Abstract
Injured plants induce a wide range of genes whose products are thought to help to repair the plant or to defend against opportunistic pathogens that might infect the wounded plant. In Arabidopsis thaliana L., oligogalacturonides (OGAs) and jasmonic acid (JA) are the main regulators of the signaling pathways that control the local and systemic wound response, respectively. RNS1, a secreted ribonuclease, is induced by wounding in Arabidopsis independent of these two signals, thus indicating that another wound-response signal exists. Here we show that abscisic acid (ABA), which induces wound-responsive genes in other systems, also induces RNS1. In the absence of ABA signaling, wounding induces only approximately 45% of the endogenous levels of RNS1 mRNA. However, significant levels of RNS1 still accumulate in the absence of ABA signaling. Our results suggest that wound-responsive increases in ABA production may amplify induction of RNS1 by a novel ABA-independent pathway. To elucidate this novel pathway, we show here that the wound induction of RNS1 is due in part to transcriptional regulation by wounding and ABA. We also show evidence of post-transcriptional regulation which may contribute to the high levels of RNS1 transcript accumulation in response to wounding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Secreted ribonucleases (RNases) are enzymes located where RNA is not thought to be readily available, such as in the vacuole or outside the cell. The T2 superfamily of secreted RNases, in particular, has been found in nearly every system examined for their presence, including fungi, viruses, bacteria, plants, and animals (Deshpande and Shankar 2002). The ubiquitous distribution of T2 RNases suggests that they have both an ancient origin and critical function(s) (Taylor and Green 1991).
Despite the apparent necessity for the activity of T2 enzymes, very few has been demonstrated regarding their biological functions. The exception is S-RNases, a class of plant T2 RNases whose activity is essential for the process of self-incompatibility in several plant families (reviewed in McCubbin and Kao 2000). Enzymes related to, but distinct from, S-RNases are also present in self-compatible plants and form a class known as S-like RNases (reviewed in Bariola and Green 1997). S-like enzymes are not involved in self-incompatibility, but seem to have important functions throughout the plant kingdom, as they are ubiquitous in plants. The A. thaliana genome contains five S-like genes, RNS1 to RNS5 (Taylor and Green 1991; G.C. MacIntosh, unpublished), and RNase activity has been demonstrated for the products of three of these (Taylor et al. 1993; Bariola et al. 1994).
Fluctuations in RNase activity levels or gene expression are useful for predicting RNase function. The discovery that growth on low concentrations of inorganic phosphate (P i) induces expression of various RNases, including Arabidopsis RNS1 and RNS2 (Bariola et al. 1994, 1999) and tomato RNases LX and LE (Nürnberger et al. 1990; Bosse and Köck 1998), led to the hypothesis that S-like RNases are part of a rescue system that plants use to recycle P i when environmental pools are limiting (Goldstein et al. 1989).
In addition to low inorganic phosphate concentration, RNases are also induced by wounding in several plant systems. For example, the transcript for RNase LE accumulates in wounded tomato leaves (Lers et al. 1998; Groß et al. 2004), and RNase NW is induced in wounded tobacco leaves (Kariu et al. 1998). We showed that RNS1 and several nuclease activities are coordinately regulated by wounding in Arabidopsis (LeBrasseur et al. 2002). The RNS1 transcript was the most highly wounding-induced transcript in two independent microarray experiments—one examined 150 genes enriched for those implicated in defense responses (Reymond et al. 2000), and the second examined 600 genes, about half of which were hypothesized to be involved in RNA metabolism and turnover (Pérez-Amador et al. 2002). The strong RNS1 transcript accumulation may indicate that RNS1 has a critical function during wounding. RNS1 transcript and activity are also increased in non-damaged tissue of wounded plants, where recycling of nutrients and degradation of bulk cellular nucleic acid, should not be necessary. We therefore proposed that RNS1 may also have a defensive function (LeBrasseur et al. 2002).
The induction of RNS1 and nuclease activities provides us with a unique perspective into Arabidopsis wound signaling mechanisms (LeBrasseur et al. 2002). Our understanding of the wound response in Arabidopsis is currently highlighted by the presence of two distinct, antagonistic pathways: JA-dependent and -independent. The JA-independent pathway controls local transcript accumulation and has been shown to be regulated by OGA elicitors probably released from injured plant cell walls (Rojo et al. 2003).
Although RNS1 and the three nuclease activities are strongly induced locally by wounding, they are not induced by treatments with OGA-rich fractions (LeBrasseur et al. 2002). The local response of RNS1 and the nucleases to wounding is also not controlled by the JA-dependent signaling pathway, as shown by the strong wound-induction of these activities in the JA-insensitive coi1 mutant. It has been proposed that JA signaling controls the systemic wound response in Arabidopsis (Titarenko et al. 1997; León et al. 2001) but the systemic induction of RNS1 did not depend on JA (LeBrasseur et al. 2002). To our knowledge, RNS1 was the first gene in Arabidopsis shown to be induced systemically by wounding in a JA-independent manner and therefore indicates the existence of an alternative long-distance signaling pathway.
It is becoming clear that JA-independent pathways are important in the regulation of wounding response, however very little is known about the signal transduction pathways controlling these responses (Howe 2004). Several molecules have been proposed to act as signals in the wounding response in plants in addition to OGAs and JA (reviewed in de Bruxelles and Roberts 2001; León et al. 2001; Howe 2004), including abscisic acid (ABA, see review by Lorenzo and Solano 2005). ABA application induces the local and systemic expression of PinII, a wound-inducible gene, in potato, tomato and tobacco (Peña-Cortés et al. 1989). Analyses of ABA-deficient mutants of potato and tomato provided further evidence for a requirement for ABA in the wound-induction of Pin genes (Peña-Cortés et al. 1989, 1991), and ABA accumulates upon wounding (Peña-Cortés et al. 1991). However, the role of ABA in the wounding response is controversial. Birkenmeier and Ryan (1998) found that exogenous ABA induces PinII expression in tomato to a much lesser extent than either wounding or JA application, and that endogenous ABA levels only increase significantly at the wound site.
