Abstract
sd1 is known as the ‘green revolution’ gene in rice because its application in rice breeding has dramatically increased rice yield. Since the ‘green revolution,’ sd1 has been extensively used to produce modern semi-dwarf varieties. The extensive use of limited dwarfing sources may, however, cause a bottleneck effect in the genetic background of rice varieties. To circumvent this problem, novel and useful sources of dwarf genes must be identified. In this study, we identified three semi-dominant dwarf mutants. These mutants were categorized as dn-type dwarf mutants according to the elongation pattern of internodes. Gibberellin (GA) response tests showed that the mutants were still responsive to GA, although at a reduced rate. Map-based cloning revealed that the dwarf phenotype in these mutants was caused by gain-of-function mutations in the N-terminal region of SLR1. Degradation of the SLR1 protein in these mutants occurred later than in the wild type. Reduced interaction abilities of the SLR1 protein in these mutants with GID1 were also observed using the yeast two-hybrid system. Crossing experiments indicated that with the use of an appropriate genetic background, the semi-dominant dwarf alleles identified in this study could be used to alleviate the deficiency of dwarfing genes for breeding applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dwarfism is an agronomically important trait in breeding for resistance to damage by wind and rain (lodging resistance) and for stable high yields via increases in harvest index (Khush 2001). The two well-known dwarf genes that most significantly contributed to the history of crop breeding are semi-dwarf1 (sd1) in rice and reduced height1 (Rht1) in wheat (Hedden 2003). In the 1960s, IR8, a high-yielding semi-dwarf rice variety possessing the sd1 gene was developed and distributed all over Asia. The introduction of high-yielding semi-dwarf varieties combined with the application of large amounts of nitrogen fertilizer led to a remarkable increase in rice production that marked the dawn of the ‘green revolution’ in rice (Hargrove and Cabanilla 1979; Khush 1999). Similarly, introduction of the Rht1 gene in wheat resulted in a semi-dwarf phenotype. Adoption of the semi-dwarf wheat varieties possessing this gene greatly increased production and led to a parallel ‘green revolution’ (Hedden 2003). These landmarks in agricultural history clearly highlight the importance of the semi-dwarf trait in crop improvement. Since then, reducing plant height has become a predominant strategy in breeding high-yielding and non-lodging crop varieties (Hargrove and Cabanilla 1979). In fact, IR64, the most widely planted rice variety, possesses the sd1 gene, and at least seven independent sd1 alleles have been used to produce semi-dwarf rice varieties (Asano et al. 2007). Both sd1 and Rht1 are still widely used to produce modern varieties of rice and wheat, respectively (Hedden 2003).
Semi-dwarf rice varieties have been extensively used not only for the production of inbred, semi-dwarf varieties but also as parents of F1 hybrid varieties. Although F1 hybrid rice varieties have greatly contributed in increasing crop production, the occurrence of excessively tall plants due to heterosis constrains the practical breeding of F1 hybrids. To resolve this constraint, both the male and female parents must carry the same recessive semi-dwarf gene. Inevitably, this strategy led to the accumulation of several parental lines carrying the sd1 gene that are available for hybrid breeding (Yuan 1996; Kikuchi and Futsuhara 1997). The extensive use of limited dwarfing sources may, however, cause a bottleneck effect in the genetic background when breeding for new varieties, and this may cause the eventual genetic vulnerability of crops to pests and diseases (Hargrove and Cabanilla 1979; Kikuchi and Futsuhara 1997). To circumvent this problem, novel and useful sources of dwarf genes must be identified. Of particular advantage would be the identification and the subsequent use of dominant dwarf mutants. When one parent carries a dominant dwarf allele, the other parent need not carry a dwarf gene. Consequently, the germplasm of the other parent would not be restricted. Moreover, F1 seed production can be increased by selecting sterile parents that are 10–20 cm lower than the pollinator parent to increase pollen shedding on the female panicle (Virmani and Edwards 1983). Thus, introduction of a dominant dwarf allele into a sterile parent will increase seed production in the F1 hybrid.
