Abstract
Light is one of the most important factors inducing morphogenesis in Neurospora crassa. The reception of light triggers the generation of reactive oxygen species (ROS) including hydrogen peroxide (H2O2). Catalase-1 (Cat-1) is one of three catalases known to detoxify H2O2 into water and oxygen. We reported that the photomorphogenetic characteristics of mutants in nucleoside diphosphate kinase-1 (NDK-1), a light signal transducer, are severely affected, and NDK-1 interacted with Cat-1 in a yeast two-hybrid assay. To disclose the function of Cat-1, we created a Cat-1 loss-of-function mutant (cat-1 RIP) by the repeat induced point-mutation (RIPing) method. No Cat-1 activity was detected in the mutant strain. Forty guanines were replaced with adenines in the cat-1 gene of cat-1 RIP, which caused 30 amino acid substitutions. The mutant strain grew normally, but its conidia and mycelia were more sensitive to H2O2 than those of the wild type. The lack of Cat-1 activity also caused a significant reduction in the conidial germination rate. Furthermore, light enhanced this reduction in cat-1 RIP more than that in the wild type. Introduction of cat-1 into the mutant reversed all of these defective phenotypes. These results indicate that Cat-1 plays an important role in supporting the survival of conidia under oxidative and light-induced stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light is one of the most important environmental factors controlling photosynthesis in plants and photomorphogenesis in plants and fungi (Kendrik and Kronenberg 1994). In Neurospora crassa, various processes of morphogenesis such as the induction of carotenoid production in mycelia (Harding and Turner 1981), protoperithecial formation (Degli-Innocenti and Russo 1984), phototropism of perithecial beaks (Harding and Melles 1984), perithecial polarity (Oda and Hasunuma 1997; Ogura et al. 2001), and circadian rhythm (Sargent and Briggs 1967; Feldman 1982), are controlled by blue light, which is associated with the generation of reactive oxygen species (ROS) (Massey 2000; Dolamns et al. 2003; Omata et al. 2006). ROS, including superoxide (•O −2 ), hydrogen peroxide (H2O2), singlet oxygen (1O2), and hydroxyl radical (•OH), can damage proteins, lipids, and nucleic acids. Many organisms have evolved strategies to remove ROS and repair the damage they cause. Catalase (EC 1.11.1.6; H2O2 oxidoreductase) is a heme-containing homotetrameric protein, and breaks down H2O2 into water and oxygen (Bravo et al. 1997). There are a number of processes producing H2O2 during the aerobic growth of an organism (Farr and Kogoma 1991). The major damage from H2O2 arises from the evolution of the highly reactive •OH, which can react with DNA, proteins, and lipids. Most aerobic organisms require catalases or peroxidases to circumvent the damage (Loewen 1997).
Genetic and biochemical studies have demonstrated that N. crassa possesses three catalases encoded by three separate structural genes, cat-1, cat-2, and cat-3 (Parvathi and Donald 1989). Catalase-1 (Cat-1) activity is pre-dominant in conidial germination and early mycelial growth. It is induced during pre-stationary growth and by ethanol treatment or heat shock (Michan et al. 2002; Diaz et al. 2001). Oxidation of Cat-1 by 1O2 gives rise to different conformers: Cat-1a is the non-modified conformer, Cat-1e is the fully modified conformer, and Cat-1b, 1c, and 1d are partially modified conformers (Lledias et al. 1998). Cat-2 is absent in rapidly growing mycelia but present at low levels in conidia and stationary-phase mycelia. It is the pre-dominant catalase in extracts derived from mycelia that have been heat-shocked for 2 h (Parvathi and Donald 1989). Cat-3 activity is pre-dominant during the late growing phase of mycelia, and at the initial stage of the conidiation process. It is induced under stress caused by treatment with H2O2, methyl viologen (paraquat), cadmium, heat shock, uric acid, and nitrate (Michan et al. 2002). Cat-1 and Cat-3 constitute the major catalases and are regulated differently during the asexual life cycle of N. crassa. It was reported that asexual development is enhanced in a cat-3-null mutant strain (cat-3 RIP). Colonies of cat-3 RIP grown in darkness produce high levels of carotenoids. Light enhances oxidative stress and further increases carotenoid synthesis in cat-3 RIP. About six times more aerial hyphae and conidia in air-exposed mycelial mats are produced in cat-3 RIP (Michan et al. 2003).
