Abstract
We have developed a novel four-element based gene tagging system in Arabidopsis to minimize the number of starter lines required to generate genome-wide insertions for saturation mutagenesis. In this system, the non-autonomous cassette, Ds(dSpm), comprises of both Ds and dSpm elements cloned one within the other along with appropriate selection markers to allow efficient monitoring of excision and re-integration of the transposons. Trans-activation of the outer borders (Ds) and selection against the negative selection marker (iaaH) linked to the cassette ensures unlinked spread of the Ds(dSpm) cassette from the initial site of integration of the T-DNA. This creates several launch pads within the genome from where the internal element (dSpm) can be subsequently mobilized to generate secondary insertions. In this study, starting from a single T-DNA integration we could spread the Ds(dSpm) cassette to 11 different locations over all the five chromosomes of Arabidopsis. The frequency of unlinked Ds transpositions in the F2 generation varied between 0.05 and 3.35%. Three of these lines were then deployed to trans-activate the internal dSpm element which led to the selection of 29 dSpm insertions. The study conclusively shows the feasibility of deploying Ds and the dSpm elements in a single construct for insertional mutagenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Assignment of function to the ever-expanding repertoire of sequence information is a major consideration in biological sciences. Another important area (in terms of functional genomics) is the identification and isolation of novel alleles distributed in the wild relatives and even distant species of crop plants, which could contribute towards cultivar improvement. Whether the aim is global assessment of gene function (as is being done in the model plant Arabidopsis thaliana and more recently in rice) or selective assessment of alleles for agronomic use, development of tools for functional analysis have critical importance for the success of such programmes.
Currently, several methods for deciphering gene function are deployed in plants. These include insertional mutagenesis, gene replacement, RNAi-based gene silencing (Chen et al. 2003; Guo et al. 2003), physical mutagenesis using fast neutrons (Li et al. 2001) and chemical mutagenesis or TILLING (McCallum et al. 2000; Till et al. 2003; Slade et al. 2005). Of these, insertional mutagenesis is one of the most favored techniques as it requires no prior sequence information of the gene and can be suitably modified to generate dominant gain-in-function mutations by activation tagging (Wilson et al. 1996; Marsch-Martinez et al. 2002) or engineered to function as a gene trap system (Sundaresan et al. 1995; Smith and Fedoroff 1995; Kiegle et al. 2000).
Two-element based transposon systems utilizing maize transposable elements Ac/Ds and Spm/dSpm have been suitably tailored for efficient gene isolation and disruption in plants (Hehl and Baker 1989; Masson and Fedoroff 1989; Masterson et al. 1989; Altmann et al. 1992; Bancroft et al. 1992). As both the transposable elements show a preference towards linked transpositions, negative selection markers were incorporated to screen for unlinked transposition events (Sundaresan et al. 1995; Tissier et al. 1999). This allowed for maximum coverage of the genome using fewer starter lines. In all the transposon-based systems described till date, only the first transposition event can be phenotypically monitored. A system that could help in monitoring subsequent jumps phenotypically after the initial transposition event would be a major advancement in gene tagging. This would make saturation mutagenesis more efficient as well as allow tagging genes or alleles of interest by using a combination of unlinked and linked transpositions. This system would be particularly helpful in plant species where generating a large collection of the initial T-DNA lines is difficult due to poor transformation frequencies.
We had earlier proposed a four-element based transposon tagging system which would allow both linked and unlinked jumps in two successive steps (Phogat et al. 2000). The four-element system uses a non-autonomous cassette, designated Ds(dSpm) or dSpm(Ds), which harbors both the Ds and dSpm border elements cloned one within the other along with appropriate marker genes to monitor the excision and re-integration of the two transposable elements. The system utilizes a negative selection marker and the outer non-autonomous element for the unlinked scattering of the transposon construct from the site of the initial T-DNA integration. This would lead to the generation of several launch pads at different locations in the genome from where the internal element can subsequently be mobilized for either generating localized mutagenesis (for allele-specific tagging) or for further scattering of the cassette for saturation mutagenesis. As the non-autonomous cassette would harbor both the Ds and the dSpm elements, their trans-activation would require their individual transposase source (Ac transposase for Ds and Spm transposase for dSpm mobilization). The system has been therefore termed as a ‘Four-element’ system. The essence of the system lies in the fact that it enables phenotypic selection of both the unlinked transpositions of the outer borders and the subsequent jumps of the internal element (dSpm) due to the presence of appropriate marker genes. In this study, we report the development of a Ds(dSpm) construct and its functionality in A. thaliana to show that the two transposable elements can be used in conjunction with gene tagging.