To date, more than 60 rice dwarf genes have been identified (Futsuhara and Kikuchi 1997), although only a few have been identified as dominant, including D53, Ssi1, Sdd(t), and Td1 (Sunohara and Kitano 2003; Sunohara et al. 2006; Wei et al. 2006; Liu et al. 2008). Although several dwarf genes in rice have been recently cloned through map-based cloning (Ashikari et al. 1999; Yamamuro et al. 2000; Komorisono et al. 2005; Ueguchi et al. 2005; Zou et al. 2006), the genes are recessive and are not available for practical use, especially since the mutants have abnormal phenotypes. In contrast, no study has reported on the cloning of dominant and useful dwarf alleles in rice, although some reports exist on the cloning and characterization of dominant dwarf mutants in other crops such as wheat, barley, and maize (Peng et al. 1999; Chandler et al. 2002). Here we report on the first map-based cloning of a semi-dominant dwarf allele and the elucidation of the mechanisms that govern the semi-dominant dwarf phenotype in rice.
Materials and methods
Plant materials and growth conditions
Wild-type rice plants (Oryza sativa japonica cvs. T65 and Kinmaze) and three mutant alleles of Slr1-d were used in this study. All alleles were identified in a mutant library generated using N-methyl-N-nitrosourea (MNU). These lines were grown under natural field conditions in the research field of Nagoya University, Togo, Aichi, Japan. Seeds of all the lines were immersed in water for 2 days and were sown in a nursery bed. One-month-old seedlings were transplanted to the paddy field at 20 × 35 cm spacing.
GA induction in shoot elongation
Seeds of the wild-type and mutant lines were sterilized with 2.5% NaClO for 30 min, washed five times in sterile distilled water, and then incubated at 4°C for 1 day. The seeds were then placed on 1% agar plates containing various concentrations of GA3 and grown under continuous fluorescent light at 30°C. After 10 days, the length of the second leaf sheath of each plant was measured.
Mapping and sequencing of Slr1-d
To map Slr1-d, 634 F2 plants derived from a cross between Slr1-d3 and Kasalath, an indica cultivar, were used for map-based cloning. To identify the mutation sites in the Slr1-d alleles, SLR1 was amplified using genomic DNA extracted from the three mutant lines. The amplified DNA fragments were sequenced directly using appropriate primers.
Plasmid construction, transformation, and growth condition
To construct HA-SLR1 in a pActNos/Hm2 vector, each of the amplified SLR1 and HA fragments containing the appropriate restriction sites were initially cloned into a pBluescript vector using an EcoRI site for SLR1 and XbaI–SmaI sites for HA. To clone the HA, the pBluescript was modified to eliminate the EcoRI site in the vector by double digestion with EcoRV and PstI, followed by blunting the ends using T4 polymerase and self-ligation. The SLR1 clone was digested with EcoRI and inserted into HA at the EcoRI site. The HA–SLR1 construct was digested with XbaI and SmaI, and inserted into pActNos/Hm2 at the XbaI and SmaI sites. The binary vectors were then introduced into Agrobacterium tumefaciens strain EHA 101 (Hood et al. 1986) by electroporation. Rice transformation was performed as described by Hiei et al. (1994) using T65, a japonica cultivar. Transgenic plants were selected on a medium containing 50 mg/L hygromycin. Hygromycin-resistant plants were transplanted to the soil and grown at 30°C under a 16-h light:8-h dark photoperiod.
Protein extraction and immunoblot analysis
Total proteins were extracted by grinding the calli with liquid nitrogen and then resuspending the ground tissue in extraction buffer [20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 0.5% Tween 20, 1 mM EDTA, 1 mM dithiothreitol (DTT)] containing the Complete protease inhibitor cocktail (Roche, Switzerland). The extracts were cleared with two centrifugations at 20 min in a microcentrifuge. The protein concentrations of the soluble fractions were determined using Bradford reagent (Bio-Rad, USA) with bovine serum albumin as the standard. For immunoblot analysis, 10 μg of proteins was separated by SDS-PAGE and transferred to a Hybond ECL membrane (Amersham-Pharmacia Biotech, USA) by semi-dry blotting. Immunoblot analysis was carried out using a 1:10,000 dilution of a rabbit anti-SLR1 serum (Itoh et al. 2002) as the primary antibody and a horseradish peroxidase-conjugated goat anti-rabbit IgG (Medical and Biological Laboratories, Japan) as the secondary antibody. Peroxidase activity was detected following the manufacturer’s instructions (Pierce, USA).
