBs inhibit Pkh-mediated phosphorylation of Pil1 (Zhang et al. 2004). How Pkh1/2 are targeted to eisosomes is currently unknown, although the presence of a phosphoinositide-binding pleckstrin homology (PH) domain in Pkh2 has been recently suggested (Olivera-Couto et al. 2011).
Slm1 and Slm2 are an essential pair of proteins that promotes actin cytoskeleton organization, sphingolipid homeostasis and cell growth (Audhya et al. 2004; Tabuchi et al. 2006). Slms are TORC2 effectors, they physically interact with and are phosphorylated by TORC2 (Audhya et al. 2004). Thus, an apparent paradox is that the expected localizations for Slms are membrane compartment occupied by TORC2 (MCTs) and not eisosomes. This conflict has been recently solved by evidence showing that Slms are dynamically exchanged between these two compartments (Berchtold et al. 2012). The molecular features that sustain Slms dynamic exchange are currently unknown. Both Slm1 and Slm2 have a PH domain that is sufficient for plasma membrane binding but not for recruitment to eisosomes (Yu et al. 2004). Slms recruitment to eisosomes is assured by the simultaneous presence of a predicted F-BAR domain and the PH domain (Olivera-Couto et al. 2011). Thus, it is possible that Slms targeting to eisosomes depends on protein-lipid interactions, whereas targeting to TORC2 depends mainly on protein–protein interactions.
Integral membrane proteins
Can1, Tat2 and Fur4 are H+-symporters that import nutrients and protons into the cell. Sur7 is the prototypical member of the Sur7/PalI family of tetra-spanning integral membrane proteins (pfam06687). In S. cerevisiae, three other proteins belonging to this family (Fmp45, Pun1 and Ynl194c) are also part of eisosomes. SUR7 has been associated with endocytosis since its overexpression partially suppresses several phenotypes caused by mutations in amphiphysin-like endocytic proteins Rvs167 and Rvs161 (Sivadon et al. 1997). Single deletion of SUR7, FMP45 or YNL194C causes slight defects in sporulation and changes in inositol phosphorylceramides membrane composition (Young et al. 2002). PUN1 gene expression is induced by different cell wall injuries, metal ion stress and during nitrogen stress-dependent filamentous growth (Lagorce et al. 2003; Xu et al. 2010; Hosiner et al. 2011). Deletion of PUN1 leads to thin cell walls with low β-glucan content, defective filamentous growth and increased heavy metal stress sensitivity (Xu et al. 2010; Hosiner et al. 2011). These phenotypes suggest that Pun1 is a stress responsive protein contributing to cell wall integrity maintenance (Xu et al. 2010; Hosiner et al. 2011). Nce102 and its paralog Fhn1 are also tetra-spanning membrane proteins. There are two interesting features that distinguish Nce102 from the other eisosomal integral membrane proteins. First, deletion of NCE102 leads to a pil1Δ-like phenotype (Grossmann et al. 2008; Frohlich et al. 2009). Second, Nce102 concentrates at eisosomes depending on sphingolipid availability and influences Pkh kinases signaling (see below).
Eisosome biogenesis and maintenance
In remarkable contrast with most yeast cellular organelles, eisosomes are static. Time-lapse microscopy monitoring of GFP-tagged versions of Sur7, Can1 and Pil1 in mother cells showed that these markers do not change their localization during a life span (Young et al. 2002; Malinska et al. 2003; Walther et al. 2006). In contrast to mother cells, live monitoring of Pil1-GFP and Sur7-GFP showed that eisosomes are assembled de novo in growing buds (Young et al. 2002; Moreira et al. 2009). Active sites of eisosome assembly are first detected at the neck of small-size buds. As the bud size increases, early eisosomes get bigger and new sites of assembly appear towards the bud tip. This vectorial pattern of eisosome assembly (advanced at the neck and nascent at the tip) reflects the polar deposition of brand new plasma membrane during bud anisotropic growth (Moreira et al. 2009). Before cytokinesis, when daughter cells are close to reaching their maximum size, active assembly of eisosomes has already concluded (Moreira et al. 2009). Pairwise correlation analysis of eisosome location demonstrated that their sites at the plasma membrane are not pre-assigned, but randomly distributed (Moreira et al. 2009). Similar features of eisosome assembly have been observed in other fungi as well, such as S. pombe and C. albicans (Kabeche et al. 2011; Reijnst et al. 2011). Although Pil1 and Lsp1 are the most likely initiators of eisosome assembly, there are still no reports that address simultaneous monitoring of more than one eisosomal protein during this process. This type of analysis would establish which components settle first and which ones colonize the preoccupied spots.
Compelling evidence indicates that Pil1 is the eisosome main organizer. Pil1 production is cell cycle-regulated in synchronicity with bud membrane expansion (Moreira et al. 2009). The absence of Pil1 causes massive eisosome disorganization. In pil1Δ cells, eisosomal integral membrane proteins disperse in the plasma membrane, associated proteins fall into the cytoplasm and furrow-like invaginations disappear (Walther et al. 2006; Grossmann et al. 2007; Stradalova et al. 2009; Aguilar et al. 2010). Still, pil1Δ cells have few eisosome “remnants” that concentrate elevated amounts of eisosomal proteins and correspond to large aberrant plasma membrane invaginations (Walther et al. 2006; Stradalova et al. 2009). In striking contrast with Pil1, Lsp1 absence did not render a noticeable phenotype indicating that Pil1 alone is sufficient to sustain eisosome formation and maintenance (Walther et al. 2006).,
Eisosome integrity does not depend on either actin or tubulin cytoskeletons since Pil1 and Can1 localization were unperturbed by latrunculin A or nocodazole treatments (Malinska et al. 2004; Walther et al. 2006). Depolarization of the plasma membrane, either by application of an external electrical field or by adding the H+ gradient uncoupler FCCP, releases Can1, Tat2 and Fur4 nutrient H+-symporters from their eisosomal location. This behavior is reversible, after repolarization H+-symporters move back to eisosomes. Notably, only these H+-symporters are sensitive to membrane depolarization since Pil1, Nce102, Sur7 and even Pma1 localizations were unperturbed under the same conditions (Grossmann et al. 2007, 2008). This differential behavior of H+-symporters suggests that their activities may be influenced by location. Accordingly, when Can1 is artificially removed from eisosomes it becomes inactive (Spira et al. 2012). It is currently unknown whether inactive mutant versions of Can1 and other H+-symporters are still concentrated at eisosomes and/or sensitive to depolarization-induced changes in localization.