Recent evidence suggests that RNS1 may be controlled by ABA signaling. A mutant screen identified an mRNA cap-binding protein, ABH1, as a negative modulator of ABA signaling in stomata (Hugouvieux et al. 2001). DNA chip analyses comparing gene expression in WT and abh1 plants identified RNS1 as one of a few transcripts that are down-regulated in abh1. These genes might function in early ABA signaling, as their transcripts represent putative targets for ABH1-dependent mRNA processing (Hugouvieux et al. 2001). As ABA is a proposed regulator of the wounding response in other plants, it could also control the OGA- and JA-independent pathway defined by RNS1 expression in Arabidopsis. In addition, the abh1 results indicate that ABA might post-transcriptionally stabilize RNS1 mRNA after wounding. Here, we show that ABA induces RNS1 expression with a timing that is similar to that of wounding. We also show that ABA is necessary to produce the full wounding response. However, ABA is only part of the signaling pathways controlling RNS1 induction in wounded Arabidopsis plants. Our results indicate that an as-yet uncharacterized ABA-independent pathway, independent of JA and OGA as well, also contributes to RNS1 induction during the wounding response. We found evidences that this novel pathway acts synergistically with ABA to regulate RNS1 induction at the transcriptional level. The possibility of post-transcriptional regulation is also discussed.
Materials and methods
Plant materials and treatments
Unless otherwise stated, the Columbia-0 ecotype of Arabidopsis thaliana L. was used throughout this study. Soil-grown plants were grown in chambers under 16 h of light in 60% relative humidity at 21°C. For seedling experiments, seeds were surface-sterilized and germinated on Arabidopsis growth medium as described (Taylor et al. 1993). The aba1-1, abi1 and abi2 seeds were kindly provided by Dr. Michael Thomashow (Michigan State University), abi4 and abi5 seeds were obtained from the Arabidopsis Biological Resource Center (ABRC). For wounding treatments, leaves of 4- to 6-week-old plants or leaves of 14-day-old seedlings were wounded using ridged flat-tipped tweezers, harvested at subsequent timepoints, and treated as previously described (LeBrasseur et al. 2002). ABA treatments were conducted on 14-day-old seedlings grown on MS-agar plates covered with plastic mesh. Seedlings were transferred to 0.5× MS medium (Sigma, Saint Louis, MO, USA) with or without 100 μM ABA (Sigma, Saint Louis, MO, USA) and harvested at subsequent timepoints. WT controls for the ABA mutant experiments were performed with the ecotype Landsberg erecta (Ler), Columbia-0 (Col) or Wassilewskija (Ws) as indicated. Experiments were performed a minimum of three times. Representative blots or gels are shown.
Plants were transformed by vacuum infiltration as previously described (Bariola et al. 1999). For each experiment, at least three independently transformed lines were used. Representative results are shown.
Cloning and sequence analysis
Standard cloning techniques were used throughout. The RNS1 promoter region was isolated previously (Howard 1996) and contains 2.6 kb of DNA upstream of the RNS1 initiation codon. This includes DNA from chromosome 2 coordinates 870957 (5′) to 873663 (3′), based on the current AGI annotation as shown at TAIR (http://www.arabidopsis.org), which corresponds to the TAIR 7 version of the Arabidopsis genome, released in April 2007. The promoter region was cloned into a Bluescript II vector (Stratagene, La Jolla, CA, USA) containing the β-glucuronidase (GUS) protein sequence (Jefferson et al. 1987) with a rbcS-E9 polyadenylation sequence (Fang et al. 1989). We removed the short stretch of 5′ UTR sequence that was present in this construct to create a 3′ end on the RNS1 promoter that corresponds to coordinate 873563. This construct was then cloned into an A. tumefaciens shuttle vector containing a kanamycin resistance gene as described before (Gil and Green 1996) and designated p2081. In plasmid p2082 the GUS coding region was replaced by luciferase (Millar et al. 1992). Construct p848 (35S-GUS-E9), containing the cauliflower mosaic virus 35S promoter in place of the RNS1 promoter, was constructed in a similar manner (Howard 1996).
The nos (nopaline synthase) promoter was amplified by PCR from pBI-121 using the primers PG-454 (5′-gatcatctgcagagaattaagg) and PG-453 (5′-gttcaaccatgggaaacgatcc). The nos-globin-E9 construct was made by replacing the 2× 35S promoter of p1185 (Diehn et al. 1998) with nos to make p2031. The RNS1 cDNA (Bariola et al. 1994) was then inserted in place of globin to make p1966. The entire nos-RNS1-E9 cassette was then cloned into the plant transformation vector pCambia 1301 (GenBank accession number AF234297), which has the hygromycin resistance plant selection marker. This clone was named p1975. The nos-globin-E9 cassette was cloned into pCambia 2301 (GenBank accession number AF234316), which confers kanamycin resistance to transformed plants, and was named p1995. The entire RNS1 transcribed region, including the full 5′ UTR and introns, was PCR-amplified. PCR products were sequenced to assure no errors were introduced and then inserted in place of globin in p2031. The orientation of the insert was confirmed and then the nos-preRNS1-E9 cassette was ligated into pCambia 2301.
Computational analysis of the proximal 1,000 nt of the promoter sequence was performed using two internet-accessible databases, PlantCARE (Lescot et al. 2002) and PLACE (Higo et al. 1999). Only elements in which the core is absolutely conserved are reported here.
RNA and protein extraction and analysis
Total RNA from Arabidopsis samples was extracted and analyzed as previously described (LeBrasseur et al. 2002). RNA blots were hybridized using a 32P-labeled RNS1 probe. To control for loading, the same RNA blots were stripped and then hybridized with a 32P-labeled probe for the Arabidopsis translation elongation factor EF-1α (EST accession number R29806) or translation initiation factor eIF-4A (Taylor et al. 1993). The COR6.6 gene, kindly provided by Dr. Michael Thomashow (Michigan State University), was used as a positive control for ABA treatments (Hajela et al. 1990). GUS and globin probes were prepared by PCR. The nos probe was prepared by polynucleotide kinase end-labeling of an antisense oligonucleotide using 32P-ATP (sequence: GATCCAGATCCGGTGCAGATTATTTGGATTGAGAGTGAATAT). All blots were exposed for 16 h, except RNS1p-GUS constructs that were exposed for 3–5 days. Blots were quantified using PhosphorImager. RNS1, GUS and nos expression data were normalized using EF-1α values (as RNS1/EF-1α; GUS/EF-1α; nos/EF-1α). The ratios from at least three independent experiments were used for the expression data shown in Figs. 2b, 5b and 6c, d. For the nos-RNS1 constructs, both the individual bands and the doublet as a whole were quantified. Results for the doublet are reported, but individual bands gave similar results.
To verify the identity of the two bands obtained with the nos-RNS1 reporter constructs, 3′ rapid amplification of cDNA ends (RACE) was performed using the 3′ RACE System (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s protocols. Gene specific primer for initial PCR was the nos probe describe above. Primers for nested PCR were GTGTTTGATCAGTCTTCTCGTAATCTTGC (RNS1) and CTGATGCATTGAACTTGACGAACGTTGTCG (E9).