Yeast two-hybrid assay
The Matchmaker Two-Hybrid System (Clontech, USA) was used for the yeast two-hybrid assay. pGBKT7-GID1 served as the bait, and pGADT7-SLR1 as the prey. Plate assays (-His) and β-galactosidase (β-gal) liquid assays were performed according to the manufacturer’s protocol (Clontech, USA) with the modification that the plate and liquid media either contained 10−4 M GA3 or not. The yeast strain AH109 was used for growth tests on -His plates, and the strain Y187 was used for the detection of β-gal activity by liquid assay.
Results
Identification of semi-dominant dwarf mutants
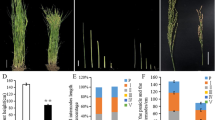
Three semi-dominant dwarf rice mutants (K304, CM211, and K344) were screened from more than 2,000 M2 lines mutagenized with MNU. K304 and K344 were derived from T65, whereas CM211 was derived from Kinmaze. All three mutants showed 50–70% reduction in plant height compared to the wild type, and had wide and dark green leaf blades (Fig. 1). Crossing K304, CM211, and K344 with their respective wild types yielded F1 plants with intermediate plant height relative to both parents (Fig. 1). In the subsequent F2 populations, the segregation ratio of dwarf to normal phenotype was 90:31 in K304, 69:23 in CM211, and 90:33 in K344, which corresponded to the expected 3:1 segregation ratio of single dominant genes (χ2 = 0.003, 0.014, and 0.133 in K304, CM211, and K344, respectively; Table 1). These results corroborate that dwarfism in K304, CM211, and K344 is controlled by a single dominant gene in each.
Gross morphology of dominant dwarf mutants at harvest. Each panel shows the wild type (T65 for K304 and K344, Kinmaze for CM211) (left), F1 plants derived from a cross between Slr1-d mutants and its wild type (center), and homozygous plants (right) of a K304 (Slr1-d1), b CM211 (Slr1-d2), and c K344 (Slr1-d3). Bar 30 cm
To investigate whether the genes responsible for the dwarf phenotypes in K304, CM211, and K344 were allelic, homozygous lines of the mutants (K304/K304, CM211/CM211, and K344/K344) were selected through progeny tests and were then crossed with each other using all possible cross-combinations. F1 and F2 populations derived from the crosses exhibited a dwarf phenotype without segregation (data not shown). The results indicated that the genes conferring the dwarf phenotype in K304, CM211, and K344 were allelic.
Characterization of dominant dwarf mutants in rice
The dwarf phenotype in rice is generally caused by a reduction in the length of the culm. Based on the elongation pattern of internodes, Takeda (1977) classified the dwarf phenotypes in rice into six types: N-, dn-, dm-, d6-, nl-, and sh-. Of these, the dn-type is defined by a reduction in the length of all internodes. The mutants K304, CM211, and K344 exhibited a reduction in the length of all internodes, characteristic of the dn-type dwarf (Fig. 2, Table 2).
Dwarfism in plants may arise due to various reasons, although defects in gibberellin (GA) biosynthesis and perception are the primary determinants of plant height. GAs comprise a large family of tetracyclic diterpenoid plant hormones that induce a wide range of plant growth responses including seed germination, stem elongation, leaf expansion, induction of flowering, and pollen maturation (Richards et al. 2001; Thomas et al. 2005). GA-related mutants exhibit dwarfism without any additional aberrant morphology. Often, leaf blades of the GA-related mutant plants are shorter, wider, and darker in color than those of the wild-type plants (Sakamoto et al. 2004). In the present study, the dominant mutants exhibited phenotypes that were reminiscent of GA-related mutants, and hence, the effects of GA application on the second leaf sheath elongation of the mutants were examined. Elongation of the second leaf sheath of the wild type was observed with the application of 10−8 to 10−7 M GA3. No such effects were observed in K304, CM211, and K344 with the application of ~10−7 M GA3 (Fig. 3). Treatment with 10−5 M GA3 resulted in the elongation of the second leaf sheath of the wild type up to 65 mm, whereas the same treatment only resulted in the elongation of the second leaf sheath of the mutants up to between 10 and 30 mm (Fig. 3). The results indicated that the mutants were still responsive to GA, albeit at a reduced rate.