Eisosome organization is highly sensitive to perturbations in plasma membrane lipid composition. Pil1 and Lsp1 bind preferentially to liposomes containing PI(4,5)P2, a phosphoinositide that is enriched in yeast plasma membrane. Eisosomes disassemble when yeast cells are depleted of PI(4,5)P2, more likely due to loss of direct interaction between Pil1 and Lsp1 with this phosphoinositide at the plasma membrane (Karotki et al. 2011). Pil1 assembly is also affected by sphingolipid-sensitive Pkh kinases activity (Walther et al. 2007; Luo et al. 2008). Pkh kinases overexpression increases the amount of phospho-Pil1, which detaches from the plasma membrane and accumulates in the cytoplasm leaving few assembled eisosomes per cell. Depletion of the sphingolipid precursors LCBs by adding the drug myriocin also leads to increased levels of phospho-Pil1 and eisosome disassembly (Walther et al. 2007; Luo et al. 2008; Frohlich et al. 2009). This is consistent with results showing loss of eisosome organization when a phospho-mimicking variant of Pil1 is the only source of Pil1. Reciprocally, in vivo inactivation of Pkh kinases leads to Pil1 hypophosphorylation and formation of large eisosomes, as does the use of a Pil1 variant lacking phosphorylation sites. (Walther et al. 2007; Luo et al. 2008). Thus, a hyperphosphorylation state of Pil1 promotes eisosomes disassembly and conversely, assembly increases if Pil1 is hypophosphorylated. However, other non-phosphorylatable variants of Pil1 are unable to form eisosomes remaining in the cytoplasm (Luo et al. 2008). A possible explanation for this discrepancy is that different sets of phosphorylated residues play different roles in protein-lipid and protein–protein interactions. Many Pil1 phosphorylated residues locate either in the concave (lipid-binding) face of the BAR domain or in areas proposed to be involved in Pil1 dimer–dimer interactions (Karotki et al. 2011; Olivera-Couto et al. 2011; Ziolkowska et al. 2011). Another player in the regulation of Pil1 phosphorylation status mediated by sphingolipids is Nce102. It has been proposed that Nce102 is a sphingolipid-responsive protein that negatively regulates Pil1 phosphorylation (Frohlich et al. 2009). Based on this model, high sphingolipid availability drives Nce102 partition towards eisosome domains where it inhibits Pkh kinases. The resulting decrease in phospho-Pil1 levels promotes eisosome assembly. Conversely, when sphingolipid availability is compromised (e.g., under myriocin treatment) Nce102 abandons eisosomes and relieves Pkh kinases inhibition leading to high levels of phospho-Pil1 and eisosomes disassembly (Frohlich et al. 2009). Cellular sterol composition also impacts on eisosome integrity. Deletion of the ergosterol biosynthetic genes ERG2, ERG24 and ERG6 lead to loss of eisosome assembly (Grossmann et al. 2008). Since yeast cells adjust their sphingolipid composition in response to changes in sterols structure it is possible that the observed phenotypes are due to altered Pkh kinases activity (Guan et al. 2009). Eisosome integrity is also lost in slm2Δ slm1 ts mutants but the mechanism involved is still unknown (Kamble et al. 2011).
Molecular and cellular functions of eisosomes, outstanding questions
Work accumulated during the last decade offers a molecular portrait of plasma membrane domain organization in fungi. At first glance, eisosome domains are the best understood case: the key players have been identified, molecular structures have been solved and a dynamic description of domain formation has already been sketched. Thus, the starting materials needed to elucidate the molecular mechanisms of eisosome-mediated membrane domain formation are already available. Still, our understanding of eisosome cellular functions remains elusive. As described below, eisosomes have been involved in many vital cellular functions. However, massive eisosome disorganization caused by PIL1 deletion is not accompanied by a noticeable decrease in cellular fitness. This conundrum may be a consequence of the lifestyle of prevalent laboratory yeast strains. In our opinion, the most pressing question is why have eisosome domains been conserved during fungi evolution? Below, we briefly summarize current topics of eisosomes molecular and cellular functionality giving prominence to still open questions, which are also listed in “Box 2”.
Membrane domain formation and maintenance
Eisosomes construct invaginated plasma membrane domains. Extensive work on different model fungi positions Pil1 as the main organizer of eisosomes (Young et al. 2002; Walther et al. 2006; Grossmann et al. 2007, 2008; Moreira et al. 2009; Stradalova et al. 2009; Vangelatos et al. 2010; Kabeche et al. 2011; Seger et al. 2011). Since Pil1 and Lsp1 self-assemble and form membrane-bound scaffolds of tubular shape an immediate question that arises is whether these proteins are sufficient to build membrane domains (Karotki et al. 2011; Olivera-Couto et al. 2011). Curvature-induced lipid segregation has been theoretically described and experimentally demonstrated (Markin 1981; Roux et al. 2005). Membrane tube pulling in giant unilamellar vesicles (GUVs) is sufficient to induce lipid segregation within ternary mixtures of sphingomyelin, phosphatidylcholine and cholesterol (Roux et al. 2005). Curvature-induced lipid sorting is amplified by sphingolipid-clustering proteins such as cholera toxin B-subunit (Sorre et al. 2009). Analogously, the phosphoinositide-binding capacity of Pil1 and Lsp1 may aid to cluster lipids into nascent domains. In vitro reconstitution of membrane domain formation using liposomes and purified proteins will be an important step in determining the minimal machinery required for eisosome-driven membrane compartmentalization.