Materials and methods
Development of the pPZP100: Ds(dSpm) construct
The Ds cassette (∼1.8 kbp Ds5′ and ∼240 bp Ds3′) containing nptII gene (under the 1′ promoter) was removed from the vector pWS32 (Genetrap Ds, described by Sundaresan et al. 1995) and transferred to the low copy vector pMuCBs (received from V. Sundaresan). The dSpm borders (1.487 kbp dSpm5′ and 740 bp dSpm3′) generated by NsiI digestion of the full-length Spm element were inserted between the 35S promoter and the coding region of the aadA gene in the vector, pLitmus38 (NEB). The aadA(dSpm) cassette was then placed between the Ds borders, alongside the nptII gene. Initially, problems were encountered in the introduction of the aadA(dSpm) fragment within the Ds borders. To circumvent this, the Ds3′ border was removed from the Ds5′-nptII-Ds3′ cassette following which the aadA(dSpm) fragment could be easily cloned. The Ds3′ border was then introduced as a separate fragment to generate the cassette Ds5′-nptII-aadA(dSpm)-Ds3′ (plasmid pMWSSD-Ds3′). The entire assembly was done such that the Ds cassette could be retrieved as a KpnI fragment from the plasmid pMuCBs.
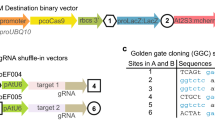
The 2′-iaaH-pA fragment was removed from plasmid pAJ6 (received from V. Sundaresan) and inserted into the KpnI-SacI site in the MCS of binary vector pPZP100 (Hajdukiewicz et al. 1994). Subsequently, the KpnI fragment from plasmid pMWSSD-Ds3′ harboring the Ds cassette was introduced to generate the binary vector pPZP100: Ds(dSpm). The region within the T-DNA borders of the binary vector pPZP100: Ds(dSpm) is schematically represented in Fig. 1.
Details of the Ds(dSpm) construct in binary vector pPZP100. The sequences cloned between the binary vector T-DNA borders have been shown. The Ds element contains an aadA gene (conferring resistance to streptomycin and spectinomycin) and an nptII gene (conferring resistance to kanamycin). Kanamycin resistance serves as a selectable marker for transgenic plants harboring the Ds(dSpm) construct and also as a re-integration marker for the Ds cassette following unlinked transposition while the aadA gene serves as an excision marker for dSpm. The iaaH gene codes for indole acetic acid hydrolase and acts as a negative selection marker to select for unlinked Ds transpositions. The arrows depict the position of the different primers used to characterize the dSpm excision events. Primers A5 and A6 are specific to pre-excision (∼460 bp), while A7 and A8 are specific to dSpm borders (∼750 bp)
Development of Ds(dSpm) transgenics
In planta transformation (Bechtold et al. 1993) of Arabidopsis thaliana ecotype Columbia was carried out using Agrobacterium tumefaciens strain GV3101 harboring the binary vector pPZP100: Ds(dSpm). Transformed lines were identified by germination on medium (1× MS salts, 1× B5 vitamins, 1% sucrose and 0.8% Difco agar) supplemented with 50 mg/l kanamycin and 200 mg/l of the bacteriostatic agent cefotaxime. The plates were placed at 4°C for 3 days after which they were shifted to the growth room (22°C with 10 h light/14 h night photoperiod). The KanR seedlings were transferred to fresh selection plates and subsequently into individual pots. Homozygous transgenic Ds(dSpm) lines were developed by selfing and used subsequently for crosses with transposase containing transgenic lines.
Transposase containing lines used for in-trans activation of Ds(dSpm) construct
Two independent, homozygous Ac transposase lines were used in the current study: sAc line harboring the sΔNaeIAc element (Bancroft et al. 1992) and Ac2 line harboring 35 S::Ac transposase fusion lines (Sundaresan et al. 1995). Both the lines have the AcTPase cassette linked to nptII and iaaH genes, but while in Ac2 the Ac TPase expression is driven by the 35 S promoter, in sAc, Ac is expressed from its own promoter (a 537 bp deletion has been created within the 5′-UTL of Ac). The Ac2 line has been shown to give >30% germinal excision frequency but a low re-insertion frequency of Ds (Swinburne et al. 1992; Long et al. 1993). The sAc line on the other hand has been reported to give a lower frequency of germinal excision (∼5%; Bancroft et al. 1992), but a higher percent of re-insertion events (∼50%; Bancroft and Dean 1993).