Total protein was extracted and RNase activities were assayed as described previously (LeBrasseur et al. 2002). Equal loading of protein gels was confirmed by Coomassie Blue staining of standard SDS-PAGE loaded with the same volume of protein extracts used for activity assays. All blots and gels are representative of at least three independent experiments.
Histochemical GUS staining and luciferase imaging
Histochemical localization of GUS activity was determined using a β-Glucuronidase Reporter Gene Staining Kit (Sigma) according to manufacturer’s recommendations. Luciferase activity was analyzed using a CCD camera (ChemiPro System, Roper Scientific, Trenton, NJ, USA) as described by Chinnusamy et al. (2002); exposure time was 20 min.
Results
RNS1 expression is induced by ABA
It has been suggested that ABA may regulate RNS1 transcript accumulation, as loss of an mRNA-binding protein, ABH1, that downregulates ABA responses leads to reduced RNS1 transcript levels (Hugouvieux et al. 2001). Transcriptional regulation of gene expression by ABA has been characterized to a large degree. Thus, we analyzed the RNS1 promoter sequence to identify putative regulatory elements (Fig. 1a, Electronic supplementary Fig. S1). A search for regions with homology to known regulatory elements identified three putative ABA-responsive elements (ABREs; Yamaguchi-Shinozaki and Shinozaki 1993, 1994), and one MYB- and three MYC-binding regions. Some members of the MYC and MYB transcription factor families are induced by drought and ABA (Abe et al. 1997). A dehydration response element (DRE) was also found in the RNS1 promoter. This element has been shown to be sufficient for a rapid response to dehydration without the involvement of ABA (Yamaguchi-Shinozaki and Shinozaki 1993, 1994). In addition, several wounding-responsive elements were found in the RNS1 promoter: a W-box (Eulgem et al. 1999) and two WUN elements (Pastuglia et al. 1997).
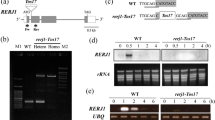
ABA induces RNS1 expression. a Structure of the RNS1 promoter. Motifs with significant similarity to previously identified cis-acting elements are shown (grey boxes). These include CAAT and TATA boxes, wound-responsive elements (W-box, WUN), a dehydration-responsive element (DRE), ABA-responsive elements (ABRE), and MYB and MYC binding sites. b Northern analysis of RNA isolated from seedlings treated with 100 μM ABA for the indicated times (h). The COR6.6 probe was used as a control for the ABA treatment, and EF-1α as a control for loading
To test whether ABA could in fact induce RNS1 expression, we treated Arabidopsis seedlings with ABA and samples were harvested at different time points. Mock-treated plants were harvested as control. Figure 1b shows that RNS1 is induced in seedlings treated with ABA with kinetics similar to that of COR 6.6, a known ABA regulated gene (Gilmour et al. 1992). Using the ABA-insensitive mutant abi2 (see below) we also showed that the induction of RNS1 by ABA is regulated by the ABI2 pathway (Supplementary Fig. S2a). In the abi2 mutants ABA is unable to induce RNS1 accumulation.
We also analyzed the induction of RNS1 activity by ABA using an in gel activity assay. In this assay extracts are resolved by semi-denaturing SDS-PAGE using gels containing RNA, later incubated in activity buffer, and finally stained to detect RNA. Clear bands represent ribonuclease activities (Supplementary Fig. S2b). Twelve hours after ABA treatment an increase in RNS1 activity is clearly observed, and it maintains similar levels after 24 h. Thus, ABA is able to induce RNS1 mRNA accumulation followed by an increase in RNS1 activity.
Both ABA-dependent and -independent pathways control RNS1 induction by wounding
Because RNS1 expression is induced by ABA, we tested whether ABA is the signal that controls the wounding pathway resulting in the accumulation of RNS1 transcript and protein. To address this question, we took advantage of a series of mutants deficient in ABA production and signaling. ABI1 and ABI2 encode protein phosphatases that participate in the transmission of the ABA signal (Leung et al. 1994, 1997). Mutant plants carrying the abi1 and abi2 alleles are insensitive to ABA. In addition, aba1-1 mutant plants possess a non-functional zeaxanthin epoxidase and cannot produce ABA (Rock and Zeevaart 1991); consequently, ABA-dependent processes are inhibited in these plants.
RNA blot analyses revealed that the wounding induction of RNS1 transcript accumulation in abi1, abi2, and aba1-1 mutants is only 32–48% that of the WT plants (Fig. 2a, b). Our results indicate that an ABA-dependent pathway is required for the full induction of RNS1 after wounding. However, wounding still induces RNS1 expression in these mutants; thus, an as-yet uncharacterized ABA-independent pathway is responsible for the induction of RNS1 in the absence of ABA signaling. As described previously (LeBrasseur et al. 2002), this pathway is also independent of JA and OGA, the two signals commonly associated with wounding responses in Arabidopsis.
Participation of ABA in the wound signaling pathway that controls RNS1 expression. Wild type (Ler) and mutants in ABA signaling (abi1, abi2) and biosynthesis (aba1-1) were examined for induction of RNS1 after wounding. a Northern blot analysis of RNA extracted from seedlings 4 h after wounding. b Average values of the quantification of the results obtained in three experiments as the ones described in a. Three independent experiments were performed; for each experiment individual bands were quantified and normalized using EF-1α as loading control, these values from the three experiments were then averaged and standard error was calculated. c Increase in RNS1 activity in response to ABA and wounding is compromised in the abi2 mutant. Wild type (Ler) and abi2 2-week-old seedlings were examined for RNase activities. Plants were wounded for 12 h, treated for 12 and 24 h with ABA or left untreated as control. Twenty micrograms of proteins were loaded in each lane
We also analyzed the role of ABA on the wound-dependent increase in RNS1 activity by in gel RNase activity assay. Figure 2c shows that the increase in RNS1 activity observed after ABA treatment is absent in the abi2 mutant. In addition, a modest decrease in activity (although this assay is not truly quantitative we estimated a reduction of ~25%) can be observed in wounded abi2 plants with respect to wounded WT plants. These results are similar to those obtained by northern blots, and confirm the existence of two pathways that control the expression of RNS1 and the increase in RNS1 activity in response to wounding. RNS1 induction by wounding is paralleled by an increase in several nuclease activities that degrade both RNA and DNA (LeBrasseur et al. 2002). Both the sustained induction of the 33-kD activities and the transient increase in the 35-kD activity still occur in all the tested ABA mutants (data not shown), indicating that the uncharacterized ABA-independent pathway is also at least partially responsible for the induction of other nuclease activities after wounding.