In our previous study, a sod-1 mutant of N. crassa, defective in Cu, Zn-superoxide dismutase (Cu, Zn-SOD), was used to examine the relationship between photomorphogenesis and ROS. We found that light-induced phenomena, including carotenoid synthesis in the mycelia and the polarity of the perithecia of the sod-1 mutant, were markedly affected (Yoshida and Hasunuma 2004). We also found that stimuli caused by the exposure of the mycelia to air or oxygen gas stimulated light-induced carotenoid synthesis (Iigusa et al. 2005). These results suggest that intracellular ROS are one of the key components of the system controlling light signal transduction. In addition, we previously isolated nucleoside diphosphate kinase-1 (NDK-1) as a candidate for a light signal transducer in N. crassa (Oda and Hasunuma 1997; Ogura et al. 2001) and also AtNDK-1 as a component of the ROS signaling machinery that interacts with the three catalases in Arabidopsis thaliana (Fukamatsu et al. 2003). Recently, our study showed that ndk-1 null mutants exhibited reduced carotenoid synthesis compared with the wild type (Lee et al. 2006), and the C-terminal half of NDK-1 interacted with Cat-1 in a yeast two-hybrid assay, and NDK-1 also formed a protein complex with Cat-1 and Cat-3 as detected by immuno-precipitation assay (Yoshida et al. 2006). These analyses suggest that there are novel regulatory systems for the response to light and for tolerating oxidative stress in plants and fungi. Catalases, especially Cat-1 and Cat-3 in N. crassa, may play crucial roles in the unidentified systems.
In order to further understand the function of Cat-1 in the regulation of ROS coupled with light signal transduction, we analyzed the effect of inactivation of Cat-1 on the morphogenesis and other characteristics of N. crassa. A Cat-1 loss-of-function mutant, cat-1 RIP, grew normally and did not show any obvious change in photomorphogenesis compared with the wild type. However, in addition to being more sensitive to H2O2, the mutant showed a significantly reduced rate of conidial germination compared to the wild type. Also, the reduction in the conidial germination rate of cat-1 RIP was enhanced when the mutant was cultured under continuous light. These defective phenotypes were reversed in the rescued strain where the cat-1 gene was introduced into cat-1 RIP.
Materials and methods
Strains, media, and growth conditions
Neurospora crassa strains, the standard wild-type 74-OR23-1A (74A; FGSC #987) and 74-OR8-1a (74a; FGSC #988), were obtained from the Fungal Genetics Stock Center (FGSC; University of Kansas Medical Center, Kansas City, KA, USA). Cultures were carried out in a liquid Vogel’s minimal medium (VM) with 1.5% sucrose at 30°C for mycelial growth. Conidia were isolated from cultures grown on glycerol complete medium. Conidial germination was carried out in liquid VM with 1.5% sucrose at 30°C for 4 h under room light.
For the experiment on the effect of light, the cultures were incubated under either continuous light (40 μE m−2 s−1) or continuous darkness at 30°C. Conidia from slant cultures, aged from 3 to 28 days (days after inoculation), were harvested and filtered to remove mycelial fragments. About 5 × 106/ml conidia were inoculated into Erlenmeyer flasks with 30 ml of liquid VM containing 1.5% sucrose, and shaken at 30°C for 4 h (∼180 rpm). Germinated (conidia with germ tubes) and non-germinated (conidia without germ tubes) conidia were enumerated using a counting chamber under a microscope.
Disruption and restoration of the cat-1 gene in N. crassa
The cat-1 gene was disrupted using the RIPing method (Selker and Garrett 1988). Two primers, each containing a Hind III site at the 5′ end (5′-aagcttgatcatgcgcttcgaccac-3′ and 5′-aagctttggacggtcgagccattg-3′), were used to amplify a 1.6-kb fragment of the second exon of cat-1 from genomic DNA (Fig. 1a). The amplified 1.6-kb Hind III-cut cat-1 fragment was TA cloned into pGEM®-T easy Vector (Promega), and then inserted into pCSN43 (Staben et al. 1989) after confirmation of the DNA sequence. The vector pCSN43-cat-1 was introduced into wild-type 74-OR23-1A as described previously (Vollmer and Yanofsky 1986). Transformants were screened on sorbose-hygromycin medium as described by Lee et al. (2006). To examine the molecular nature of the introduced cat-1gene fragment, Southern blotting was performed using the cat-1-inserted fragment as a probe. Then the conidia of positive transformants were inoculated into the protoperithecia from the wild type on synthetic crossing medium (Davis and De Serres 1970). Once perithecia were well developed and ascospores had been ejected, the ascospores were harvested and then spread on Vogel’s sorbose medium (VSM, VM supplemented with 2% sorbose, 0.05% glucose, 0.05% fructose, and 2% agar) after heat treatment, and the colonies were isolated individually and grown on liquid VM. To select Cat-1 loss-of-function candidates from the RIP-induced strains, catalase activities were checked in-gel after separating the proteins by non-denaturing polyacrylamide gel electrophoresis (PAGE) as described by Lledias et al. (1998). Non-denaturing iso-electric focusing (IEF) was also performed as described by Lledias et al. (1998). After three crosses with the wild type (FGSC #987A), individual colonies were isolated. Among the 60 randomly picked progenies, 32 colonies were defective in Cat-1, indicating 1:1 segregation. The phenotype of one of these candidates, cat-1 RIP, mating type A, was characterized in more detail.