For the Spm transposase, A. thaliana (Col) transgenic line (Spm404) harboring the construct 35 STnpA-35 STnpD-bar-iaaH (received from Monika Frey) was used.
Mapping of T-DNA and transposon integration sites
Genomic sequences flanking the insertion lines were amplified using Directional Genome Walking PCR (Mishra et al. 2002) or TAIL-PCR (Liu et al. 1995) with slight modifications in either of the protocols. Genomic DNA was isolated by CTAB method (Rogers and Bendich 1994) or by using DNeasy Plant Mini kit (Qiagen). PCR reactions were carried out using arbitrary primers and gene-specific nested primers. The arbitrary primers AD1, AD2, AD5, AD6 and AD8 (Liu et al. 1995; Liu and Whittier 1995; Tsugeki et al. 1996) and RWP1-RWP4 (Mishra et al. 2002) were deployed for TAIL-PCR and Genome Walking PCR, respectively. Each gene-specific primer set comprised of three nested primers, one each to be used in the three successive PCR reactions. The primary-specific primer of each set was biotinylated so that the same primer sets could be deployed for Genome walking PCR. Two sets of Ds3′ and Ds5′ end-specific primers (previously described by Parinov et al. 1999) were used. The iaaH specific primers consisted of iaaH1 (5′-CCgCAA ACC ATC CCA gTC TgT A-3′), iaaH 1.2 (5′-gCT TCA CAA CgC gCT ATC AgA g-3′) and iaaH1.3 (5′-TCC gTT TCA ggT gTT CTA ggC T-3′). The LB set consisted of RRB1 (5′-ATT CAA TTC ggC gTT AAT TCA gTA CA-3′), RRB1.2 (5′-ACg TCC gCA ATg TgT TAT TAA gTT g-3′) and RRB1.3 (5′-AAg CgT CAA TTT gTT TAC ACC ACA A-3′). The dSpm3′-specific primers included the following: dSpm3-1a (5′-ggC TTA TTT CAg TAA gAg Tg-3′), dSpm3-2a (5′-gTA AgA gTg Tgg ggT TTT ggC C-3′) and dSpm3-3a (5′-Tgg CCg ACA CTC CTT ACC TTT T-3′). The dSpm 5′-specific set comprised of dSpm5-1 (5′-TAT ACT ggA Cgg Cgg gCA gg-3′), dSpm5-2 (5′-ggA gAg AAg AAg CAC gAC ggC-3′) and dSpm5-3 (5′-Agg ATT ggg gAA TTT Agg gTA ACA T-3′).
The amplified PCR products obtained with either method were electrophoresed on agarose gels; the band of interest was eluted (GFXTM PCR, DNA and Gel band purification kit, Amersham Biosciences) and sequenced using ABI PrismTM 310 Genetic Analyzer (Applied Biosystems). The flanking sequences obtained were then subjected to BLASTN search of the NCBI Genbank database to map the insertions on the Arabidopsis genome.
Molecular characterization of the dSpm excision events
PCR amplifications were carried out on tissue samples as described by Klimyuk et al. 1993. The dSpm pre-excision specific primers, A5 (5′-CgC TCg ATg ACg CCA ACT ACC TC-3′) and A6 (5′- gCC CAg gTA gCT TAC TgA TgT-3′), were designed to amplify approximately 460 bp product (Fig. 1). Primers used for analyzing re-integration of the dSpm, A7 (5′-CAA CgA ATC CTg CAT ACC CAA A-3′) and A8 (5′-ACC Tgg gCg CAT ATA AgA gTg Tgg-3′), were designed from within the dSpm borders to amplify approximately 750 bp product (Fig. 1).
The PCR reaction mix comprised of the specific primers (50 pmoles each), 200 μM dNTPs (NEB), 1× Taq polymerase buffer (Finnzyme), 1 U of Finnzyme Taq polymerase and 0.05% nonidet in a final volume of 50 μl. The PCR parameters were as follows: initial denaturation at 94°C for 5 min followed by 30 cycles of 94°C for 1 min, 55°C for 1 min, 72°C for 1 min. A final extension at 72°C was given for 5 min.