In an initial attempt to dissect the ABA-dependent pathway controlling RNS1 expression we analyzed whether any of the most common transcription factors involved in regulation of ABA-dependent transcription was necessary for wound induction of RNS1. Three different classes of transcription factors have been identified through genetic screenings of plants with reduced sensitivity to ABA (reviewed by Finkelstein et al. 2002). The abi3 mutation corresponds to a B3-domain transcription factor (Giraudat et al. 1992), while abi4 and abi5 correspond to APETALA2 domain (Finkelstein et al. 1998) and bZIP domain (Finkelstein and Lynch 2000) transcription factors, respectively. Microarray analyses indicate that ABI3 does not control RNS1 expression (Suzuki et al. 2003). Thus, we tested whether ABI4 or ABI5 were responsible for ABA-dependent induction of RNS1. WT and mutant abi4 and abi5 plants were wounded and RNA was extracted 4 h later. We found that neither ABI4 nor ABI5 are necessary for full induction of RNS1 by wounding (Supplementary Fig. S3). These results suggest that another transcription factor is responsible for ABA-dependent induction of RNS1 by wounding. Alternatively, post-transcriptional processes could be invoked to explain this regulation.
Evidence for transcriptional and post-transcriptional control of RNS1 by wounding and ABA
As a first step toward dissecting the regulatory mechanisms that control RNS1 gene expression, we investigated whether RNS1 transcript accumulation is controlled at the transcriptional and/or the post-transcriptional level. To this end we made transgenic Arabidopsis lines carrying the constructs depicted in Fig. 3. Transcriptional regulation was analyzed using the construct RNS1p-GUS (Fig. 3b), in which a 2.6-kb fragment corresponding to the RNS1 promoter region controlled the expression of the β-glucuronidase (GUS) reporter gene. The same reporter driven by the CaMV 35S promoter was used as control (35S-GUS; Fig. 3a). Transformed plants were analyzed by RNA gel blots.
Constructs used to examine the regulation of RNS1. Several constructs were used to transform wild-type Arabidopsis plants. Transgenic lines were then used to analyze the expression of the reporters under various conditions. LUC Luciferase coding region, GUS β-glucuronidase coding region, 35S CaMV 35S promoter; nos nopaline synthase promoter; RNS1p RNS1 promoter, E9 3′ end of the pea E9 gene, preRNS1 transcribed region of RNS1 including UTRs and introns
As shown in Fig. 4, RNS1 is regulated at the transcriptional level. In untreated leaves of plants transformed with the RNS1p-GUS construct, the GUS transcript is not detected. But 4 h after wounding, the GUS transcript is clearly expressed in wounded leaves. The control 35S-GUS lines showed no response to wounding. Similarly, GUS accumulation is also observed in plants treated with ABA. These results show that the RNS1 promoter is sufficient to provide a transcriptional response to wounding and ABA.
The RNS1 promoter confers wound- and ABA-inducibility to reporter transcripts. Leaves of transgenic Arabidopsis plants expressing the GUS reporter under the control of either 2.6 kb of genomic sequence upstream of the RNS1 transcription start site or the constitutive 35S promoter were harvested 4 h after wounding or ABA treatment (W and A, respectively). Untreated plants were used a control (C) for wounding and buffer treated plants (C) were used as control for ABA treatments. Blots were probed with GUS, then stripped and probed with EF-1α (to control for loading). 35S-GUS plants were used as controls to demonstrate that GUS is not stabilized by wounding. For each experiment, at least three independently transformed lines were used. Representative results are shown
Although endogenous levels of RNS1 transcript are induced both by wounding and ABA, after 4 h endogenous RNS1 expression is fivefold higher in wounded plants than in ABA-treated plants (Fig. 5a, b). Side-by-side comparison of the levels of GUS reporter transcript showed that GUS expression is similar or higher in RNS1p-GUS plants treated with ABA compared to those that were wounded (Fig. 5a, b). Thus, although the RNS1 promoter used in these studies is sufficient to provide transcriptional control in response to both stimuli, other regulatory mechanisms seem to contribute to the different levels of induction of the RNS1 transcript from the native gene.
Differential accumulation in response to wounding and ABA of endogenous or reporter genes under the control of the RNS1 promoter. a Northern blot analysis of transcript accumulation corresponding to the endogenous RNS1 (upper panels) or the GUS reporter under the control of the RNS1 promoter (lower panels). Blots were treated as in Fig. 4. b Quantification of data shown in a. Data represent the average normalized ratio from at least four independent experiments involving eight independent transgenic plant lines. For each blot, the normalized values were calculated by dividing the GUS (or the RNS1) transcript level in response to wounding and to ABA to that of the EF-1α transcript. Only the +wounding or +ABA transcript level (each divided by that of EF-1α) was used to calculate the normalized ratio for a given experiment
To examine the possibility of post-transcriptional regulation by wounding and ABA, we designed constructs containing either the RNS1 cDNA or genomic DNA under the control of the nopaline synthase (nos) promoter (Fig. 3d, e). Specifically, we fused the mature RNS1 transcribed region (RNS1cDNA) or a genomic clone corresponding to the coding region plus 5′ and 3′ UTR and intron sequences of RNS1 (pre-RNS1) to the nos promoter (designated nos-RNS1cDNA and nos-preRNS1, respectively; Fig. 3d and e). As a control for this set of constructs, we used the human β-globin gene under the control of the nos promoter (nos-globin, Fig. 3c). These constructs contain a ‘tag’ of 42 nucleotides transcribed from the nos promoter. Thus, blots were probed with an oligonucleotide complementary to the nos tag to distinguish between RNS1 transcribed from the transgene and the endogenous RNS1 copy.
Analysis of the nos-RNS1cDNA lines revealed no difference between nos signal in wounded and unwounded leaves (Fig. 6a, c), although endogenous RNS1 was induced by wounding (not shown). In the nos-preRNS1 plants, however, a reproducible increase in nos signal was seen (Fig. 6a, c). The two bands detected in the preRNS1 and RNS1cDNA lanes could be the result of alternative polyadenylation sites, as these constructs contain both the endogenous RNS1 and E9 3′ end polyadenylation signals. This was confirmed by 3′RACE analysis, which identified two transcript ends corresponding to the two alternative polyadenylation sites (data not shown). Control nos-globin lines indicate that wounding does not induce the nos promoter (in fact, a slight but reproducible repressive effect was seen). It is therefore possible that some level of post-transcriptional regulation of RNS1 mRNA exists that requires either the entire UTR regions or intron sequences or both, whereas the cDNA sequence alone is not sufficient. This increase of approximately 2.5-fold (Fig. 6c) might provide a second layer of induction in addition to the transcriptional effect described above.