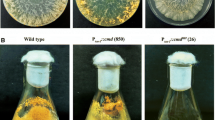
Isolation and analysis of the mutant cat-1 RIP. a Cloning of a cat-1 gene fragment used for the RIPing procedure. A 1.6-kb fragment from the second exon of cat-1 was cloned. b Catalase activity in native PAGE. Proteins (300 μg) extracted from conidia and mycelia of the wild type (wt), the restored strain (PTC1), and cat-1 RIPwere loaded. c Catalase activity in gels after isoelectric focusing (IEF). IEF was done under non-denaturing conditions with 300 μg of protein extracted from conidia and mycelia of wt, PTC1, and cat-1 RIP. d cat-1 mRNA accumulation in wt and cat-1 RIP. Total RNA (10 μg per sample) was hybridized with a 32P-labeled cat-1 probe (the cloned 1.6-kb fragment). 25S rRNA was used to evaluate RNA loading in the Northern blot. The abundance of cat-1 mRNA was calculated relative to that of 25S rRNA. Relative values of cat-1 RIPwere calculated using the values of wt. The value is the average of results from three independent experiments, with standard error bars. e Morphogenesis of 5-day-old wt, PTC1, and cat-1 RIPgrown on glycerol complete slant medium. They were cultured at 30°C under constant light (20 μE m−2 s−1)
A full-length cat-1 cDNA was used to restore cat-1 in the mutant. The RT-PCR method for preparing cDNA was described by Lee et al. (2006). Two primers, 5′-atgcaacgactcgcgttgcattat-3′ and 5′-ttagtacgcaatcatggagtgcaagcc-3′, were used. Then the cDNA fragment was inserted into pTREB after confirming the sequence (Mullaney et al. 1985; Ogura et al. 2001). The vector pTREB-cat-1 was introduced into the mutant as described previously (Vollmer and Yanofsky 1986). Transformants were screened on sorbose-Ignite medium (Ignite was supported by FGSC. Nitrogen-free Vogel’s medium supplemented with 2% sorbose, 2% sucrose, 0.5% proline, 2% agar, and 0.03% Ignite was used for basal medium, and Nitrogen-free Vogel’s medium supplemented with 2% sorbose, 2% sucrose, 0.5% proline, 18.2% sorbitol, and 1% agar was used for top medium) (McCluskey 2003). After checking the catalase activities, Southern blotting was performed to examine the molecular nature of the restored cat-1 gene. A rescued strain, PTC1 (pTREB-c at- 1), was finally determined from these candidates and characterized in more detail.
Enzyme activity assay for Catalases
One milliliter of conidial suspension (106 conidia/ml) was inoculated into 50 ml of liquid VM with 1.5% sucrose in a 250-ml Erlenmeyer flask. After being shaken (∼180 rpm) at 30°C for 24 h, the mycelial cultures were harvested on Whatman 540 filter paper by filtration. Conidia were harvested from 5-day-old cultures grown on glycerol complete medium. Then the mycelia and conidia were disrupted in 50 mM potassium phosphate buffer (pH 7.0) by homogenization and by sonication, respectively. The disrupted cell suspensions were centrifuged at 10,000g for 10 min at 4°C. The resulting supernant (300 μg of protein) was loaded onto a 6% native polyacrylamide slab gel as described (Lledias et al. 1998). Non-denaturing IEF was performed in a 3.5% acrylamide gel in capillaries, containing 5% ampholine pH 5-7, 1% ampholine pH 3.5–10, and 15% glycerol. Samples (300 μg of protein) were loaded onto non-denaturing IEF gels. H3PO4 (20 mM) was used as an anolyte and NaOH (20 mM) as a catholyte. After electrophoresis, catalase activity was determined as described by Lledias et al. (1998).
Northern blot analysis
Total RNA was prepared using a small-scale RNA extraction method (Sokolovsky et al. 1990). Five micrograms of total RNA was separated by glycerol-gel electrophoresis and transferred to a piece of Gene Screen Plus membrane (NEN Research Product). The cDNA probe for cat-1 was the same as the fragment used in vector construction. Hybridization with 32P-labeled probes was carried out overnight at 60°C using Church’s phosphate buffer (Church and Kieffer-Higgins 1998). Radio-isotopic signals were visualized by autoradiography using X-OMAT AR film (Kodak).