Map-based cloning of the dwarf gene
To elucidate the molecular mechanism of the dominant genes conferring dwarfism in the mutants, map-based cloning of the dominant gene controlling dwarfism in K344, which showed the most severe phenotype, was carried out. In total, 634 F2 plants generated by crossing K344 with Kasalath were used to map the location of the gene. Plant height of the F2 population showed continuous and transgressive segregation (data not shown), so only plants exhibiting a severe dwarf phenotype were selected and used for mapping. Linkage analysis indicated that the dominant dwarf gene was tightly linked with one STS marker, TG1093, and was localized between the STS markers, ddw77 and ddw132, on the long arm of chromosome 3 (Fig. 4a). Cross-referencing in the Rice Annotation Project Database (Rice Annotation Project 2008) showed more than 120 predicted genes in this region. Survey of the predicted proteins within this candidate region showed that one gene encodes the DELLA protein, SLR1. DELLA proteins encoded by SLR1 and its orthologs in Arabidopsis (GAI, RGA, RGL1, RGL2, and AGL3), maize (d8), barley (SLN1), and grape (VvGAI) have a conserved function as repressors of GA signaling (Sun and Gubler 2004). Previous studies showed that in-frame deletion and specific point mutations within the N-terminal domain (DELLA and TVHYNP motifs and nearby sequences) result in constitutive DELLA protein function, thus conferring a dominant and GA-insensitive dwarf phenotype (Peng et al. 1997, 1999). Since the dwarf mutants in the current study were controlled by a dominant gene and had reduced GA responsiveness, the SLR1 gene was considered a very likely candidate for the dominant gene controlling dwarfism in K304, CM211, and K344.
Map-based cloning of K344 and sequence comparison of DELLA proteins. a High-resolution linkage and physical map of the K344 gene. Horizontal lines represent chromosome 3 and a physical map around the K344 locus of chromosome 3, near 126 cM. The vertical bars represent the molecular markers, and the numbers of recombinant plants are indicated between markers. b Structure of the SLR1 gene and mutation sites of the Slr1-d alleles. c Comparison of the deduced amino acid sequences of DELLA proteins. Amino acid sequences of DELLA proteins from rice (SLR1), Arabidopsis (GAI1 and RGA1), maize (D8), barley (SLN1), wheat (Rht1-D1), and lycophyte (SmDELLA1) were aligned using ClustalW, followed by manual alignment. The open triangles indicate mutation sites of Slr1-d mutants
Molecular analysis of the dominant dwarf mutants
To verify whether the dwarf phenotypes were caused by mutations within SLR1, DNA sequence analysis of SLR1 in the mutants was carried out. As expected, all mutants had a single nucleotide substitution in the N-terminal domain of SLR1. Based on these results and those of subsequent transgenic analysis, we renamed K304 as Slr1-d1, CM211 as Slr1-d2, and K344 as Slr1-d3. These 1-bp substitutions induced an amino acid substitution of Val-49 to Met in the DELLA domain of Slr1-d2 (CM211), and Leu-99 to Phe and Met-106 to Lys in the TVHYNP domain of Slr1-d3 (K344) and Slr1-d1 (K304), respectively (Fig. 4b). All amino acid residues substituted in Slr1-d mutants are likely to be important for DELLA protein function because they are completely conserved in all of the DELLA proteins from different plant species (Fig. 4c). To confirm that these mutations cause the dwarf phenotypes, transgenic plants that highly and constitutively express mutant SLR1 protein under the control of rice Actin1 promoter were generated and compared in terms of plant height. All transgenic plants expressing the mutant SLR1 proteins showed a more severe dwarf phenotype than those expressing the wild-type SLR1 protein (Fig. 5). The severity of the dwarf phenotype in the transgenic plants was correlated with the severity of dwarfism in the mutants (compare Figs. 5, 1), thereby confirming that the dominant dwarf mutants were caused by the gain-of-function of rice DELLA protein, SLR1.