Differential response of RNS1 transcribed sequences to wounding and ABA. a Pools of T2 Arabidopsis seedlings expressing either the entire RNS1 transcribed region (left panels), the RNS1 cDNA (center), or the globin transcript (right) under the control of the constitutive nos promoter were wounded and harvested 3 h later. An oligonucleotide corresponding to a transcribed portion of nos was used as a probe in order to distinguish the transgene from endogenous RNS1. Blots were then stripped and probed with RNS1 and EF-1α (to control for loading). b Same as (a) except that the plants were treated with 100 μM ABA and harvested 4 h later. c Average values of the quantification (see Fig. 2) of the results obtained in three experiments as the ones described in a. Nos signal was corrected for loading differences (NOS/EF-1α); and it is shown as ratio of wounded versus unwounded expression [(NOS/EF-1α)wounded/(NOS/EF-1α)unwounded]. d Average values of the quantification of the results obtained in three experiments as the ones described in b. Nos signal is shown as ratio of ABA-treated versus buffer-treated expression. For each experiment, at least three independently transformed lines were used. Representative results are shown
Post-transcriptional regulation was not observed after treatment with ABA (Fig. 6b, d). Transcript levels in ABA-treated nos-preRNS1 and nos-RNS1cDNA plants resemble those in untreated plants. This disparity in post-transcriptional regulation might explain why endogenous RNS1 is induced to higher levels by wounding than by ABA.
Tissue specific, developmental and stress regulated activity of the RNS1 promoter
Analysis of specific patterns of expression can also provide clues to dissect the mechanisms that control RNS1 expression. Analysis of RNS1 promoter activity could also be used to identify transcription factors with similar expression patterns that may participate in this control. To analyze promoter activity, we used plants expressing the RNS1p-GUS construct described in Fig. 3. Plants expressing a similar construct in which the GUS reporter was replaced by luciferase (RNS1p-LUC) were also made.
Plants at different stages, from germination to maturity, were subjected to GUS staining (Fig. 7a–f). In the absence of stress the RNS1 promoter is active early during germination (Fig. 7a–c). GUS staining was detected in cotyledons as early as one day after germination (Fig. 7a), and almost disappeared 3–4 days after germination. Seven-day-old seedlings showed expression in root tips (Fig. 7c) and hydathodes (Fig. 7b), and some expression could be observed in vascular tissue (Fig. 7b). In adult leaves, RNS1 expression was limited to hydathodes (Fig. 7d). In flowers, RNS1 expression was only observed in anthers (Fig. 7e–f).
Tissue specific, developmental and stress regulated activity of the RNS1 promoter. Patterns of RNS1 promoter-driven GUS expression in seedlings at different ages or in different tissues: (a) 1-day-old seedling, (b–c) cotyledon and root of 7-day-old seedling, respectively, (d) 4-week-old leaf, (e–f) mature flower
Activity of the RNS1 promoter in response to wounding and ABA was analyzed using RNS1p-LUC plants. As described previously, the RNS1 promoter responds to wounding and ABA stimuli. Luciferase expression was observed throughout ABA-treated plants (Supplementary Fig. S4c), and in local and systemic tissues of wounded plants (Supplementary Fig. S2d). Note that RNS1 promoter activity is higher next to the wound (the wounds can be observed in the visible light picture, Supplementary Fig. S2b). We were unable to detect significant luciferase activity upon dehydration of plants expressing RNS1p-LUC. However, wounding combined with dehydration produced a stronger luciferase signal than did wounding alone (data not shown).
Discussion
The regulation of RNS1 transcript accumulation defines a novel pathway for the wounding response in Arabidopsis. The induction of RNS1 activity is independent of the two signals that have been proposed to control wounding responses in this plant—JA and OGAs. This pathway is also independent of other defense response regulators like ethylene (Reymond et al. 2000) and salicylic acid (SA; LeBrasseur et al. 2002). In this report we examined whether ABA controls this novel pathway. We showed that treatment of plants with ABA or wounding induces the expression of RNS1 within the same timeframe. Accumulating evidence, points to ABA as a component of the wounding response in plants. Although the exact nature of its contribution has not been defined, it is known that ABA accumulates upon wounding (Peña-Cortés et al. 1991). Our results with ABA mutants indicate that ABA is necessary for full induction of RNS1 during the wounding response. Thus ABA role during wounding seems to be to regulate the amplitude of the wounding response for RNS1 and likely other genes that could be co-regulated by the same pathway.
ABA is also likely to mediate the induction of dehydration-responsive genes that occurs locally following wounding. The cDNA microarray analysis carried out by Reymond et al. (2000) suggests that dehydration may also directly control wound gene induction, at least in Arabidopsis. Many of the wound-induced genes identified in that study were also induced by dehydration. It is likely that the extent of tissue damage incurred by the plant will determine the extent to which dehydration and ABA influence gene expression during a wound response (de Bruxelles and Roberts 2001). An alternative view was presented by Cheong et al. (2002), who suggested that drought and cold response pathways are activated in response to wounding. Their hypothesis is based on microarray experiments showing that the transcription factor DREB1B/CBF and several of its downstream targets are induced after wounding.
The use of mutants that either do not produce or cannot respond to ABA allowed us to show that ABA is one part of a signaling cascade that mounts a comprehensive wound response. ABA mutants consistently showed a weaker induction of RNS1 expression, indicating that ABA is necessary for full RNS1 induction. However, this hormone is not absolutely necessary for RNS1 wound induction, as 40–50% of the increase still occurs in the absence of ABA signaling (Fig. 2b). These results indicate the existence of an ABA-independent pathway that is responsible for a substantial portion of the induction of RNS1 and nuclease activities after wounding. Based on our and others’ previous results (Reymond et al. 2000; LeBrasseur et al. 2002), this pathway is also non-responsive to JA, OGAs, SA or ethylene. The existence of this pathway led to the previous assertion that wounding control of RNS1 was independent of ABA (LeBrasseur et al. 2002). However, it is now clear that intact ABA production and ABA signaling pathways are necessary for full induction of RNS1, as indicated by the results obtained with aba1-1, and abi1 and abi2, respectively.
Our experiments indicate that there is a synergy between different signals contributing to the induction of RNS1 expression. Although RNS1 is not significantly induced by dehydration, there seems to be a stronger induction by wounding when plants are dehydrated. It is possible that the putative DRE is not functional, or alternatively, this element present in the RNS1 promoter may function only in a cooperative manner with other elements in the promoter. Synergistic interactions have been described before. For example, the stress-responsive gene RD29A is rapidly induced after dehydration by an ABA-independent pathway, which is followed by a strong ABA-dependent induction. This regulation was explained by the existence of separate cis-acting elements in the RD29A promoter, including DRE and ABRE elements (Yamaguchi-Shinozaki and Shinozaki 1993, 1994).