Assay of sensitivity to H2O2
For assaying the sensitivity of conidia to H2O2, two methods were carried out. (1) About 250 conidia were inoculated on VSM, and incubated at 30°C for 2 days. Then 10 ml of H2O2 was added to each culture. After a 10-min treatment, the H2O2 was discarded and incubation was continued for 3 more days. Conidia from each group (ten colonies in one group) were harvested and counted, then compared with those of untreated controls (Michan et al. 2003). (2) About 250 conidia from a 5-day-old culture were mixed with top medium (VM supplemented with 2% sorbose, 0.05% glucose, 0.05% fructose, and 0.8% agar), then plated on basal medium (VM supplemented with 2% sorbose, 0.05% glucose, 0.05% fructose, and 2% agar), which contained 0.5, 1, 1.5 or 2 mM H2O2. After 3 days of incubation at 30°C, the numbers of colonies formed on the medium were counted and compared with those of untreated controls (Schmit and Brody 1976).
For assaying the sensitivity of mycelia to H2O2, conidia (1 × 106) were inoculated into 20 ml of liquid VM in 8.5-cm Petri dishes, and incubated at 30°C for 36 h in darkness. After removal of the medium, the flat mycelial mat was cut into small pieces with a Pasteur pipette. Mycelial pieces of approximately the same size were inoculated into liquid VM which contained 0.5, 1, 2 or 4 mM H2O2. After shaking (180 rpm) at 30°C for 30 h, the mycelia were harvested on filter paper by vacuum filtration, and dried for 3 h at 60°C. Then the dry weights of mycelia were measured.
Results
Isolation of Cat-1 loss-of-function mutant in N. crassa
The repeat induced point-mutation (RIPing) method has been used with great success to generate null mutants of N. crassa by introducing a copy of the gene to be disrupted into the genome and crossing the transformed strain. With the RIPing process, methylation of cytosine and subsequent deamination generate C-T transitions, and eventually result in G-A transitions that can inactivate every repeated DNA sequence (Cambareri et al. 1989). We created a Cat-1 loss-of-function mutant using the RIPing method (see Materials and methods) to investigate the biological function of Cat-1 in N. crassa. A 1.6-kb fragment from the second exon of cat-1 was used in the RIPing procedure (Fig. 1a). The RIP-induced strains were examined for catalase activity by native PAGE. Because Cat-1c and Cat-3 were located at the same position in the native PAGE gel, native IEF was performed to separate them and confirm the absence of Cat-1 activity. Eventually we isolated one candidate, cat-1 RIP, which showed no Cat-1 activity either in mycelia or in conidia. As a control, Cat-2 and Cat-3 activities were detected at similar levels in the wild type and cat-1 RIP(Fig. 1b, c). The mRNA of cat-1 was accumulated in the mutant. However, the abundance of cat-1 in cat-1 RIP was about half of that in the wild type (Fig. 1d). These results indicated that cat-1 RIP was a loss-of-function mutant with no detectable activity by Cat-1 in conidia or in mycelia. To determine whether the phenotypes of cat-1 RIP are caused only by the disruption of cat-1 or by additional mutations, we generated a rescued strain, PTC1, where the cat-1 gene was restored in the mutant (Fig. 1b, c). On morphogenesis including the formation of mycelia, aerial hyphae, and conidia, the slant cultures of cat-1 RIP did not differ significantly from those of the wild type and the rescued strain (Fig. 1e). We also assayed the growth speed and conidial banding of cat-1 RIP. These characteristics of cat-1 RIP were also the same as those of the wild type and PTC1 (data not shown).
The cat-1 in the mutant was sequenced in its entirety and compared with that of the wild type. Forty guanines of the cat-1 DNA of the wild type were replaced with adenines in the cat-1 DNA of cat-1 RIP(Supplemental data 1). The Cat-1 amino acid sequence, deduced from the DNA analysis, showed that 30 amino acids in the wild type were substituted in cat-1 RIP. Among these amino acids, four tryptophans (W248, W286, W316, and W327) were substituted with stop codons. All of these substitutions except one, valine (V587), were carried out in the catalytic domain (Fig. 2). We assumed that the Cat-1 protein in cat-1 RIP was truncated during translation and the catalytic domain was seriously damaged, which caused the loss of Cat-1 activity in cat-1 RIP.