Accumulation of SLR1 protein in Slr1-d mutants
The DELLA protein acts as a repressor of GA signaling, and its GA-dependent degradation is an essential event in GA signaling. Previous studies showed that gain-of-function mutation in the N-terminal domain of the DELLA protein, such as RGA and GAI in Arabidopsis and SLN1 in barley, gives the mutant protein resistance to GA-dependent degradation resulting in a GA-insensitive dwarf phenotype (Dill et al. 2001; Gubler et al. 2002). To examine the extent of GA responsiveness of Slr1-d proteins compared to the wild-type SLR1, immunoblot analysis using polyclonal anti-SLR1 antibodies was performed. Slr1-d calli were used for the experiment since rice callus cells contained very low levels of bioactive GA (Itoh et al. 2005), thus eliminating the need to pretreat these materials with a GA synthesis inhibitor to exclude the possibility of differences in GA content between varieties. Concordant with the results presented by Itoh et al. (2005), the GA-dependent degradation of SLR1 triggered by GA3 occurred within 60 min in the wild-type callus (Fig. 6). In the Slr1-d mutants, however, complete degradation of SLR1 proteins was not observed within 60 min. Furthermore, the rate of degradation corresponded to the severity of dwarfism in the mutants; that is, degradation of SLR1 protein in Slr1-d2, the mutant with the mildest dwarf phenotype, occurred the earliest, followed by that in Slr1-d1 and finally by that in Slr1-d3, the mutant exhibiting the most severe dwarfism (compare Figs. 6, 1). These results indicate that mutations in the N-terminal domain of SLR1 confer resistance against GA-dependent degradation, making the mutants only moderately sensitive to GA.
Degradation of SLR1 induced by GA3 treatment of the Slr1-d protein. Gel-blot analysis of SLR1 in the callus of the wild type, Slr1-d1, Slr1-d2, and Slr1-d3. These calluses were incubated with 10−5 M GA3 for the indicated times. A 10-μg aliquot of protein extract was loaded per lane and probed with anti-SLR1 antibody. The loading control of Coomassie Brilliant Blue staining is shown
Interaction between Slr1-d and GID1
Recent studies showed that DELLA proteins interact with the GA receptor, GID1, in a GA-dependent manner. The interaction occurs as a first step in GA signaling and is necessary for subsequent degradation of DELLA proteins. GID1 proteins in rice and Arabidopsis can interact with DELLA proteins in a GA-dependent manner in yeast cells (Ueguchi-Tanaka et al. 2005, 2007; Griffiths et al. 2006; Nakajima et al. 2006; Willige et al. 2007). A yeast two-hybrid assay was used to determine whether the interaction between Slr1-d and GID1 was affected in the Slr1-d mutants. Slr1-d proteins were expressed as GAL4 transactivation domain (AD) fusion proteins and the GID1 as GAL4 DNA-binding domain (BD) fusion proteins in yeast. Growth tests on -His plates using the yeast strain AH109, with or without 10−4 M GA3, were performed. All yeast cells expressing Slr1-d and GID1 grew on -HIS plates in the presence of GA3, whereas none of the yeast cells grew on -HIS plates without GA3. No apparent differences in the growth of yeast cells expressing the wild-type SLR1 and Slr1-d were observed, indicating that all of the Slr1-d proteins interacted with the GID1 protein in a GA-dependent manner (Fig. 7b). To assess the extent of interaction between Slr1-d and GID1, β-gal activity was measured by liquid assay using yeast strain Y187, with or without 10−4 M GA3. All Slr1-d weakly interacted with GID1 relative to the wild type (8.6, 0.22, 6.8, and 15.8 Miller units in Slr1-d1, Slr1-d2, Slr1-d3, and the wild type, respectively) (Fig. 7a). Results of the yeast two-hybrid assays confirmed that the extent of interaction between Slr1-d proteins and GID1 was reduced in the mutants.
Distribution of culm length in another genetic background
The culm length of Slr1-d2, the mutant with the mildest dwarf phenotype, was about 50 cm (Fig. 2, Table 2) and therefore may be too short for direct practical use. However, because Slr1-d mutants showed no other unfavorable phenotype except for low fertility in Slr1-d3, if crossed with tall varieties, these mutants might be useful as new dwarf gene resources. To test whether Slr1-d2 would have progeny with plant heights suitable for breeding purposes when crossed with a variety of different genetic backgrounds, Slr1-d2 was crossed with Kasalath, one of the tall indica varieties. Culm length showed a continuous and transgressive segregation in F2 populations (Fig. 8). We observed plants showing suitable culm length (around 70 cm) for practical use in both F1 and F2 populations.