One of the most striking differences between budding yeast Pil1 and Lsp1 is the inability of the latter to organize eisosomes in the absence of the former. Why are Pil1 and Lsp1 so similar but behaving so differently? In Ashbya gossypii, this functional divergence is also observed for the highly similar pair (74 % amino acid identity) of Pil1-Lsp1 orthologs (Seger et al. 2011). Elucidation of the intrinsic differences between S. cerevisiae Pil1 and Lsp1 proteins will shed light on this issue. Lsp1 phosphorylation sites have not been characterized yet and Pil1 and Lsp1 phosphatases, if they exist, are still unidentified. The current crystal structure of Lsp1 lacks the first 50 amino acid residues of the poorly conserved N-terminus (Ziolkowska et al. 2011). In both Pil1 and Lsp1, this region is important for in vitro assembly and membrane binding (Karotki et al. 2011). It is currently unknown whether Pil1/Lsp1 N-termini, like N-terminal extra amphipatic helices in BAR domain proteins, confer differential membrane-sculpting capacities (Zimmerberg and Kozlov 2006). Also, whether these regions interact with different subsets of eisosomal proteins remains to be determined.
Another unsolved issue is how integral membrane proteins are targeted to eisosomes. The different behaviors of eisosome integral membrane proteins suggest the existence of at least two mechanisms operating in eisosome targeting. Can1 and Tat2 are being continuously exchanged between eisosomes and MCP (Brach et al. 2011). These symporters abandon eisosomes upon plasma membrane depolarization. Thus, either functional- or membrane potential-dependent conformational changes affecting protein–protein and/or protein–lipid interactions may mediate this displacement (Grossmann et al. 2007). In contrast, Sur7 localization is not affected by plasma membrane depolarization. Whether the other members of the Sur7 family that localize at eisosomes behave similarly is currently unknown. Mug33, a fission yeast member of the Sur7/PalI family provides an interesting clue in this regard. Wild-type Mug33 resides at the plasma membrane but does not colocalize with Pil1. Deletion of Mug33 C-terminal cytoplasmic tail redirects the resultant protein to eisosomes. This suggests that the remaining N-terminal portion, which strictly spans the four transmembrane domains, contains enough structural information for eisosome targeting (Snaith et al. 2011). Similarly, the structural determinants that keep Pma1 in the MCP network await further characterization.
Eisosomes and endocytosis
Clathrin-mediated endocytosis in budding yeast depends on actin dynamics and involves the progressive assembly of more than 60 proteins at the plasma membrane (Kaksonen et al. 2006). Colocalization analyses using the lipid dye FM4-64 and the plasma membrane protein cargo Hxt2 suggested that Pil1 marks sites for endocytosis (Walther et al. 2006). The endocytic rate of the a-factor mating pheromone receptor Ste3 is diminished in pil1Δ lsp1Δ double mutants (Walther et al. 2006). Loss of Pil1 leads to several defects in assembly of endocytic mediators (e.g., Abp1, Las17, Rvs161/Rvs167, Sjl2) at endocytic sites (Murphy et al. 2011). Genetic interactions between SUR7, PIL1, LSP1 and known endocytic mediators (e.g., RVS161, RVS167, SJL1, SJL2) reinforced the link between eisosomes and endocytosis (Sivadon et al. 1997; Walther et al. 2006; Fiedler et al. 2009; Aguilar et al. 2010; Karotki et al. 2011). However, colocalization analyses of different markers of clathrin-actin-mediated endocytosis (e.g., Abp1, Sla1, Ede1, Sla2, Rvs161) indicated that endocytic events occur within MCPs independently of eisosome organization (Grossmann et al. 2008; Brach et al. 2011). Moreover, PIL1 or NCE102 deletion resulted in accelerated endocytosis of Can1 and Fur1 (Grossmann et al. 2008). These results supported the idea that eisosomes protect these symporters from being internalized (Grossmann et al. 2008). However, an independent analysis of Can1 and Tat2 endocytic rates found no differences between pil1Δ and wild-type cells (Brach et al. 2011). Besides these contrary results, these reports agree showing that calthrin-actin-dependent endocytic mediators do not gather at eisosomes. Moreover, the lack of colocalization between different calthrin-actin-dependent endocytic mediators and eisosomes has also been reported in both A. gosypii and S. pombe (Kabeche et al. 2011; Seger et al. 2011). Overall, the role of eisosomes in endocytosis is under serious criticism and further research is needed to unambiguously solve this issue. Recently, a clathrin-independent actin-dependent endocytic pathway has been described in S. cerevisiae. This novel pathway depends on the GTPase Rho1 and the formin Bni1 and seems to be Abp1- and Las17-independent (Prosser et al. 2011). No specific cargo for this pathway has been identified yet and the spatial/functional relationship with eisosomes remains to be determined.
Eisosomes and signaling
Pkh kinases and Slm1/Slm2-TORC2 are involved in cell growth, heat stress-response, actin cytoskeleton organization and sphingolipid homeostasis. It has been proposed that these shared functions are channeled through the AGC kinases orthologs Ypk1 and Ypk2. To be fully activated, Ypk1 and Ypk2 require dual phosphorylation by Pkh kinases and TORC2 (Roelants et al. 2002; Kamada et al. 2005; Aronova et al. 2008). The lack of lipid-binding motifs in Ypk1 and Ypk2 made it difficult to explain how these kinases were activated at the plasma membrane. However, it has been shown that TORC2 effectors Slm1 and Slm2 recruit Ypk1 to the plasma membrane for phosphorylation by TORC2 which, in turn, facilitates the subsequent activation of Ypk1 by Pkh1/2 (Niles et al. 2012). In agreement with these findings, it has been shown that under plasma membrane stress (e.g., hiposmotic shock, sphingolipid synthesis inhibition) Slm1 abandons eisosomes and interacts with TORC2 to further promote recruitment and activation of Ypk1 (Berchtold et al. 2012). Thus, in this scenario, eisosomes act as reservoirs of signaling molecules that are released according to need. It is currently unknown whether relocalization of Pkh kinases is also required for plasma membrane stress-mediated Ypk1 activation.