Alignment of deduced amino acid sequences of wt and cat-1 RIP. Substituted amino acids in cat-1 RIPare shown below the corresponding wild-type residues. Trp248, Trp286, Trp316, Trp327 were substituted with termination codons which are indicated by triangles. Two conserved domains are boxed. Box I represents the catalytic domain. Box II represents the GATase1-like domain
Sensitivity of cat-1 RIP to H2O2
We analyzed the sensitivity of conidia of cat-1 RIP to H2O2 by examining the conidial formation rates of colonies treated with H2O2 and the colony-forming ability of conidia on VSM containing H2O2. To determine the conidial formation, we treated 2-day-old colonies grown in Petri dishes for 10 min with 5–20 mM of H2O2 solution, respectively. The number of conidia formed by colonies that survived was analyzed 3 days later. Both cat-1 RIP and the wild type showed a continuous decrease in the colony survival rate with the increase in the concentration of H2O2, the pattern of the decrease being very similar (data not shown). However, significantly fewer conidia were formed by colonies of cat-1 RIP than those of the wild type and PTC1 under H2O2 stress. With 10 mM H2O2, the colonies of cat-1 RIP showed a rate of conidial formation about 18 times lower than that of the wild type and 14 times lower than that of PTC1 (Fig. 3a). Similar results were obtained in the experiment carried out on solid VM without sorbose (data not shown). To determine the colony-forming ability of conidia on VSM containing H2O2, the dose-response effect was assayed with 0.5–2 mM H2O2. With the increase in the concentration of H2O2, the colony-forming ability of conidia from cat-1 RIP decreased more rapidly than that of the wild type. With 1 mM H2O2, the relative rate of formed colonies in cat-1 RIP was less than half of that in the wild type (Fig. 3b). PTC1 exhibited similar patterns to the wild type (Fig. 3a, b). These results indicate that conidia of cat-1 RIP were more sensitive to H2O2 than those of the wild type and PTC1.
Sensitivity of cat-1 RIPto hydrogen peroxide (H2O2). a Conidiation patterns of wt, PTC1, and cat-1 RIPon solid VSM (VM supplemented with 2% sorbose) with or without H2O2 treatment. Representative patterns of conidiation on colonies are shown in (a) to (f). Each number of conidia per group (ten randomly picked colonies as one group) is the average of three groups. b Percentages of colonies which were formed on medium containing H2O2. Conidia from 3-day-old cultures were plated on VSM containing 0.5, 1, 1.5, or 2 mM H2O2 as indicated above. After 3 days, the numbers of colonies formed were counted and compared with those of untreated controls. Each value is the average of results from three independent experiments, with standard error bars. c Dry weight of mycelia grown in liquid medium containing H2O2. Pieces of mycelia of approximately the same size were inoculated into liquid VM which contained 0.5, 1, 2, or 4 mM H2O2. After 30 h of shaking at 30°C, the mycelia were harvested and dried for 3 h at 60°C. Each value is the average of results from three independent experiments, with standard error bars
We also analyzed the sensitivity of mycelia of cat-1 RIPto H2O2 by measuring the dry masses of mycelia grown by shaking cultures in liquid medium containing H2O2. The dose response effect of H2O2 was assayed with 0.5–4 mM H2O2. With the increase in the concentration of H2O2, cat-1 RIPproduced less biomass than the wild type and PTC1. The mycelial dry weights of cat-1 RIP were less than half of those of the wild type and PTC1 with 1 and 2 mM H2O2 (Fig. 3c). The results indicate that mycelia of cat-1 RIP also were more sensitive to H2O2 than those of the wild type and the rescued strain.
Large reduction in conidial germination rate of cat-1 RIP
During the formation of conidia, Cat-1 increases many times over and reaches a very high concentration in conidia (Diaz et al. 2001). The mutant did not show a significant change in the kinetics of conidial germination (Supplemental data 2). However, we found that cat-1 RIPhad a greatly reduced conidial germination rate compared with the wild type and PTC1. Under normal conditions, at 30°C and under constant light (20 μE m−2 s−1), the mutant and the wild type formed conidia at the same rate (data not shown). However, only about 32% of conidia harvested from 5-day-old cat-1 RIP could germinate after being shaken for 4 h at 30°C in liquid medium, compared to about 60% from the wild type and PTC1 (Fig. 4). A similar result was obtained with the colony formation rate of conidia on solid VSM (data not shown). Interestingly, the reduction in the colony formation rate of cat-1 RIP was not affected by methyl viologen, temperature stress, or ultraviolet (UV) irradiation (data not shown). These results indicate that Cat-1 plays important roles in supporting the survival of conidia.