Results
Experimental strategy
The four-element system is supposed to function in two-steps (Fig. 2). In the first step, Ds(dSpm) and Ac transposase lines were crossed to trans-activate the Ds cassette. Seeds from selfed F1 lines were screened for the presence of unlinked Ds transpositions based on KanRNAMR phenotype. Since both Ac and the Ds(dSpm) cassettes are linked to the iaaH gene, the selection for a NAMR phenotype would ensure the enrichment of unlinked jumps while simultaneously selecting against the Ac transposase, thereby generating stable insertions.
In the second step, the trans-activation of the dSpm borders was initiated from the primary transposition site to generate secondary transpositions by crossing the lines harboring the transposed Ds cassette with a Spm transposase containing line (Fig. 2). Resulting F2 or F3 seeds were selected on streptomycin containing medium to select for germinal dSpm excision events (seedlings with totally green phenotype on the selection medium). Re-integration of the excised dSpm element in such lines was analyzed by PCR.
Generation of the Ds(dSpm) starter lines
A total of 261 independent transgenic lines were generated in A. thaliana with the pPZP100: Ds(dSpm) construct (Fig. 1). On the basis of the segregation analysis of the F1 seedlings for kanamycin resistance, 65 lines were identified showing an inheritance pattern of T-DNA integrated at a single locus. These lines were further analyzed by Southern hybridization to determine the copy number of T-DNA using probes specific for the left and right border flanks. Five transgenic lines (∼5% of the total lines analyzed) were found to harbor a single copy of the construct.
In order to map the location of the T-DNA insertions in the transgenic lines, the genomic region flanking the insertions was amplified using either TAIL-PCR or Genome Walking PCR. Primers specific to either the Ds3′ border (RB end) or LB region were used in different combinations with the arbitrary primers. The flanking sequences obtained were then subjected to BLASTN search (NCBI Genbank database) to map the insertions on the Arabidopsis genome. We could successfully map four of these single copy lines. The sites of integrations have been summarized in Table 1. Integrations were located on chromosome 2, 3 and 5. Homozygous lines of these events were developed and used in further crosses.
Selection for unlinked transpositions of the Ds element was based on the negative selection marker iaaH, which is also linked to the Ac transposase source (Fig. 2). Thus, it was preferable to use only those lines for crosses which had the Ac and the Ds construct on different chromosomes (i.e., they assorted independently of each other). As the physical location of the T-DNA in the sAc and the Ac2 lines was not known, this was assessed using a genetic approach. The F2 progeny of crosses between the four Ds(dSpm) lines and the two Ac lines were selected on kanamycin and NAM. Crosses showing KanSNAMR phenotype in 1/16th of their progeny indicated independently assorting loci. Thus the F1 progeny of crosses between 4E26 × sAc and 4E59 × Ac2 were taken up for further large-scale transposition experiments.
Functionality of Ds in the Ds(dSpm) cassette
Unlinked Ds transpositions were identified by screening ∼2,000 seeds collected from individual F1 lines on germination medium supplemented with NAM (3.5 μM) and kanamycin (50 mg/l). The putative transposants (exhibiting the double resistant phenotype) were shifted to fresh selection plates to confirm the phenotype and subsequently transferred to soil for further characterization. We identified 19 F1 lines harboring unlinked Ds transpositions in their progeny from a total of 103 F1 lines generated from crosses between Ds(dSpm) line 4E59 and Ac2. The percentage of F2 seeds per F1 line exhibiting a KanRNAMR phenotype varied from 0.05 to 3.5%, of which 0.05% was the most frequent value exhibited by about three-fifths of the total number of F1 plants. F2 seeds from ∼100 F1 plants from the cross between 4E26 and sAc were analyzed for transposition events. No Ds transpositions were observed in this case.
As linked Ds insertions leading to disruption of the iaaH gene can also result in a KanRNAMR phenotype, the absence of iaaH gene in the 19 Ds transposants was tested by PCR. Two sets of iaaH-specific primers and primers from an internal PROLIFERA gene (as an internal control) were used for the PCR reaction (described by Martienssen and Springer, http://www.arabidopsis.org/home.html). Eighteen of the above KanRNAMR lines (one each from the individual F1 lines) lacked the iaaH gene (data not shown) and were taken up for further characterization.