Pkh kinases, TORC2 and Ypk1 are also involved in aminophospholipid flippase mediated activation (Roelants et al. 2010). Phosphatidylethanolamine and phosphatidylserine are returned to the inner leaflet of the plasma membrane via lipid translocases or flippases. The membrane-associated protein kinase Fpk1, a flippase activator, is phosphorylated and inactivated by Ypk1 in a Slm1/Slm2- and Pkh1/2-dependent manner (Roelants et al. 2010; Niles et al. 2012). Fluorescent microscopy imaging showed that Fpk1 localizes diffusely in the cytoplasm, at endosomal/TGN structures and at the plasma membrane (Nakano et al. 2008; Roelants et al. 2010). It is currently unknown whether eisosomes participate in these signaling events.
In both A. nidulans and S. cerevisiae, a signaling pathway that includes the Sur7/PalI family member PalI (Rim9 in S. cerevisiae) mediates ambient pH sensing (Penalva et al. 2008). PalI/Rim9 associates with the plasma membrane sensor PalH (Rim21 and Dfg16 in S. cerevisiae). This pathway, dubbed Pal/RIM, requires components of the ESCRT machinery and arrestin-like proteins. Live fluorescent microscopy monitoring of S. cerevisiae cells showed that activation of this pathway leads to sequential recruitment of arrestins, Pal/RIM and ESCRT proteins into plasma membrane static foci that are distributed in an eisosome-like pattern (Herrador et al. 2010; Galindo et al. 2012). These signaling foci are not involved in actin-clathrin-mediated endocytosis and whether or not they are related with eisosomes is currently unknown (Galindo et al. 2007).
Most of the mentioned signaling components are very low abundant proteins with a highly dynamic behavior making live monitoring a technically difficult task. However, the power of yeast genetics combined with novel microscopy techniques, such as live stochastic optical reconstruction microscopy (live STORM), image correlation spectroscopy (ICS), fluorescence lifetime imaging (FLIM) and fluorescence cross correlation spectroscopy (FCCS), will help dissecting the specific roles of eisosomes as signaling platforms.
References
Aguilar PS, Frohlich F, Rehman M, Shales M, Ulitsky I, Olivera-Couto A, Braberg H, Shamir R, Walter P, Mann M, Ejsing CS, Krogan NJ, Walther TC (2010) A plasma-membrane E-MAP reveals links of the eisosome with sphingolipid metabolism and endosomal trafficking. Nat Struct Mol Biol 17:901–908
Alvarez FJ, Douglas LM, Rosebrock A, Konopka JB (2008) The Sur7 protein regulates plasma membrane organization and prevents intracellular cell wall growth in Candida albicans. Mol Biol Cell 19:5214–5225
Aronova S, Wedaman K, Aronov PA, Fontes K, Ramos K, Hammock BD, Powers T (2008) Regulation of ceramide biosynthesis by TOR complex 2. Cell Metab 7:148–158
Audhya A, Loewith R, Parsons AB, Gao L, Tabuchi M, Zhou H, Boone C, Hall MN, Emr SD (2004) Genome-wide lethality screen identifies new PI4,5P2 effectors that regulate the actin cytoskeleton. EMBO J 23:3747–3757
Bagnat M, Simons K (2002) Cell surface polarization during yeast mating. Proc Natl Acad Sci USA 99:14183–14188
Bagnat M, Keranen S, Shevchenko A, Simons K (2000) Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc Natl Acad Sci USA 97:3254–3259
Bastiani M, Parton RG (2010) Caveolae at a glance. J Cell Sci 123:3831–3836
Berchtold D, Walther TC (2009) TORC2 plasma membrane localization is essential for cell viability and restricted to a distinct domain. Mol Biol Cell 20:1565–1575
Berchtold D, Piccolis M, Chiaruttini N, Riezman I, Riezman H, Roux A, Walther TC, Loewith R (2012) Plasma membrane stress induces relocalization of Slm proteins and activation of TORC2 to promote sphingolipid synthesis. Nat Cell Biol 14:542–547
Bernardo SM, Lee SA (2010) Candida albicans SUR7 contributes to secretion, biofilm formation, and macrophage killing. BMC Microbiol 10:133
Brach T, Specht T, Kaksonen M (2011) Reassessment of the role of plasma membrane domains in the regulation of vesicular traffic in yeast. J Cell Sci 124:328–337
Chen L, Davis NG (2000) Recycling of the yeast a-factor receptor. J Cell Biol 151:731–738
de Bony J, Lopez A, Gilleron M, Welby M, Laneelle G, Rousseau B, Beaucourt JP, Tocanne JF (1989) Transverse and lateral distribution of phospholipids and glycolipids in the membrane of the bacterium Micrococcus luteus. Biochemistry 28:3728–3737
Demel RA, Jansen JW, van Dijck PW, van Deenen LL (1977) The preferential interaction of cholesterol with different classes of phospholipids. Biochim Biophys Acta 465:1–10
Deng C, Xiong X, Krutchinsky AN (2009) Unifying fluorescence microscopy and mass spectrometry for studying protein complexes in cells. Mol Cell Proteomics 8:1413–1423
Douglas LM, Wang HX, Keppler-Ross S, Dean N, Konopka JB (2012) Sur7 promotes plasma membrane organization and is needed for resistance to stressful conditions and to the invasive growth and virulence of Candida albicans. MBio 3(1). doi:10.1128/mBio.00254-11
Douglass AD, Vale RD (2005) Single-molecule microscopy reveals plasma membrane microdomains created by protein–protein networks that exclude or trap signaling molecules in T cells. Cell 121:937–950
Fiedler D, Braberg H, Mehta M, Chechik G, Cagney G, Mukherjee P, Silva AC, Shales M, Collins SR, van Wageningen S, Kemmeren P, Holstege FC, Weissman JS, Keogh MC, Koller D, Shokat KM, Krogan NJ (2009) Functional organization of the S. cerevisiae phosphorylation network. Cell 136:952–963
Fishov I, Woldringh CL (1999) Visualization of membrane domains in Escherichia coli. Mol Microbiol 32:1166–1172
Friant S, Lombardi R, Schmelzle T, Hall MN, Riezman H (2001) Sphingoid base signaling via Pkh kinases is required for endocytosis in yeast. EMBO J 20:6783–6792