Reduction in conidial germination rate of cat-1 RIP. Conidia of wt, PTC1, and cat-1 RIPgrown at 30°C under constant light (20 μE m−2 s−1) were shaken (∼180 rpm) at 30°C for 4 h in liquid VM. Germinated and non-germinated conidia were photographed and enumerated with a counting chamber under a microscope. Representative germinated and non-germinated conidia are specified by arrow and circle, respectively. Each value is the average of results from three independent experiments, with standard error bars
High sensitivity to light in conidial germination of cat-1 RIP
To analyze the effect of light on cat-1 RIP, we assayed the accumulation of carotenoid in mycelia, light-induced conidiation, protoperithecial formation, and perithecial polarity of cat-1 RIP. Compared with the wild type, the mutant showed no obvious differences in these characteristics and the kinetics of conidial germination (data not shown). However, we found that conidia from cat-1 RIP were more sensitive to light. Only about 30% of conidia from cat-1 RIP could germinate, compared to about 55% of conidia from the wild type and PTC1 if they were cultured under continuous light (40 μE m−2 s−1) for 3 days only, while those strains cultured under darkness did not show any obvious difference in the rate of conidial germination (Fig. 5a). A similar result was obtained with 5-day-old conidia (data not shown). This suggests that light has a damaging effect on conidial germination and the absence of Cat-1 enhances the reduction in the germination rate of conidia. Whereas, with the lengthening of the culturing period to more than 1 week, the conidia of the mutant also exhibited a lower germination rate than those of the wild type and PTC1 although they were cultured under darkness. However, on the whole, the mutant conidia exhibited a significantly lower germination rate and lost the ability to germinate much more rapidly than the conidia of the wild type and PTC1 under light. At 21 days, we could not detect any germination of the conidia from the mutant, but the wild type and the rescued strain still retained a conidial germination rate of about 6% (Fig. 5b). These results clearly indicate that Cat-1 has important functions in protecting conidial germination under stress caused by light.
Sensitivity to light in conidial germination of cat-1 RIP. Wt, PTC1, and cat-1 RIPwere cultured under either continuous light (40 μE m−2 s−1) or continuous darkness. Conidia were shaken at 30°C for 4 h (∼180 rpm) in liquid VM. Germinated and non-germinated conidia were photographed and enumerated with a counting chamber under a microscope. Each value is the average of results from three independent experiments, with standard error bars. a Effect of light on the germination rate of conidia from 3-day-old cultures. b Effect of light on germination rate of conidia from aging cultures. Conidia of wt, PTC1, and cat-1 RIPat different points (time after inoculation) as indicated above were used
Discussion
We isolated a loss-of-function mutant to investigate the biological function of Cat-1 in N. crassa. The mRNA accumulation of cat-1 in the mutant was reduced to half of that in the wild type (Fig. 1d). We assume that the point mutations in the DNA sequence of cat-1 RIP caused a premature translation termination which eventually affected the mRNA’s stability as described by Jacobson and Peltz (1996). From the deduced amino acid sequence, Cat-1 in cat-1 RIP was truncated and seriously damaged especially in the catalytic domain (Fig. 2), which was assumed to cause the loss of activity.
The mutant did not show any obvious morphogenetic difference from the wild type and PTC1. However, our previous study suggested that intracellular ROS could affect photomorphogenetic characteristics in N. crassa (Yoshida and Hasunuma 2004; Iigusa et al. 2005). Therefore, other factors and enzymes appear to be used in place of the defective Cat-1 to regulate ROS levels during development in cat-1 RIP. There are another two catalases (Cat-2 and Cat-3) in the mycelia of N. crassa, and they possibly compensate for the missing Cat-1. Michan et al. (2003) reported that a cat-3 null mutant strain was prone to an increase in asexual development. However, we could not detect any increase in Cat-2 and Cat-3 activities either in conidia or in mycelia of cat-1 RIP(Fig. 1b, c). While under osmotic stress and starved of a source of carbon, more Cat-3 activity was induced in cat-1 RIP than in the wild type (data not shown). Other H2O2-disposing enzymes such as peroxiredoxins and/or peroxidases and a dissipating substance such as carotenoid could also have a compensating effect, although the carotenoids accumulated at the same rate in mycelia of the wild type and cat-1 RIP (data not shown). It has been reported that Cat-1 accounted for most of the activity during the germination of conidia and elongation of mycelia, and Cat-3 was pre-dominant during the late growing phase of mycelia (Michan et al. 2002). So we assume that Cat-3 is associated with the growth of mycelia and Cat-1 with the germination of conidia, which explains the normal morphogenesis of cat-1 RIP.
Cat-1 is an enormously efficient enzyme, which is not inhibited by molar concentrations of H2O2. It also exhibits an unusual resistance to denaturation caused by temperature and various denaturing reagents (Diaz et al. 2001). The high concentration of Cat-1 in conidia and the resistance to denaturation and to high concentrations of H2O2 enable conidia to survive and germinate under adverse environmental conditions. From our results, both mycelia and conidia of cat-1 RIP were sensitive to H2O2 compared to those of the wild type (Fig. 3). The conidial germination rate of cat-1 RIPwas much lower than that of the wild type although both strains were cultured under normal conditions (Fig. 4). These results suggest that Cat-1 has characteristics that are especially suitable for conidia and support their survival. It has been reported that conidia of a catalase (catA, cat-1 homologous catalase)-deficient mutant strain of Aspergillus nidulans were sensitive to H2O2 (Navarro et al. 1996) and heat exposure (Noventa-Jordao et al. 1999).