Discussion
To date, more than 60 dwarf mutants of rice have been identified (Kikuchi and Futsuhara 1997) and some of them have been cloned through map-based cloning (Ashikari et al. 1999; Yamamuro et al. 2000; Komorisono et al. 2005; Ueguchi-Tanaka et al. 2005; Zou et al. 2006). However, because these mutants show malformed phenotypes such as short grain, extreme dwarfism, and sterility, sd1 is still the gene that is predominantly being used to produce semi-dwarf varieties (Hedden 2003; Asano et al. 2007). Identification of dominant dwarf genes would provide a new genetic resource that can be tapped for producing inbred semi-dwarf varieties and also for generating F1 hybrids. Cloning of the ‘green revolution’ genes, sd1 and Rht1, revealed that both are related to GA. The SD1 gene encodes the GA biosynthetic enzyme, GA20 oxidase 2, and mutations in this gene cause a reduction in the endogenous GA level, consequently resulting in a semi-dwarf phenotype in sd1 mutants (Ashikari et al. 2002; Sasaki et al. 2002; Spielmeyer et al. 2002). In contrast, Rht1 encodes a GA signal suppressor DELLA protein and deletion in the N-terminal region of the native RHT1 constitutively suppresses GA signaling, consequently resulting in a dominant semi-dwarf phenotype (Peng et al. 1997, 1999). Both cases underline the importance of GA in the regulation of developmental processes critical to agriculture, making the GA pathway a prime target for improving crop yield (Hedden 2003).
In this study, novel dominant mutants were identified: Slr1-d1, Slr1-d2, and Slr1-d3. All Slr1-d mutants had reduced plant height via the inhibition of elongation of all internodes, which is characteristic of dn-type dwarf rice mutants. Based on GA response tests, we concluded that Slr1-d mutants were still responsive to GA, albeit at a reduced rate. Cloning of the gene revealed that all Slr1-d mutants had a 1-bp substitution in the rice DELLA gene, SLR1, and transgenic analysis confirmed that these mutations were responsible for the dominant dwarf phenotype. Immunoblot analysis revealed that degradation of the SLR1 protein in Slr1-d mutants occurs later than in the wild type, with the rate of degradation corresponding to the severity of dwarfism in the mutants. DELLA proteins interact with the GA receptor, GID1, and this interaction eventually leads to the induction of DELLA protein degradation via the 26S proteasome pathway (McGinnis et al. 2003; Sasaki et al. 2003). Reduced interaction abilities of the SLR1 protein in the Slr1-d mutants with GID1 were confirmed using the yeast two-hybrid system. However, the extent of interaction did not correspond to the severity of dwarfism in the mutants. In particular, even under the presence of GA, SLR1 from Slr1-d2, the mutant with the mildest dwarf phenotype, showed no or very weak interaction with GID1. This may have been due to the unstable or low expression level of SLR1 protein of Slr1-d2 in Y187. Previous studies using a deletion series of the DELLA protein in yeast two-hybrid analysis also revealed that DELLA proteins and GID1 interact via the N-terminal of the DELLA protein (Ueguchi-Tanaka et al. 2007; Griffiths et al. 2006; Nakajima et al. 2006; Willige et al. 2007). In this study, results of interaction studies between GID1 and the SLR1 proteins from the Slr1-d mutants having single amino acid substitutions in the DELLA and TVHYNP domain reconfirmed the essential role of the N-terminal domain in the interaction between SLR1 and GID1. Based on these results, we concluded that the dwarf phenotypes of the Slr1-d mutants are caused by the inefficient GA-dependent degradation of the SLR1 protein due to the reduced interaction with GID1.
Fu et al. (2001) produced transgenic rice plants expressing the mutant form of Arabidopsis DELLA protein, gai, a protein resistant to GA-dependent degradation due to its lack of 17 amino acids in the DELLA domain. The authors pointed out the possible application of gai transgenic plants in breeding. However, because cultivation of transgenic plants in the field is restricted, identification and subsequent application of novel dwarf genes would still be more advantageous compared to the transgenic approach. Slr1-d2, the mutant with the mildest dwarf phenotype, may be too short for direct practical use. However, crossing experiments between Slr1-d2 and Kasalath revealed that culm length showed continuous and transgressive segregation in the resultant F2 populations, and plants with suitable culm length (around 70 cm) for practical use were also observed in F1 and F2 populations. The results indicate that with the use of an appropriate genetic background, the dominant dwarf genes identified in this study could provide a powerful tool to resolve the deficiency in dwarf genes for breeding. Alternatively, exploiting milder Slr1-d alleles using a more extensive screening approach or the tilling method (Suzuki et al. 2008) may yield new genetic sources for dwarf breeding. The practical application of this gene in breeding is currently under way.