Frohlich F, Moreira K, Aguilar PS, Hubner NC, Mann M, Walter P, Walther TC (2009) A genome-wide screen for genes affecting eisosomes reveals Nce102 function in sphingolipid signaling. J Cell Biol 185:1227–1242
Galindo A, Hervas-Aguilar A, Rodriguez-Galan O, Vincent O, Arst HN Jr, Tilburn J, Penalva MA (2007) PalC, one of two Bro1 domain proteins in the fungal pH signalling pathway, localizes to cortical structures and binds Vps32. Traffic 8:1346–1364
Galindo A, Calcagno-Pizarelli AM, Arst HN Jr, Penalva MA (2012) An ordered pathway for the assembly of ESCRT-containing fungal ambient pH signalling complexes at the plasma membrane. J Cell Sci 125:1784–1795. doi:10.1242/jcs.098897
Georgiev AG, Sullivan DP, Kersting MC, Dittman JS, Beh CT, Menon AK (2011) Osh proteins regulate membrane sterol organization but are not required for sterol movement between the ER and PM. Traffic 12:1341–1355
Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, Weissman JS (2003) Global analysis of protein expression in yeast. Nature 425:737–741
Grossmann G, Opekarova M, Malinsky J, Weig-Meckl I, Tanner W (2007) Membrane potential governs lateral segregation of plasma membrane proteins and lipids in yeast. EMBO J 26:1–8
Grossmann G, Malinsky J, Stahlschmidt W, Loibl M, Weig-Meckl I, Frommer WB, Opekarova M, Tanner W (2008) Plasma membrane microdomains regulate turnover of transport proteins in yeast. J Cell Biol 183:1075–1088
Guan XL, Souza CM, Pichler H, Dewhurst G, Schaad O, Kajiwara K, Wakabayashi H, Ivanova T, Castillon GA, Piccolis M, Abe F, Loewith R, Funato K, Wenk MR, Riezman H (2009) Functional interactions between sphingolipids and sterols in biological membranes regulating cell physiology. Mol Biol Cell 20:2083–2095
Hancock JF (2006) Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol 7:456–462
Hansen CG, Nichols BJ (2010) Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol 20:177–186
Hayer A, Stoeber M, Bissig C, Helenius A (2010) Biogenesis of caveolae: stepwise assembly of large caveolin and cavin complexes. Traffic 11:361–382
Herrador A, Herranz S, Lara D, Vincent O (2010) Recruitment of the ESCRT machinery to a putative seven-transmembrane-domain receptor is mediated by an arrestin-related protein. Mol Cell Biol 30:897–907
Hosiner D, Sponder G, Graschopf A, Reipert S, Schweyen RJ, Schuller C, Aleschko M (2011) Pun1p is a metal ion-inducible, calcineurin/Crz1p-regulated plasma membrane protein required for cell wall integrity. Biochim Biophys Acta 1808:1108–1119
Jin H, McCaffery JM, Grote E (2008) Ergosterol promotes pheromone signaling and plasma membrane fusion in mating yeast. J Cell Biol 180:813–826
Johannes L, Mayor S (2010) Induced domain formation in endocytic invagination, lipid sorting, and scission. Cell 142:507–510
Kabeche R, Baldissard S, Hammond J, Howard L, Moseley JB (2011) The filament-forming protein Pil1 assembles linear eisosomes in fission yeast. Mol Biol Cell 22:4059–4067
Kaiser HJ, Orlowski A, Rog T, Nyholm TK, Chai W, Feizi T, Lingwood D, Vattulainen I, Simons K (2011) Lateral sorting in model membranes by cholesterol-mediated hydrophobic matching. Proc Natl Acad Sci USA 108:16628–16633
Kaksonen M, Toret CP, Drubin DG (2006) Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 7:404–414
Kamada Y, Fujioka Y, Suzuki NN, Inagaki F, Wullschleger S, Loewith R, Hall MN, Ohsumi Y (2005) Tor2 directly phosphorylates the AGC kinase Ypk2 to regulate actin polarization. Mol Cell Biol 25:7239–7248
Kamble C, Jain S, Murphy E, Kim K (2011) Requirements of Slm proteins for proper eisosome organization, endocytic trafficking and recycling in the yeast Saccharomyces cerevisiae. J Biosci 36:79–96
Karotki L, Huiskonen JT, Stefan CJ, Ziolkowska NE, Roth R, Surma MA, Krogan NJ, Emr SD, Heuser J, Grunewald K, Walther TC (2011) Eisosome proteins assemble into a membrane scaffold. J Cell Biol 195:889–902
Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, Kasai RS, Kondo J, Fujiwara T (2005) Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct 34:351–378
Kusumi A, Suzuki KG, Kasai RS, Ritchie K, Fujiwara TK (2011) Hierarchical mesoscale domain organization of the plasma membrane. Trends Biochem Sci 36:604–615
Lagorce A, Hauser NC, Labourdette D, Rodriguez C, Martin-Yken H, Arroyo J, Hoheisel JD, Francois J (2003) Genome-wide analysis of the response to cell wall mutations in the yeast Saccharomyces cerevisiae. J Biol Chem 278:20345–20357
Lauwers E, Andre B (2006) Association of yeast transporters with detergent-resistant membranes correlates with their cell-surface location. Traffic 7:1045–1059
London E, Brown DA (2000) Insolubility of lipids in triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochim Biophys Acta 1508:182–195
Lopez D, Kolter R (2010) Functional microdomains in bacterial membranes. Genes Dev 24:1893–1902
Luo G, Gruhler A, Liu Y, Jensen ON, Dickson RC (2008) The sphingolipid long-chain base-Pkh1/2-Ypk1/2 signaling pathway regulates eisosome assembly and turnover. J Biol Chem 283:10433–10444
Malinska K, Malinsky J, Opekarova M, Tanner W (2003) Visualization of protein compartmentation within the plasma membrane of living yeast cells. Mol Biol Cell 14:4427–4436
Malinska K, Malinsky J, Opekarova M, Tanner W (2004) Distribution of Can1p into stable domains reflects lateral protein segregation within the plasma membrane of living S. cerevisiae cells. J Cell Sci 117:6031–6041
Markin VS (1981) Lateral organization of membranes and cell shapes. Biophys J 36:1–19
Matsumoto K, Kusaka J, Nishibori A, Hara H (2006) Lipid domains in bacterial membranes. Mol Microbiol 61:1110–1117