As we mentioned before, light is a major environmental signal for organisms in all kingdoms of life. Intracellular ROS are considered to be one of the key components of the machinery for light signal transduction (Yoshida and Hasunuma 2004; Iigusa et al. 2005). Besides detoxifying H2O2, Cat-1 can also be modified specifically by binding 1O2 which is considered to be part of a hyperoxidant state that develops at the start of conidial germination (Lledias et al. 1998, 1999; Peraza and Hansberg 2002; Diaz et al. 2004). From our results, the 3-day-old conidia of cat-1 RIP showed an apparently lower germination rate than those of the wild type and the rescued strain when they were cultured under continuous light, while there was no difference between the conidia from those strains cultured under darkness (Fig. 4b). Because light is considered to generate ROS, we speculate that the oxidative damage in the dark has not reached a minimal threshold beyond which the conidial germination of the mutant is significantly affected, but this threshold is easily surpassed under light. Additionally, the conidium is a closed dormant structure, so the oxidative damage from outside accumulates with time, and Cat-1 helps to mitigate this damage. With more and more damage being accumulated, Cat-1 acts to slow down the reduction in the survival rate of conidia although it cannot stop this reduction completely. In conclusion, Cat-1 supports the survival of conidia under oxidative stress, which is produced by basal metabolic activity in the dark and is enhanced under light. Recently, Schriner et al. (2005) reported that overexpression of a catalase recruited to mitochondria extended the murine life span.
In our previous study, AtNDK-1 interacted with catalases and played a role in the response to ROS in A. thaliana (Fukamatsu et al. 2003). In N. crassa, NDK-1 could also respond to light and oxidative stress (Oda and Hasunuma 1997; Ogura et al. 2001; Yoshida and Husunuma 2004). Moreover, NDK-1 controlled Cat-1 and Cat-3 at the post-transcriptional level and interacted with Cat-1 in a yeast two-hybrid assay (Yoshida et al. 2006). Thus, further investigation of the characteristics of cat-1 RIP and the interaction of Cat-1 with NDK-1 or other proteins from a biochemical and a genetic point of view is required for a precise understanding of the molecular mechanism by which Cat-1 acts during signal transduction evoked by ROS and light.
References
Bravo J, Fita I, Gouet P, Jouve HM, Melik-Adamyan W, Murshudov GN (1997) Structure of catalase. In: Scandalios JG (ed) Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor, New York, pp 407–445
Cambareri EB, Jensen BC, Schabtach E, Selker EU (1989) Repeat induced G-C to A-T mutations in Neurospora. Science 244:1571–1575
Church GM, Kieffer-Higgins S (1998) Multiplex DNA sequencing. Science 240:185–188
Davis RH, De Serres FJ (1970) Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol 71:79–143
Degli-Innocenti F, Russo VE (1984) Isolation of new white collar mutants of Neurospora crassa and studies on their behaviour in the blue-light-induced formation of protoperithecia. J Bacteriol 159:757–761
Diaz A, Rangel P, Montes de Oca Y, Lledias F, Hansberg W (2001) Molecular and kinetic study of catalase-1, a durable large catalase of Neurospora crassa. Free Radic Biol Med 31:1323–1333
Diaz A, Horjales E, Rudino-pinera E, Arreola R, Hansberg W (2004) Unusual Cys-Tyr covalent bond in a large catalase. J Mol Biol 342:971–985
Dolamns DEJGJ, Fukumura D, Jain RK (2003) Photodynamic therapy for cancer. Nat Rev 1:380–387
Farr SB, Kogoma T (1991) Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev 55:561–585
Feldman JF (1982) Genetic approaches to circadian clocks. Annu Rev Plant Physiol 33:583–608
Fukamatsu Y, Yabe N, Hasunuma K (2003) Arabidopsis NDK1 is a component of ROS signaling by interacting with three catalases. Plant Cell Physiol 44:982–989
Harding RW, Melles S (1984) Genetic analysis of the phototropism of Neurospora crassa perithecial beaks using white collar and albino mutants. Plant Physiol 72:996–1000
Harding RW, Turner RV (1981) Photoregulation of the carotenoid biosynthetic pathways in albino and white collar mutants of Neurospora crassa. Plant Physiol 68:745–749
Iigusa H, Yoshida Y, Hasunuma K (2005) Oxygen and hydrogen peroxide enhance light-induced carotenoid synthesis in Neurospora crassa. FEBS Lett 579:4012–4016
Jacobson A, Peltz SW (1996) Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu Rev Biochem 65:693–739
Kendrick RE, Kronenberg GHM (1994) Photomorphogenesis in plants. Kluwer, Dordrecht, Netherlands