Localization of the Ds transpositions on Arabidopsis chromosomes
Genomic DNA from the double resistant (KanRNAMR) plants was subjected to Directional Genome Walking PCR. Sets of outwardly directed, nested primers specific to both ends of Ds were used in the PCR reactions. Flanking sequences could be amplified from 11 of the 18 KanRNAMR plants using either the Ds3′ or the Ds5′ end-specific primers. For the other lines, either no amplification was observed or the overlapping border sequence was missing in the amplified fragments. Such inconclusive events were not analyzed further. Mapping the Ds insertions showed that these were distributed on all the five chromosomes (Fig. 3). The exact sites of integration in the genome are summarized in Table 1. Therefore, starting from a single Ds insertion (on chromosome 3) in the Ds(dSpm) line 4E59 insertions on all the five chromosomes of A. thaliana could be achieved. This demonstrated the functionality of the Ds cassette. These insertions would serve as new launch sites within the genome from where the dSpm can be trans-activated to generate further insertions.
Distribution of the Ds(dSpm) cassette in the Arabidopsis genome. The integrated map localizes the sites of integration of the Ds cassette in all the KanRNAMR lines generated in the study. Filled triangle indicates the position of the Ds cassette in the parental line 4E59, while the open triangles represent the site of the transposed Ds element. The numbers represent the lines containing these insertions. The scale bar represents distance in megabase pairs (Mbp)
Trans-activation of the dSpm element in the Ds transposants
dSpm transposition was initiated in the Ds transposed lines Ds59-1010, Ds59-191, Ds59-231 and Ds59-251 (Fig. 3) by crossing with the Spm404 line. As the allelic status of the Ds cassette in the transposed lines was not determined prior to crossing, the F1 seeds were selected for KanR to ensure the presence of the Ds construct. The Spm404 line is sensitive to kanamycin. F2 seeds from individual F1 lines were screened on streptomycin plates to analyze dSpm transpositions. On the basis of the presence of a completely green phenotype on selection medium, the germinal excision frequency was estimated to range between 0.005 and 0.03%. Majority of the F2 seedlings exhibited a variegated phenotype. Seedlings exhibiting green or large sectors of green (early excisions) phenotype were rescued on antibiotic-free medium and later transferred to soil for selfing. Approximately, 2,000 F3 seeds collected from individual plants were again subjected to streptomycin assay to select for germinal dSpm excisions. Seedlings exhibiting green phenotype were transferred to fresh selection plates for further characterization.
The re-insertion of the excised element was ascertained through PCR using dSpm-specific primers. To rule out the possibility of the dSpm-specific amplifications arising from the un-excised dSpm allele, the seedlings were first subjected to PCR with primers specific for the pre-excision allele (Fig. 1). From each F2 line, 20–30 green seedlings were analyzed and seedlings exhibiting no amplification products were analyzed further for the presence of the re-inserted dSpm element using primers designed from within the element (Fig. 1). Seedlings harboring the transposed dSpm element were transferred to soil. Genomic DNA isolated from these transposants was then subjected to TAIL-PCR to amplify the genomic region flanking the insertions using dSpm-specific primers designed from both the 5′ and 3′ border regions. We could localize dSpm insertions from three of the Ds transposant lines: Ds59–1010, Ds59-231 and Ds59-251. In case of the line Ds59-191, the rate of germinal dSpm excisions observed in the F3 generation was found to be very low for most of the F2 lines screened. Hence, this line was not used for further screenings. The distribution of the transposed dSpm elements on the Arabidopsis genome is shown in Fig. 4, while the exact sites of integrations are summarized in Table 2. From the Ds59-1010 line, 19 insertions could be selected of which three were present on the same chromosome. Seven dSpm insertions were characterized from the Ds59-251 line and three from the Ds59-231 line. Some of the lines had multiple insertions.
Distribution of the dSpm element in the Arabidopsis genome. The lollipop structure indicates the site of integration of the Ds(dSpm) transposant and the triangles indicate transposed dSpm elements. The shade of the parental line and all the dSpm transpositions originating from it are identical. The scale bar represents distance in megabase pairs (Mbp)
Discussion
Our results show that the two transposon systems namely Ac/Ds and Spm/dSpm can work in conjunction and that the four-element system can functionally generate genome-wide independent insertions using a very limited number of starter lines. Starting from a single transgenic line containing the Ds(dSpm) construct, the transposon cassette could be distributed on all the different chromosomes of Arabidopsis thereby creating several new launch pads within the genome from where the internal dSpm could be activated in-trans to generate secondary transpositions. As the secondary transposition can be phenotypically selected, it saves the huge effort that is required in selecting new insertions.