McLaughlin S, Murray D (2005) Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438:605–611
Moor H, Muhlethaler K (1963) Fine structure in frozen-etched yeast cells. J Cell Biol 17:609–628
Moreira KE, Walther TC, Aguilar PS, Walter P (2009) Pil1 controls eisosome biogenesis. Mol Biol Cell 20:809–818
Mouritsen OG, Bloom M (1984) Mattress model of lipid-protein interactions in membranes. Biophys J 46:141–153
Mueller NS, Wedlich-Soldner R, Spira F (2012) From mosaic to patchwork: Matching lipids and proteins in membrane organization. Mol Membr Biol. doi:10.3109/09687688.2012.687461
Mulholland J, Preuss D, Moon A, Wong A, Drubin D, Botstein D (1994) Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J Cell Biol 125:381–391
Munro S (2003) Lipid rafts: elusive or illusive? Cell 115:377–388
Murphy ER, Boxberger J, Colvin R, Lee SJ, Zahn G, Loor F, Kim K (2011) Pil1, an eisosome organizer, plays an important role in the recruitment of synaptojanins and amphiphysins to facilitate receptor-mediated endocytosis in yeast. Eur J Cell Biol 90:825–833
Nakano K, Yamamoto T, Kishimoto T, Noji T, Tanaka K (2008) Protein kinases Fpk1p and Fpk2p are novel regulators of phospholipid asymmetry. Mol Biol Cell 19:1783–1797
Niles BJ, Mogri H, Hill A, Vlahakis A, Powers T (2012) Plasma membrane recruitment and activation of the AGC kinase Ypk1 is mediated by target of rapamycin complex 2 (TORC2) and its effector proteins Slm1 and Slm2. Proc Natl Acad Sci USA 109:1536–1541
Oh Y, Bi E (2011) Septin structure and function in yeast and beyond. Trends Cell Biol 21:141–148
Okuzaki D, Satake W, Hirata A, Nojima H (2003) Fission yeast meu14+ is required for proper nuclear division and accurate forespore membrane formation during meiosis II. J Cell Sci 116:2721–2735
Olivera-Couto A, Grana M, Harispe L, Aguilar PS (2011) The eisosome core is composed of BAR domain proteins. Mol Biol Cell 22:2360–2372
Penalva MA, Tilburn J, Bignell E, Arst HN Jr (2008) Ambient pH gene regulation in fungi: making connections. Trends Microbiol 16:291–300
Poveda JA, Fernandez AM, Encinar JA, Gonzalez-Ros JM (2008) Protein-promoted membrane domains. Biochim Biophys Acta 1778:1583–1590
Prosser DC, Drivas TG, Maldonado-Baez L, Wendland B (2011) Existence of a novel clathrin-independent endocytic pathway in yeast that depends on Rho1 and formin. J Cell Biol 195:657–671
Rankin BR, Moneron G, Wurm CA, Nelson JC, Walter A, Schwarzer D, Schroeder J, Colon-Ramos DA, Hell SW (2011) Nanoscopy in a living multicellular organism expressing GFP. Biophys J 100:L63–L65
Reijnst P, Walther A, Wendland J (2011) Dual-colour fluorescence microscopy using yEmCherry-/GFP-tagging of eisosome components Pil1 and Lsp1 in Candida albicans. Yeast 28:331–338
Robinson JM, Karnovsky MJ (1980) Evaluation of the polyene antibiotic filipin as a cytochemical probe for membrane cholesterol. J Histochem Cytochem 28:161–168
Roelants FM, Torrance PD, Bezman N, Thorner J (2002) Pkh1 and Pkh2 differentially phosphorylate and activate Ypk1 and Ykr2 and define protein kinase modules required for maintenance of cell wall integrity. Mol Biol Cell 13:3005–3028
Roelants FM, Baltz AG, Trott AE, Fereres S, Thorner J (2010) A protein kinase network regulates the function of aminophospholipid flippases. Proc Natl Acad Sci USA 107:34–39
Roux A, Cuvelier D, Nassoy P, Prost J, Bassereau P, Goud B (2005) Role of curvature and phase transition in lipid sorting and fission of membrane tubules. EMBO J 24:1537–1545
Schroeder RJ, Ahmed SN, Zhu Y, London E, Brown DA (1998) Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J Biol Chem 273:1150–1157
Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K (2003) Resistance of cell membranes to different detergents. Proc Natl Acad Sci USA 100:5795–5800
Seger S, Rischatsch R, Philippsen P (2011) Formation and stability of eisosomes in the filamentous fungus Ashbya gossypii. J Cell Sci 124:1629–1634
Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387:569–572
Simons K, Sampaio JL (2011) Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol 3:a004697
Sivadon P, Peypouquet MF, Doignon F, Aigle M, Crouzet M (1997) Cloning of the multicopy suppressor gene SUR7: evidence for a functional relationship between the yeast actin-binding protein Rvs167 and a putative membranous protein. Yeast 13:747–761
Snaith HA, Thompson J, Yates JR 3rd, Sawin KE (2011) Characterization of Mug33 reveals complementary roles for actin cable-dependent transport and exocyst regulators in fission yeast exocytosis. J Cell Sci 124:2187–2199
Sorre B, Callan-Jones A, Manneville JB, Nassoy P, Joanny JF, Prost J, Goud B, Bassereau P (2009) Curvature-driven lipid sorting needs proximity to a demixing point and is aided by proteins. Proc Natl Acad Sci USA 106:5622–5626
Spira F, Mueller NS, Beck G, von Olshausen P, Beig J, Wedlich-Soldner R (2012) Patchwork organization of the yeast plasma membrane into numerous coexisting domains. Nat Cell Biol 14(6):640–648. doi:10.1038/ncb2487
Stradalova V, Stahlschmidt W, Grossmann G, Blazikova M, Rachel R, Tanner W, Malinsky J (2009) Furrow-like invaginations of the yeast plasma membrane correspond to membrane compartment of Can1. J Cell Sci 122:2887–2894
Streiblova E (1968) Surface structure of yeast protoplasts. J Bacteriol 95:700–707
Sun Y, Taniguchi R, Tanoue D, Yamaji T, Takematsu H, Mori K, Fujita T, Kawasaki T, Kozutsumi Y (2000) Sli2 (Ypk1), a homologue of mammalian protein kinase SGK, is a downstream kinase in the sphingolipid-mediated signaling pathway of yeast. Mol Cell Biol 20:4411–4419