References
Asano K, Takashi T, Miura K, Qian Q, Kitano H, Matsuoka M, Ashikari M (2007) Genetic and molecular analysis of utility of sd1 alleles in rice breeding. Breed Sci 57:53–58
Ashikari M, Wu J, Yano M, Sasaki T, Yoshimura A (1999) Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the alpha-subunit of GTP-binding protein. Proc Natl Acad Sci USA 96:10284–10289
Ashikari M, Sasaki A, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush GS, Kitano H, Matsuoka M (2002) Loss-of-function of a rice gibberellin biosynthetic gene, GA20 oxidase (GA20ox-2), led to the rice ‘green revolution’. Breed Sci 52:143–150
Chandler PM, Marion-Poll A, Ellis M, Gubler F (2002) Mutants at the Slender1 locus of barley cv Himalaya. Molecular and physiological characterization. Plant Physiol 129:181–190
Dill A, Jung HS, Sun TP (2001) The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc Natl Acad Sci USA 98:14162–14167
Fu X, Sudhakar D, Peng J, Richards DE, Christou P, Harberd NP (2001) Expression of Arabidopsis GAI in transgenic rice represses multiple gibberellin responses. Plant Cell 13:1791–1802
Futsuhara Y, Kikuchi F (1997) Inheritance of morphological characters. 2. Inheritance of dwarfism. In: Matsuo T et al (eds) Science of the rice plant, vol 3. Food and Agricultural Policy Research Center, Tokyo, Japan, pp 300–308
Griffiths J, Murase K, Rieu I, Zentella R, Zhang ZL, Powers SJ, Gong F, Phillips AL, Hedden P, Sun TP, Thomas SG (2006) Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18:3399–3414
Gubler F, Chandler PM, White RG, Llewellyn DJ, Jacobsen JV (2002) Gibberellin signaling in barley aleurone cells. Control of SLN1 and GAMYB expression. Plant Physiol 129:191–200
Hargrove TR, Cabanilla VL (1979) The impact of semi-dwarf varieties on Asian rice-breeding programs. Bioscience 29:731–735
Hedden P (2003) The genes of the green revolution. Trends Genet 19:5–9
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Hood EE, Helmer GL, Fraley RT, Chilton MD (1986) The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J Bacteriol 168:1291–1301
Itoh H, Ueguchi-Tanaka M, Sato Y, Ashikari M, Matsuoka M (2002) The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell 14:57–70
Itoh H, Sasaki A, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Hasegawa Y, Minami E, Ashikari M, Matsuoka M (2005) Dissection of the phosphorylation of rice DELLA protein, SLENDER RICE1. Plant Cell Physiol 46:1392–1399
Khush GS (1999) Green revolution: preparing for the 21st century. Genome 42:646–655
Khush GS (2001) Green revolution: the way forward. Nat Rev Genet 2:815–822
Kikuchi F, Futsuhara Y (1997) Inheritance of morphological characters. 2. Inheritance of semi-dwarf. In: Matsuo T et al (eds) Science of the rice plant, vol 3. Food and Agricultural Policy Research Center, Tokyo, Japan, pp 309–317
Komorisono M, Ueguchi-Tanaka M, Aichi I, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M, Sazuka T (2005) Analysis of the rice mutant dwarf and gladius leaf 1. Aberrant katanin-mediated microtubule organization causes up-regulation of gibberellin biosynthetic genes independently of gibberellin signaling. Plant Physiol 138:1982–1993
Liu BM, Wu YJ, Fu XD, Qian Q (2008) Characterizations and molecular mapping of a novel dominant semi-dwarf gene Sdd(t) in rice (Oryza sativa). Plant Breed 127:125–130
McGinnis KM, Thomas SG, Soule JD, Strader LC, Zale JM, Sun TP, Steber CM (2003) The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 15:1120–1130
Nakajima M, Shimada A, Takashi Y, Kim YC, Park SH, Ueguchi-Tanaka M, Suzuki H, Katoh E, Iuchi S, Kobayashi M, Maeda T, Matsuoka M, Yamaguchi I (2006) Identification and characterization of Arabidopsis gibberellin receptors. Plant J 46:880–889
Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP (1997) The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev 11:3194–3205
Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pelica F, Sudhakar D, Christou P, Snape JW, Gale MD, Harberd NP (1999) ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 400:256–261