From ∼103 F1 lines generated by crossing a single Ds(dSpm) line 4E59 (insertion on chromosome 3) with Ac transposase, we could select for 18 unlinked Ds transpositions, of which 11 could be readily mapped on the genome. Two of these insertions were found on the same chromosome, loosely linked to the site of initial integration in the parental line. The other nine insertions were found distributed in the other four chromosomes. The frequency of unlinked Ds transpositions (KanRNAMR progeny) in the progeny of F1 plants varied between 0.05 and 3.5%, of which 0.05% constituted the most frequent transposition frequency shown by ∼60% of the F1 lines that were analyzed. In an earlier report (Sundaresan et al. 1995), a similar range (0.1–2.6%) of unlinked Ds transposition was reported wherein ∼33% of the F1 lines exhibited 0.1% transposition frequency. Thus, in the present study the most common encountered transposition frequency of 0.05% was two-fold lower than the earlier report. One of the possible reasons for this drop could be the increased size (∼8 kbp) of the Ds construct used in this study as compared to the construct used by Sundaresan et al. (1995). A Ds construct comparable to the size of the Ds(dSpm) construct has been used by Suzuki et al. (2001). However, the frequencies of unlinked transpositions have not been clearly described in this study. The size of the Ds(dSpm) cassette used in the present study can be reduced further by deploying smaller Ds and dSpm borders. Significant variation in the frequency of Ds transpositions from different single copy T-DNA donor sites has been observed (Smith et al. 1996), suggesting that chromatin structure influences transposition frequency. This can also be a possible reason for the lower frequency of transpositions observed in this study since the results presented are from a single T-DNA insertion.
After the initial unlinked spread of the Ds(dSpm) construct, the internally placed dSpm element was activated in-trans in four of the Ds transposant lines. These four lines gave a germinal frequency ranging between 0.005 and 0.03%. The frequency, although low, is comparable to the frequency range of 0.0009–4.7% reported by Tissier et al. (1999) wherein most of the plants exhibited germinal excision frequency of less than 1%. The frequencies recorded in this study are in the lower range of the earlier report which could be due to the fewer number of plants that were analyzed.
Of the 29 dSpm insertions studied, six were linked and the rest were unlinked transposition events. The Spm element has been reported to transpose to linked sites at a frequency of 60% in maize and around 45% in tobacco (Cardon et al. 1993). An earlier study in Arabidopsis (Tissier et al. 1999) has recorded more than 80% of the transpositions to be linked. However, our study shows the opposite; around 80% of the dSpm transpositions belong to unlinked sites. These insertions were spread on all the five chromosomes of Arabidopsis. Hence, even with a minimum of three Ds(dSpm) launch pads, dSpm can be suitably trans-activated to generate a large number of insertions distributed throughout the genome. dSpm elements from the rest of the Ds launch sites can be similarly trans-activated to generate more insertions for saturating the genome.
Currently, saturation mutagenesis using transposons is achieved by either unlinked dispersal of the transposon construct from a few starter lines or localized mutagenesis using several starter lines. The first strategy of genome-wide coverage using unlinked transpositions is an arduous task since the frequency of such transpositions is low (Sundaresan et al. 1995; Tissier et al. 1999). The second strategy is applicable to model systems like Arabidopsis but may not be feasible in plants less amenable to genetic transformation. Many crop plants belong to the latter category. The difficulty in using the second strategy is further compounded by the need to identify single-copy starter lines. In such situations a four-element system can be advantageous since a minimum of two transgenic lines containing the Ds(dSpm) construct, apart from the lines expressing the specific transposases, will be sufficient to initiate a saturation mutagenesis program in a species. Although, in a two-element system, the Ds or the dSpm insertions resulting from the initial transpositions can be re-activated to generate further insertions, the absence of a visual excision marker makes the task of identifying new transposition events tedious. Moreover, many of the launch sites are rendered transposition-incompetent due to the perturbations in the borders during the initial transposition (Ito et al. 2002). In the present strategy this difficulty is overcome by activating a second transposon carrying its own borders and independent transposition markers.