Tabuchi M, Audhya A, Parsons AB, Boone C, Emr SD (2006) The phosphatidylinositol 4,5-biphosphate and TORC2 binding proteins Slm1 and Slm2 function in sphingolipid regulation. Mol Cell Biol 26:5861–5875
Takeo K (1984) Lack of invaginations of the plasma membrane during budding and cell division of Saccharomyces cerevisiae and Schizosaccharomyces pombe. FEMS Microbiol Lett 22:97–100
Tanos B, Rodriguez-Boulan E (2008) The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene 27:6939–6957
Valdez-Taubas J, Pelham HR (2003) Slow diffusion of proteins in the yeast plasma membrane allows polarity to be maintained by endocytic cycling. Curr Biol 13:1636–1640
van Meer G, Stelzer EH, Wijnaendts-van-Resandt RW, Simons K (1987) Sorting of sphingolipids in epithelial (Madin–Darby canine kidney) cells. J Cell Biol 105:1623–1635
Vangelatos I, Roumelioti K, Gournas C, Suarez T, Scazzocchio C, Sophianopoulou V (2010) Eisosome organization in the filamentous ascomycete Aspergillus nidulans. Eukaryot Cell 9:1441–1454
Walther TC, Brickner JH, Aguilar PS, Bernales S, Pantoja C, Walter P (2006) Eisosomes mark static sites of endocytosis. Nature 439:998–1003
Walther TC, Aguilar PS, Frohlich F, Chu F, Moreira K, Burlingame AL, Walter P (2007) Pkh-kinases control eisosome assembly and organization. EMBO J 26:4946–4955
Wang HX, Douglas LM, Aimanianda V, Latge JP, Konopka JB (2011) The Candida albicans Sur7 protein is needed for proper synthesis of the fibrillar component of the cell wall that confers strength. Eukaryot Cell 10:72–80
Xu T, Shively CA, Jin R, Eckwahl MJ, Dobry CJ, Song Q, Kumar A (2010) A profile of differentially abundant proteins at the yeast cell periphery during pseudohyphal growth. J Biol Chem 285:15476–15488
Young ME, Karpova TS, Brugger B, Moschenross DM, Wang GK, Schneiter R, Wieland FT, Cooper JA (2002) The Sur7p family defines novel cortical domains in Saccharomyces cerevisiae, affects sphingolipid metabolism, and is involved in sporulation. Mol Cell Biol 22:927–934
Yu JW, Mendrola JM, Audhya A, Singh S, Keleti D, DeWald DB, Murray D, Emr SD, Lemmon MA (2004) Genome-wide analysis of membrane targeting by S. cerevisiae pleckstrin homology domains. Mol Cell 13:677–688
Zhang X, Lester RL, Dickson RC (2004) Pil1p and Lsp1p negatively regulate the 3-phosphoinositide-dependent protein kinase-like kinase Pkh1p and downstream signaling pathways Pkc1p and Ypk1p. J Biol Chem 279:22030–22038
Zimmerberg J, Kozlov MM (2006) How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol 7:9–19
Ziolkowska NE, Karotki L, Rehman M, Huiskonen JT, Walther TC (2011) Eisosome-driven plasma membrane organization is mediated by BAR domains. Nat Struct Mol Biol 18:854–856
Acknowledgments
We thank Héctor Yuyo Romero for helping with phylogenetic reconstructions and analysis. We also thank Arlinet Kierbel and Gustavo Pesce for collaborating with stimulating discussions and critical reading of the manuscript. This work was supported by the Agencia Nacional de Investigación e Innovación (INNOVA URUGUAY-DCI-ALA/2007/19.040 URU-UE, P.S.A.; Sistema Nacional de Becas, A.O.-C.; and Sistema Nacional de Investiga-dores, P.S.A.) and the Programa de Desarrollo de Ciencias Básicas (A.O.-C. and P.S.A).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Hohmann.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Box 1: Eisosome conservation and organization in other fungi
There is currently no evidence for eisosome presence in organisms outside the fungi kingdom. However, structurally comparable domains called caveolae are found in the plasma membrane of mammalian cells. Caveolae are 60–80 nm flask-shaped stable invaginations formed by scaffolding of the integral membrane proteins caveolins and the membrane-associated proteins cavins (Hansen and Nichols 2010). Like eisosomes, caveolae concentrate sterols and phosphoinositides and have been implicated in plasma membrane stress-mediated signaling and regulation of lipid homeostasis (Bastiani and Parton 2010; Hansen and Nichols 2010). Besides these appealing similarities, the repertoire of known core components and mechanism of assembly of both domains are quite dissimilar (Moreira et al. 2009; Hayer et al. 2010). Eisosomes and caveolae might, therefore, represent a case of convergent evolution.
Conservation of different eisosomal components across all fungi is disparate. Most eisosomal components are present in ascomycetes, Pil1 and Lsp1 and few others are found in basidiomycetes and no eisosomal proteins were found in zygomycetes or chytridiomycetes (Olivera-Couto et al. 2011). A phylogenetic analysis across the ascomycota phylum shows that most eisosomal proteins share a history filled with multiple events of gene duplication and gene loss, a common feature in this phylum (see Online Resource 1). In addition to extensive work in budding yeast, eisosomes are being actively characterized in four other ascomycetes, annotation of eisosomal proteins and their phylogenetic relationships are summarized in Table 2 and Online Resource 1:
Aspergillus nidulans: In ungerminated conidia of this filamentous fungus, eisosome organization is similar to budding yeast: Pil1/Lsp1 orthologs (PilA and PilB) and Sur7 ortholog (SurG) all colocalize at the spore periphery forming a dense net of foci (Vangelatos et al. 2010). In contrast, in actively growing hyphae eisosome organization is markedly different: PilA forms foci, whereas PilB is cytoplasmic and SurG shows vacuolar and endosomal localization. PilA is required for organization of SurG peripheral foci but not of PilB foci. On the other hand, SURG deletion leads to loss of PilB (but not PilA) peripheral foci (Vangelatos et al. 2010).