Lee B, Yoshida Y, Hasunuma K (2006) Photomorphogenetic characteristics are severely affected in nucleoside diphosphate kinase-1 (ndk-1)-disrupted mutants in Neurospora crassa. Mol Gen Genomics 275:9–17
Lledias F, Rangel P, Hansberg W (1998) Oxidation of catalase by singlet oxygen. J Biol Chem 273:10630–10637
Lledias F, Rangel P, Hansberg W (1999) Singlet oxygen is part of a hyperoxidant state generated during spore germination. Free Radic Biol Med 26:1396–1404
Loewen PC (1997) Bacterial catalases. In: Scandalios JG (ed) Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor, New York, pp 273–308
Massey V (2000) The chemical and biological versatility of riboflavin. Biochem Soc Trans 28:283–296
McCluskey K (2003) The Fungal Genetics Stock Center: from molds to molecules. Adv Appl Microbiol 52:245–262
Michan S, Lledias F, Baldwin JD, Natvig DO, Hansberg W (2002) Regulation and oxidation of two large monofunctional catalases. Free Radic Biol Med 33:521–532
Michan S, Lledias W, Hansberg W (2003) Asexual development is increased in Neurospora crassa CAT-3 null mutant strains. Eukaryot Cell 2:798–808
Mullaney EJ, Hamer JE, Roberti KA, Yelton MM, Timberlake WE (1985) Primary structure of the trpC gene from Aspergillus nidulans. Mol Gen Genet 199:37–45
Navarro RE, Stringer MA, Hansberg W, Timberlade WE, Aguirre J (1996) catA, a new Aspergilus nidulans gene encoding a developmentally regulated catalase. Curr Genet 29:352–359
Noventa-Jordao MA, Couto RM, Goldman MHS, Aguirre J, Lyer S, Caplan A, Terenzi HF, Goldman GH (1999) Catalase activity is necessary for heat-shock recovery in Aspergillus nidulans germlings. Micorbiology 145:3229–3234
Oda K, Hasunuma K (1997) Genetic analysis of signal transduction through light-induced protein phosphorylation in Neurospora crassa. Mol Gen Genet 256:593–601
Ogura Y, Yoshida Y, Yabe N, Hasunuma K (2001) A point mutation in nucleoside diphosphate kinase results in a deficient light response for perithecial polarity in Neurospora crassa. J Biol Chem 276:21228–21234
Omata Y, Lewis JB, Rotenberg S, Lockwood PE, Messer RL, Noda M, Hsu SD, Sano H, Wataha JC (2006) Intra- and extracellular reactive oxygen species generated by blue light. J Biomed Mater Res A 77(3):470–477
Parvathi C, Donald ON (1989) Evidence for three differentially regulated catalase genes in Neurospora crassa: effects of oxidative stress, heat shock, and development. J Bacteriol 171:2646–2652
Peraza L, Hansberg W (2002) Neurospora crassa catalases, singlet oxygen and cell differentiation. Biol Chem 383:569–575
Sargent ML, Briggs WR (1967) The effects of blue light on a circadian rhythm of conidiation in Neurospora. Plant Physiol 42:1504–1510
Schmit JC, Brody S (1976) Biochemical genetics of Neurospora crassa conidial germination. Bacteriol Rev 40:1–41
Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Remmen HV, Wallace DC, Rabinovitch PS (2005) Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308:1909–1911
Selker EU, Garrett PW (1988) DNA sequence duplications trigger gene inactivation in Neurospora crassa. Proc Natl Acad Sci USA 85:6870–6874
Sokolovsky V, Kaldenhoff R, Ricci M, Russo VEA (1990) Fast and reliable mini-prep RNA extraction from Neurospora crassa. Fungal Genet Newslett 67:41–43
Staben C, Jensen B, Singer M, Pollock J, Schechtman M, Kinsey J, Selker E (1989) Use of a bacterial hygromycin B resistance gene as a dominant selectable marker in Neurospora crassa transformation. Fungal Genet Newslett 36:79–81
Vollmer SJ, Yanofsky C (1986) Efficient cloning of genes of Neurospora crassa. Proc Natl Acad Sci USA 83:4869–4873
Yoshida Y, Hasunuma K (2004) Reactive oxygen species affect photo-morphogenesis in Neurospora crassa. J Biol Chem 279:6986–6993
Yoshida Y, Ogura Y, Hasunuma K (2006) Interaction of nucleoside diphosphate kinase and catalases for stress and light responses in Neurospora crassa. FEBS Lett 580(13):3282–3286
Acknowledgments
We are grateful to Winslow R. Briggs for critical discussions. This work is supported by a grant-in-aid from the Japan Society for the Promotion of Science, the Kihara Memorial Yokohama Foundation for the Advancement of Life Science, the Mishima Kaiun Memorial Foundation, and the Yamada Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Perez-Martin.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, N., Yoshida, Y. & Hasunuma, K. Loss of Catalase-1 (Cat-1) results in decreased conidial viability enhanced by exposure to light in Neurospora crassa . Mol Genet Genomics 277, 13–22 (2007). https://doi.org/10.1007/s00438-006-0170-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-006-0170-4