We found that RNS1 induction by wounding and ABA is controlled, at least in part, at the transcriptional level. The best characterized transcription factors that participate in ABA regulation are ABI3, ABI4 and ABI5. Although their activity has been mostly studied during seed development, it is clear that these transcription factors can also act in vegetative tissues (Arenas-Huertero et al. 2000; Rohde et al. 2000; Brocard et al. 2002). However, our results and those of Suzuki et al. (2003) indicate that ABI3, ABI4 and ABI5 are not responsible for transcriptional control during wounding induction of RNS1. Recently, other transcription factors with the ability to bind ABREs have been described (see review by Yamaguchi-Shinozaki and Shinozaki 2005). Analysis of RNS1 expression patterns can provide clues to identify transcription factors that could regulate its expression. Comparison of RNS1 promoter activity with known expression patterns of other ABRE binding proteins shows a striking similarity between RNS1 and AREB1 expression (Fujita et al. 2005). AREB1 is a basic domain/leucine zipper factor that binds ABREs and functions as a trans-activator to regulate ABRE-dependent ABA signaling that enhances drought tolerance in vegetative tissues (Fujita et al. 2005). As RNS1, AREB1 is expressed in roots, hydathodes and anthers (Fujita et al. 2005); thus, it is possible that AREB1 also participates in the control of RNS1 expression. In addition, the MYB transcription factor PHR1 has been shown to regulate RNS1 expression in response to phosphate-starvation conditions (Rubio et al. 2001).
The RNS1 promoter alone is able to provide some wound and ABA responsiveness to reporter genes. The RNS1 promoter has a modular structure similar to that of other ABA-responsive genes, suggesting that similar synergistic interactions control RNS1 expression. The promoter contains ABA-responsive elements, such as ABRE, MYB and MYC regions, and an ABA-independent, dehydration-responsive DRE element, which might mediate a quick response to dehydration even before the peak of ABA production is reached (Yamaguchi-Shinozaki and Shinozaki 1993, 1994). The binding of DREB1B to this element after its wound induction (Cheong et al. 2002) might provide a direct link between wounding and dehydration responses. These promoter elements could also cooperate with several wounding-responsive elements in the RNS1 promoter to produce the full induction of RNS1 after mechanical damage, as shown for other genes (Narusaka et al. 2004). In addition to this synergy, ABREs might be promiscuous; signals other than ABA might activate ABRE-mediated transcription (reviewed by Nambara and Marion-Poll 2003). It is thus possible that ABREs act as nodes in signaling crosstalk. This possibility is supported by recent work showing that ABREs are over-represented in the promoter regions of genes corresponding to several different stress cDNA collections (Mahalingam et al. 2003). However, the presence of multiple ABRE elements in a promoter is not a random event, since only 137 genes out of 26,207 genes in the Arabidopsis genome have multiple ABREs (Huang and Wu 2006). Interactions between ABA and JA signaling during the wound response might be mediated by AtMYC2 (review by Lorenzo and Solano 2005). This hypothesis would explain microarray results that show overlaps between wounding responses and those observed after pathogen attacks, abiotic stress, and hormonal treatments (Reymond et al. 2000; Cheong et al. 2002).
The transcriptional responsiveness of the reporter constructs shown in Figs. 3 and 7 supports the functionality of the ABA- and wounding-responsive elements in the RNS1 promoter. However, transcriptional activity of the promoter is insufficient to explain the differences in transcript accumulation of the endogenous RNS1 after wounding and ABA treatment (Fig. 5). Analysis of the expression of the RNS1 transcript, including UTRs and introns and driven by a constitutive promoter, showed that untranslated sequences also respond to wounding (Fig. 6a, b). The simplest explanation of this result is that sequences in the RNS1 mRNA stabilize the transcript in response to wounding. In plants, several stress and hormonal responses affect mRNA stability (reviewed by Gutiérrez et al. 1999). Our results might be the first evidence of post-transcriptional control during the wounding response since, to our knowledge, changes in mRNA stability in response to this stress have not been described before.
A transcription regulatory element in the transcribed region, however, cannot be ruled out. For example, a transcriptional enhancer could be located in the RNS1 transcribed region. Most studies on transcriptional regulation and promoter elements that control ABA and wounding responses have focused on the promoter region upstream of the transcription start site (see, for example, reviews by Farmer et al. 2003; Yamaguchi-Shinozaki and Shinozaki 2005).
Several recently identified ABA-hypersensitive mutants, such as abh1, have mutations in RNA-binding proteins. A double-stranded RNA-binding protein, HYL1 (Lu and Fedoroff 2000), and an Sm-like snRNP protein, SAD1 (Xiong et al. 2001), control ABA regulation of seed germination. Plants carrying homozygous mutations in either of these genes are hypersensitive to ABA, suggesting that both proteins are negative regulators of ABA signaling. Another RNA-binding protein, AKIP1, was identified as a specific target of the ABA-activated protein kinase AAPK (Li et al. 2002). These results prompted the idea that ABA signaling is linked to RNA metabolism (reviewed by Fedoroff 2002).
The induction of the RNS1 RNase by ABA is further support for ABA-regulation of RNA metabolism. Interestingly, although RNS1 is induced by ABA, its expression is downregulated in the abh1 mutant. Based on this finding, Hugouvieux et al. (2001) proposed that RNS1 and other downregulated transcripts could be negative regulators of ABA signaling. Following this hypothesis, RNS1 would be induced by ABA early during the wounding response, and work in a negative feedback loop to regulate such response. In addition, in view of the reduced level of RNS1 in the abh1 mutant, it was suggested that RNS1 itself is a target for post-transcriptional regulation by ABA (Hugouvieux et al. 2001). We were unable to detect regulation of the RNS1 cDNA or pre-RNA by ABA. However, our experimental set-up could have interfered with this regulation. ABH1 is a cap-binding protein; therefore interactions with the 5′ UTR of target transcripts could be expected. Our transgene transcripts carry a 5′ nos tag to differentiate them from endogenous RNS1. This tag could disrupt interaction between ABH1 or an associated factor with the 5′ UTR of RNS1. Thus, more experiments will be necessary before we can discard a role of post-transcriptional regulation of RNS1 by ABA.
Our initial results and the tools developed in this work open a new avenue to the study of post-transcriptional regulation during wounding, an area mostly overlooked so far. It also provides a means to test directly the commonly accepted idea that ABA regulation has a large post-transcriptional component. In addition to helping us to dissect the complex signaling pathways leading to RNS1 induction, our work has begun to address the role of ABA in the regulation of wounding response and the function of RNS1 as part of the signal or response to wounding and ABA.