Ashbya gossypii: Eisosomes in this filamentous fungus share several common features with their S. cerevisiae counterparts: Pil1 and Lsp1 form static and stable foci that are assembled de novo at active sites of cellular growth (Seger et al. 2011). Also, eisosomes organization depends on the presence of Pil1 (but not of Lsp1). Unlike S. cerevisiae, deletion of NCE102 in A. gossypii does not affect eisosome organization, suggesting that there is no connection between Nce102 and Pkh kinases signaling. In addition to loss of eisosome organization, PIL1 deletion in A. gossypii leads to severe reduction of polar surface expansion and formation of abnormal bulged hyphae (Seger et al. 2011). The ortholog of YMR086w/YKL105c, SEG1, is needed to maintain eisosome stability (Seger et al. 2011).
Candida albicans: Studies in this human pathogen, which exhibits both budding yeast and hyphae morphologies, uncovered novel functional roles for Sur7. Like in S. cerevisiae, in C. albicans Pil1, Lsp1, Sur7 and Fmp45 form static foci in budding cells and also in hyphae (Alvarez et al. 2008; Reijnst et al. 2011; Wang et al. 2011). C. albicans eisosomes are absent at the tips of growing hyphae or buds, suggesting that de novo assembly is also restricted to areas of active growth (Reijnst et al. 2011). In contrast to S. cerevisiae, deletion of C. albicans SUR7 ortholog leads to several phenotypes, including lack of septin localization at bud necks, defective growth polarization, ectopic growth of cell wall, defective biofilm formation and, more importantly, decreased virulence in a mouse model of infection (Alvarez et al. 2008; Bernardo and Lee 2010; Wang et al. 2011; Douglas et al. 2012). Since septins control the correct positioning of actin patches and cell wall-synthesizing enzymes, it has been proposed that many of the phenotypes observed in sur7Δ cells are due to defects in septin organization (Alvarez et al. 2008). How Sur7 is implicated in septin organization remains to be elucidated. Notably, SUR7 deletion does not affect Lsp1 foci organization (Alvarez et al. 2008). Currently, there is no published evidence about the phenotypes caused by deletion of PIL1 or LSP1 in C. albicans. Thus, it is currently unknown whether C. albicans eisosomes mediate plasma membrane organization and if they are needed to sustain Sur7 functions.
Schizzosaccharomyces pombe: Both frozen-etch EM and fluorescent microscopy data showed that fission yeast has 1–2 μm long eisosomes, much larger than those present in S. cerevisiae (Moor and Muhlethaler 1963; Streiblova 1968; Takeo 1984; Kabeche et al. 2011). Like S. cerevisiae, eisosomes are formed de novo behind the active sites of growth. Eisosomes are also stable and static (Kabeche et al. 2011). However, during cell division eisosomes are actively removed from the future zone of septation. This clearance includes breakage, disassembly and even directional movement of eisosomes away from the cell division zone (Kabeche et al. 2011). Gene swapping experiments suggest that there is a Pil1-independent mechanism that regulates eisosome assembly in both fission and budding yeasts. In an S. pombe pil1Δ pil2Δ strain, expression of S. cerevisiae PIL1-GFP leads to formation of 1–2 μm long filaments that are indistinguishable from those observed in wild-type fission yeast cells. Conversely, expression of S. pombe Pil1-GFP in a pil1Δ S. cerevisiae strain renders Pil1-GFP puncta that colocalize with endogenous Lsp1 (Kabeche et al. 2011).
Like Nce102 in S. cerevisiae, S. pombe Fhn1 presents a dual localization being dispersed along the plasma membrane and concentrated at Pil1 filaments. Similarly, in pil1Δ cells Fhn1 disperses homogeneously in the plasma membrane and Pil1 filaments are less numerous and shorter in fhn1Δ cells (Kabeche et al. 2011). Surprisingly, S. pombe Slm1 and Sur7 orthologues do not colocalize with Pil1 or depend on Pil1 for their localization stressing the functional divergence that exists in budding and fission yeast’s Pil1 (Kabeche et al. 2011). There is a third, more distantly related Pil1/Lsp1 ortholog, Meu14, which is required for maturation of the forespore membrane (FSM) during sporulation (Okuzaki et al. 2003). The FSM is a double-membrane envelope that extends and engulfs each of the haploid nuclei produced by meiosis during sporulation. The extending FSM adopts a cup-like shape and Meu14 localizes at the rim, which is called the leading edge. In Meu14-depleted cells, the leading edges are abnormally assembled and FSMs are morphologically aberrant (Okuzaki et al. 2003). A high sensitivity search for distant orthologs suggested that, like Pil1 and Lsp1, Meu14 is a BAR domain-containing protein (our unpublished results). Thus, it is tempting to speculate that the molecular function of Meu14 is to maintain the high curvature of the FSM leading edge.
Box 2: Outstanding questions
-
Is there a physiological or genetic context in which eisosome domain organization is essential for cell growth?
-
Are Pil1 and Lsp1 sufficient to segregate proteins and lipids?
-
What are the molecular features that distinguish eisosome targeting of H+ -symporters from proteins of the Sur7 and Nce102 families?
-
Which are the molecular functions of still uncharacterized eisosomal proteins?
-
Which are the Pil1 and Lsp1 phosphatases?
-
What keeps eisosomes static?
-
How is eisosome assembly controlled?
-
Which are the molecular features that distinguish Pil1 from Lsp1?
-
Is there an eisosome-dependent endocytic pathway?
-
What is the molecular mechanism that releases Slm1 from eisosomes upon plasma membrane stress?
Rights and permissions
About this article
Cite this article
Olivera-Couto, A., Aguilar, P.S. Eisosomes and plasma membrane organization. Mol Genet Genomics 287, 607–620 (2012). https://doi.org/10.1007/s00438-012-0706-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-012-0706-8