Although the present study has conclusively shown the functionality of the Ds(dSpm)-based four-element system for gene tagging, the system can be further modified to optimize its functionality in Arabidopsis as well as in other plant species. The kanamycin resistance conferring nptII gene, currently placed outside the dSpm cassette (Fig. 1), can be cloned within the dSpm borders, thereby allowing the use of nptII both as a transformation as well as an integration marker for Ds and dSpm transpositions. With this type of construct the KanRNAMR phenotype would help in selecting unlinked Ds transpositions, while the StrepRKanR plants would be indicative of dSpm transpositions. The use of a herbicide resistance conferring marker (viz., bar gene) will allow field selection of transpositions. Incorporation of a negative selection marker more effective than iaaH would further facilitate the screening process. We found iaaH to be a very cumbersome selection marker since it does not kill the plants carrying it. The selection is based on morphological changes. Using proficient pro-herbicide to herbicide conversion based negative selection marker like SUI (Tissier et al 1999; Marsch-Martinez et al. 2002; Greco et al. 2004) would be a better choice. The use of markers like SUI would also allow field level selections and thus extend the use of the four-element system to crop species. Fluorescence-based non-destructive selection markers like GFP (green fluorescent protein) and RFP (red fluorescent protein) have been shown to work efficiently in Arabidopsis (Kumar et al. 2005). These can also be used to enhance the screening efficiency. Additionally, in-cis expression of the Ac Tpase (Tissier et al. 1999), especially under an inducible promoter (Nishal et al. 2005), can reduce the number of generations involved and also increase the frequency of selecting unlinked jumps. An inducible promoter would limit the extent of the transposase activity, thus reducing the background mutations which result from the continued presence of the transposase.
A further improvement in the Ds(dSpm) construct could be the use of a dSpm(Ds) construct in which dSpm is used for unlinked transpositions and Ds is used subsequently for linked transpositions for saturation mutagenesis. The present study has conclusively shown that dSpm transposes to unlinked sites at a much higher frequency than what has been reported for the Ds element (Ito et al. 2002). The Ac/Ds transposable elements have also been shown to be more versatile in terms of frequency of transposition in evolutionary very diverse species (Ramachandran and Sundaresan 2001). Novel transposon elements discovered in specific species (Ramachandran and Sundaresan 2001) could be used in conjunction with Ds for developing four-element systems tailored for specific plant species.
Although a number of strategies for mutagenesis are available, systems based on transposable elements like the four-element system described in this study will remain very cost-effective and versatile methods for saturation mutagenesis and site-specific mutagenesis.
References
Altmann I, Schmidt R, Willmitzer L (1992) Establishment of a gene tagging system in Arabidopsis thaliana based on the maize transposable element Ac. Theor Appl Genet 84:371–383
Bancroft I, Dean C (1993) Transposition pattern of the maize element Ds in Arabidopsis thaliana. Genetics 134:1221–1229
Bancroft I, Bhatt AM, Sjodin C, Scofield S, Jones JDG, Dean C (1992) Development of an efficient two-element transposon tagging system in Arabidopsis thaliana. Mol Gen Genomics 233:449–461
Bechtold N, Ellis J, Pelletier G (1993) In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci Paris 316:1194–1199
Cardon GH, Frey M, Saedler H, Gierl A (1993) Definition and characterization of an artificial En/Spm-based transposon tagging system in transgenic tobacco. Plant Mol Biol 23:157–178
Chen S, Hofius D, Sonnewald U, Bornke F (2003) Temporal and spatial control of gene silencing in transgenic plants by inducible expression of double-stranded RNA. Plant J 36:731–740
Greco R, Ouwerkerk PBF, Taal AJC, Sallaud C, Guiderdoni E, Meijer AH, Hoge JHC, Pereira A (2004) Transcription and somatic transposition of the maize En/Spm transposon system in rice. Mol Gen Genomics 270:514–523
Guo H-S, Fei J-F, Xie Q, Chua N-H (2003) A chemical-regulated inducible RNAi system in plants. Plant J 34:383–392
Hajdukiewicz P, Svab Z, Maliga P (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25:989–994