Abbreviations
- ABA:
-
Abscisic acid
- ABRE:
-
ABA-responsive element
- DRE:
-
Dehydration response element
- JA:
-
Jasmonic acid
- OGA:
-
Oligogalacturonides
- RNase:
-
Ribonuclease
- SA:
-
Salicylic acid
References
Abe H, Yamaguchi-Shinozaki K, Urao T, Iwasaki T, Hosokawa D, Shinozaki K (1997) Role of Arabidopsis MYC and MYB homologs in drought- and abscisic acid-regulated gene expression. Plant Cell 9:1859–1868
Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, León P (2000) Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev 14:2085–2096
Bariola PA, Green PJ (1997) Plant ribonucleases. In: D’Alessio G, Riordan JF (eds) Ribonucleases: structures and functions. Academic, New York, pp 163–190
Bariola PA, Howard CJ, Taylor CB, Verburg MT, Jaglan VD, Green PJ (1994) The Arabidopsis ribonuclease gene RNS1 is tightly controlled in response to phosphate limitation. Plant J 6:673–685
Bariola PA, MacIntosh GC, Green PJ (1999) Regulation of S-like ribonuclease levels in Arabidopsis. Antisense inhibition of RNS1 or RNS2 elevates anthocyanin accumulation. Plant Physiol 119:331–342
Birkenmeier GF, Ryan CA (1998) Wound signaling in tomato plants—evidence that ABA is not a primary signal for defense gene activation. Plant Physiol 117:687–693
Bosse D, Köck M (1998) Influence of phosphate starvation on phosphohydrolases during development of tomato seedlings. Plant Cell Environ 21:325–332
Brocard IM, Lynch TJ, Finkelstein RR (2002) Regulation and role of the Arabidopsis Abscisic Acid-Insensitive 5 Gene in abscisic acid, sugar, and stress response. Plant Physiol 129:1533–1543
Cheong YH, Chang H-S, Gupta R, Wang X, Zhu T, Luan S (2002) Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol 129:661–677
Chinnusamy V, Stevenson B, Lee B-H, Zhu J-K (2002) Screening for gene regulation mutants by bioluminescence imaging. Science’s STKE, http://www.stke.org/cgi/content/full/sigtrans;2002/140/pl10
de Bruxelles GL, Roberts MR (2001) Signals regulating multiple responses to wounding and herbivores. Crit Rev Plant Sci 20:487–521
Deshpande RA, Shankar V (2002) Ribonucleases from T2 family. Crit Rev Microbiol 28:79–122
Diehn SH, Chiu W-L, De Rocher EJ, Green PJ (1998) Premature polyadenylation at multiple sites within a Bacillus thuringiensis toxin gene-coding region. Plant Physiol 117:1433–1443
Eulgem T, Rushton PJ, Schmelzer E, Hahlbrock K, Somssich IE (1999) Early nuclear events in plant defence signalling: rapid gene activation by WRKY transcription factors. EMBO J 18:4689–4699
Fang R, Nagy F, Sivasubramaniam S, Chua N (1989) Multiple cis-regulatory elements for maximal expression of the cauliflower mosaic virus 35S promoter in transgenic plants. Plant Cell 1:141–150
Farmer EE, Alméras E, Krishnamurthy V (2003) Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr Opin Plant Biol 6:372–378
Fedoroff NV (2002) RNA-binding proteins in plants: the tip of an iceberg? Curr Opin Plant Biol 5:452–459
Finkelstein R, Lynch T (2000) The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 12:599–609
Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM (1998) The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA2 domain protein. Plant Cell 10:1043–1054
Finkelstein RR, Gampala SS, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14(suppl):S15–S45
Fujita Y, Fujita M, Satoh R, Maruyama K, Parvez MM, Seki M, Hiratsu K, Ohme-Takagi M, Shinozaki K, Yamaguchi-Shinozaki K (2005) AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell 17:3470–3488
Gil P, Green PJ (1996) Multiple regions of the Arabidopsis SAUR-AC1 gene control transcript abundance: the 3′ untranslated region functions as an mRNA instability determinant. EMBO J 15:1678–1686
Gilmour SJ, Artus NN, Thomashow MF (1992) cDNA sequence analysis and expression of two cold-regulated genes of Arabidopsis thaliana. Plant Mol Biol 18:13–21
Giraudat J, Hauge B, Valon C, Smalle J, Parcy F, Goodman H (1992) Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell 4:1251–1261
Goldstein AH, Baertlein DA, Danon A (1989) Phosphate starvation stress as an experimental system for molecular analysis. Plant Mol Biol Rep 7:7–16
Groß N, Wasternack C, Köck M (2004) Wound-induced RNaseLE expression is jasmonate and systemin independent and occurs only locally in tomato (Lycopersicon esculentum cv. Lukullus). Phytochemistry 65:1343–1350
Gutiérrez RA, MacIntosh GC, Green PJ (1999) Current perspectives on mRNA stability in plants: multiple levels and mechanisms of control. Trends Plant Sci 4:429–438
Hajela RK, Horvath DP, Gilmour SJ, Thomashow MF (1990) Molecular cloning and expression of cor (cold-regulated) genes in Arabidopsis thaliana. Plant Physiol 93:1246–1252
Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database:1999. Nucleic Acids Res 27:297–300
Howard CJ (1996) Identification and characterization of ribonucleases in Arabidopsis thaliana. Ph.D thesis, Michigan State University, USA
Howe GA (2004) Jasmonates as signals in the wound response. J Plant Growth Regul 23:223–237
Huang MD, Wu WL (2006) Genome-wide in silico identification and experimental confirmation of abscisic acid-regulated genes in Arabidopsis. Plant Sci 170:986–993
Hugouvieux V, Kwak JM, Schroeder JI (2001) An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis. Cell 106:477–487
Jefferson RA, Kavanagh TA, Bevan MA (1987) GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Kariu T, Sano K, Shimokawa H, Itoh R, Yamasaki N, Kimura M (1998) Isolation and characterization of a wound-inducible ribonuclease from Nicotiana glutinosa leaves. Biosci Biotechnol Biochem 62:1144–1151
LeBrasseur ND, MacIntosh GC, Pérez-Amador MA, Saitoh M, Green PJ (2002) Local and systemic wound-induction of RNase and nuclease activities in Arabidopsis: RNS1 as a marker for a JA-independent systemic signaling pathway. Plant J 29:393–403
León J, Rojo E, Sánchez-Serrano JJ (2001) Wound signaling in plants. J Exp Bot 52:1–9