Rice Annotation Project (2008) The Rice Annotation Project Database (RAP-DB): 2008 update. Nucleic Acids Res 36(Database issue):D1028–D1033
Richards DE, King KE, Ait-ali T, Harberd NP (2001) How gibberellin regulates plant growth and development: a molecular genetic analysis of gibberellin signaling. Annu Rev Plant Physiol Plant Mol Biol 52:67–88
Sakamoto T, Miura K, Itoh H, Tatsumi T, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Agrawal GK, Takeda S, Abe K, Miyao A, Hirochika H, Kitano H, Ashikari M, Matsuoka M (2004) An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol 134:1642–1653
Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush GS, Kitano H, Matsuoka M (2002) Green revolution: a mutant gibberellin-synthesis gene in rice. Nature 416:701–702
Sasaki A, Itoh H, Gomi K, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Jeong DH, An G, Kitano H, Ashikari M, Matsuoka M (2003) Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 299:1896–1898
Spielmeyer W, Ellis MH, Chandler PM (2002) Semi-dwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc Natl Acad Sci USA 99:9043–9048
Sun TP, Gubler F (2004) Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol 55:197–223
Sunohara H, Kitano H (2003) New dwarf mutant controlled by a dominant gene, Twisted dwarf1. Rice Genet Newsl 20:20–21
Sunohara H, Miura K, Wu X, Saeda T, Mizuno S, Ashikari M, Matsuoka M, Kitano H (2006) Effects of Ssi1 gene controlling dm-type internode elongation pattern on lodging resistance and panicle characters in rice. Breed Sci 56:261–268
Suzuki T, Eiguchi M, Kumamaru T, Satoh H, Matsusaka H, Moriguchi K, Nagato Y, Kurata N (2008) MNU-induced mutant pools and high performance TILLING enable finding of any gene mutation in rice. Mol Genet Genomics 279:213–223
Takeda K (1977) Internode elongation and dwarfism in some graminaeous plants. Gamma Field Symp 16:1–18
Thomas SG, Rieu I, Steber CM (2005) Gibberellin metabolism and signaling. Vitam Horm 72:289–338
Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow TY, Hsing YI, Kitano H, Yamaguchi I, Matsuoka M (2005) GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437:693–698
Ueguchi-Tanaka M, Nakajima M, Katoh E, Ohmiya H, Asano K, Saji S, Hongyu X, Ashikari M, Kitano H, Yamaguchi I, Matsuoka M (2007) Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin. Plant Cell 19:2140–2155
Virmani SS, Edwards IB (1983) Current status and future prospects for breeding hybrid rice and wheat. Adv Agron 36:145–214
Wei LR, Xu JC, Li XB, Qian Q, Zhu LH (2006) Genetic analysis and mapping of the dominant dwarfing gene D-53 in rice. J Integr Plant Biol 48:447–452
Willige BC, Ghosh S, Nill C, Zourelidou M, Dohmann EM, Maier A, Schwechheimer C (2007) The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell 19:1209–1220
Yamamuro C, Ihara Y, Wu X, Noguchi T, Fujioka S, Takatsuto S, Ashikari M, Kitano H, Matsuoka M (2000) Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell 12:1591–1606
Yuan LP (1996) Hybrid rice breeding in China. In: Virmani SS, Siddiq EA, Muralidharan K (eds) Advances in hybrid rice technology. IRRI, Los Baños Philippines, pp 27–33
Zou J, Zhang S, Zhang W, Li G, Chen Z, Zhai W, Zhao X, Pan X, Xie Q, Zhu L (2006) The rice HIGH-TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. Plant J 48:687–698
Acknowledgments
The mutant lines used in this study were obtained from the National Institute of Genetics supported by the National Bioresource Project, MEXT, Japan. This work was supported by a grant-in-aid from the Ministry of Agriculture, Forestry and Fisheries of Japan (Green Technology Project IP-1003) and a research fellowship from the Japan Society for the Promotion of Science for Young Scientists.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Tyagi.
Rights and permissions
About this article
Cite this article
Asano, K., Hirano, K., Ueguchi-Tanaka, M. et al. Isolation and characterization of dominant dwarf mutants, Slr1-d, in rice. Mol Genet Genomics 281, 223–231 (2009). https://doi.org/10.1007/s00438-008-0406-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-008-0406-6