Lers A, Khalchitski A, Lomaniec E, Burd S, Green PJ (1998) Senescence-induced RNases in tomato. Plant Mol Biol 36:439–449
Lescot M, Déhais P, Moreau Y, De Moor B, Rouzé P, Rombauts S (2002) PlantCARE: a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30:325–327
Leung J, Bouvier-Durand M, Morris P-C, Guerrier D, Chefdor F, Giraudat J (1994) Arabidopsis ABA response gene ABI1: features of a calcium-modulated protein phosphatase. Science 264:1448–1452
Leung J, Merlot S, Giraudat J (1997) The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9:759–771
Li JX, Kinoshita T, Pandey S, Ng CKY, Gygi SP, Shimazaki K, Assmann SM (2002) Modulation of an RNA-binding protein by abscisic-acid-activated protein kinase. Nature 418:793–797
Lorenzo O, Solano R (2005) Molecular players regulating the jasmonate signalling network. Curr Opin Plant Biol 8:532–540
Lu C, Fedoroff N (2000) A mutation in the Arabidopsis HYL1 gene encoding a dsRNA binding protein affects responses to abscisic acid, auxin, and cytokinin. Plant Cell 12:2351–2365
Mahalingam R, Gomez-Buitrago A, Eckardt N, Shah N, Guevara-Garcia A, Day P, Raina R, Fedoroff NV (2003) Characterizing the stress/defense transcriptome of Arabidopsis. Genome Biol 4:R20
McCubbin AG, Kao TH (2000) Molecular recognition and response in pollen and pistil interactions. Annu Rev Cell Dev Biol 16:333–364
Millar AJ, Short S, Hiratsuka K, Chua N-H, Kay SA (1992) Firefly luciferase as a reporter of regulated gene expression in plants. Plant Mol Bio Rep 10:324–337
Nambara E, Marion-Poll A (2003) ABA action and interactions in seeds. Trends Plant Sci 8:213–217
Narusaka Y, Narusaka M, Seki M, Umezawa T, Ishida J, Nakajima M, Enju A, Shinozaki K (2004) Crosstalk in the responses to abiotic and biotic stresses in Arabidopsis: analysis of gene expression in cytochrome P450 gene superfamily by cDNA microarray. Plant Mol Biol 55:327–342
Nürnberger T, Abel S, Jost W, Glund K (1990) Induction of an extracellular ribonuclease in cultured tomato cells upon phosphate starvation. Plant Physiol 92:970–976
Pastuglia M, Roby D, Dumas C, Cock JM (1997) Rapid induction by wounding and bacterial infection of an S gene family receptor-like kinase gene in Brassica oleracea. Plant Cell 9:49–60
Peña-Cortéz H, Sánchez-Serrano JJ, Merens R, Willmitzer L (1989) Abscisic acid is involved in the wound-induced expression of the proteinase inhibitor II gene in potato and tomato. Proc Natl Acad Sci USA 86:9851–9855
Peña-Cortéz H, Willmitzer L, Sánchez-Serrano JJ (1991) Abscisic-acid mediates wound induction but not developmental-specific expression of the proteinase inhibitor-II gene family. Plant Cell 3:963–972
Pérez-Amador MA, Leon J, Green PJ, Carbonell J (2002) Induction of the arginine decarboxylase ADC2 gene provides evidence for the involvement of polyamines in the wound response in Arabidopsis. Plant Physiol 130:1454–1463
Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12:707–719
Rock CD, Zeevaart JAD (1991) The aba mutant of Arabidopsis thaliana is impaired in epoxy-carotenoid biosynthesis. Proc Natl Acad Sci USA 88:7496–7499
Rohde A, Kurup S, Holdsworthc M (2000) ABI3 emerges from the seed. Trends Plant Sci 5:418–419
Rojo E, Solano R, Sánchez-Serrano JJ (2003) Interactions between signaling compounds involved in plant defense. J Plant Growth Regul 22:82–98
Rubio V, Linhares F, Solano R, Martin AC, Iglesias J, Leyva A, Paz-Ares J (2001) A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev 15:2122–2133
Suzuki M, Ketterling MG, Li QB, McCarty DR (2003) Viviparous1 alters global gene expression patterns through regulation of abscisic acid signaling. Plant Physiol 132:1664–1677
Taylor CB, Green PJ (1991) Genes with homology to fungal and S-gene RNases are expressed in Arabidopsis thaliana. Plant Physiol 96:980–984
Taylor CB, Bariola PA, DelCardayré SB, Raines RT, Green PJ (1993) RNS2: a senescence-associated RNase of Arabidopsis that diverged from the S-RNases before speciation. Proc Natl Acad Sci USA 90:5118–5122
Titarenko E, Rojo E, León J, Sánchez-Serrano JJ (1997) JA-dependent and -independent signalling pathways control wound-induced gene activation in Arabidopsis thaliana. Plant Physiol 115:817–826
Xiong LM, Gong ZZ, Rock CD, Subramanian S, Guo Y, Xu WY, Galbraith D, Zhu JK (2001) Modulation of abscisic acid signal transduction and biosynthesis by a Sm-like protein in Arabidopsis. Dev Cell 1:771–781
Yamaguchi-Shinozaki K, Shinozaki K (1993) Characterization of the expression of a desiccation-responsive rd29 gene of Arabidopsis thaliana and analysis of its promoter in transgenic plants. Mol Gen Genet 236:331–340
Yamaguchi-Shinozaki K, Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low temperature, or high-salt stress. Plant Cell 6:251–264
Yamaguchi-Shinozaki K, Shinozaki K (2005) Organization of cis-acting regulatory elements in osmotic- and cold-stress responsive promoters. Trends Plant Sci 10:88–94
Acknowledgments
The authors would like to thank Dr. Daniel Cook and Dr. Michael Thomashow (Michigan State University) for helpful discussions and for sharing ABA mutant seeds and the COR6.6 clone. We also thank Dr. Alan Myers (Iowa State University) for critical reading of the manuscript. This work was supported by the National Science Foundation (grant no. 0096394, 0228144, and 0445638 to P.J.G.), the US Department of Energy (grant no. DE-FG02-91ER20021 to P.J.G.), and the Roy J. Carver Charitable Foundation (grant no. 06-2323 to G.C.M.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Westhoff.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hillwig, M.S., LeBrasseur, N.D., Green, P.J. et al. Impact of transcriptional, ABA-dependent, and ABA-independent pathways on wounding regulation of RNS1 expression. Mol Genet Genomics 280, 249–261 (2008). https://doi.org/10.1007/s00438-008-0360-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-008-0360-3