Hehl R, Baker B (1989) Induced transposition of Ds by a stable Ac in crosses of transgenic tobacco plants. Mol Gen Genomics 217:53–59
Ito T, Motohashi R, Kuromori T, Mizukado S, Sakurai T, Kanahara H, Seki M, Shinozaki K (2002) A new resource of locally transposed Dissociation elements for screening gene-knockout lines in silico on the Arabidopsis genome. Plant Physiol 129:1695–1699
Kiegle E, Moore CA, Haseloff J, Tester MA, Knight MR (2000) Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J 23:267–278
Klimyuk VI, Carroll BJ, Thomas CM, Jones JDG (1993) Alkali treatment for rapid preparation of plant material for reliable PCR analysis. Plant J 3:493–494
Kumar CS, Wing RA, Sundaresan V (2005) Efficient insertional mutagenesis in rice using the maize En/Spm elements. Plant J 44:879–892
Li X, Song Y, Century K, Straight S, Ronald P, Dong X, Lassner M, Zhang Y (2001) A fast neutron deletion mutagenesis-based reverse genetics system for plants. Plant J 27:235–242
Liu YG, Whittier RF (1995) Thermal asymmetric interlaced PCR: Automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25:674–681
Liu YG, Mitsukawa N, Oosumi T, Whittier RF (1995) Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J 8:457–463
Long D, Swinburne J, Martin M, Wilson K, Sundberg E, Lee K, Coupland G (1993) Analysis of the frequency of inheritance of transposed Ds elements in Arabidopsis after activation by a CaMV 35 S promoter fusion to the Ac transposase gene. Mol Gen Genomics 241:627–636
Marsch-Martinez N, Greco R, Arkel GV, Herrera-Estrella L, Pereira A (2002) Activation tagging using En-I maize transposon system in Arabidopsis. Plant Physiol 129:1544–1556
Masson P, Fedoroff N (1989) Mobility of the maize Suppressor-mutator element in transgenic tobacco cells. Proc Natl Acad Sci USA 86:2219–2223
Masterson RV, Furtek DB, Grevelding C, Schell J (1989) A maize Ds transposable element containing a dihydrofolate reductase gene transposes in Nicotiana tabacum and Arabidopsis thaliana. Mol Gen Genomics 219:461–466
McCallum CM, Comai L, Greene EA, Henikoff S (2000) Targeting induced local lesions in genomes (TILLING) for plant functional genomics. Plant Physiol 123:439–442
Mishra RN, Singla-Pareek SL, Nair S, Sopory SK, Reddy MK (2002) Directional genome walking using PCR. Biotechniques 33:830–834
Nishal B, Tantikanjana T, Sundaresan V (2005) An inducible targeted tagging system for localized saturation mutagenesis in Arabidopsis. Plant Physiol 137:3–12
Parinov S, Sevugan M, Ye D, Yang WC, Kumaran M, Sundaresan V (1999) Analysis of flanking sequences from Dissociation insertion lines: a database for reverse genetics in Arabidopsis. Plant Cell 11:2263–2270
Phogat S, Burma PK, Pental D (2000) A four-element based transposon system for allele specific tagging in plants—theoretical considerations. J. Biosci 25(1):57–63
Ramachandran S, Sundaresan V (2001) Transposons as tools for functional genomics. Plant Physiol Biochem 39:243–252
Rogers SO, Bendich AJ (1994) Extraction of total cellular DNA from plants, algae and fungi. In: Gelvin SB, Schilperoot RA (eds) Plant Mol Biol Manual. Kluwer Academic Publishers, Dordrecht, pp D:1–D:8
Slade AJ, Fuerstenberg SI, Loeffler D, Steine MN, Facciotti D (2005) A reverse genetic, nontransgenic approach to wheat crop improvement by TILLING. Nat Biotechnol 23:75–81
Smith DL, Fedoroff NV (1995) LRP1, a gene expressed in lateral and adventitious root primordia of Arabidopsis. Plant Cell 7:735–745
Smith D, Yanai Y, Liu Y-G, Ishiguro S, Okada K, Shibata D, Whittier RF, Fedoroff NV (1996) Characterization and mapping of Ds-GUS T-DNA lines for targeted insertional mutagenesis. Plant J 10:721–732
Sundaresan V, Springer P, Volpe T, Haward S, Jones JDG, Dean C, Ma H, Martienssen R (1995) Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev 9:1797–1810
Suzuki Y, Uemura S, Saito Y, Murofushi N, Schmitz G, Theres K, Yamaguchi I (2001) A novel transposon tagging element for obtaining gain-of-function mutants based on a self-stabilizing Ac derivative. Plant Mol Biol 45:123–131
Swinburne J, Balcells L, Scofield SR, Jonathan DGJ, Coupland G (1992) Elevated levels of Activator transposase mRNA are associated with high frequencies of Dissociation excision in Arabidopsis. Plant Cell 4:583–595
Till BJ, Reynolds SH, Greene EA, Codomo CA, Enns LC, Johnson JE, Burtner C, Odden AR, Young K, Taylor NE, Henikoff JG, Comai L, Henikoff S (2003) Large-scale discovery of induced point mutations with high throughput TILLING. Genome Res 13:524–530
Tissier AF, Marillonnet S, Klimyuk V, Patel K, Torres MA, Murphy G, Jones JDG (1999) Multiple independent defective suppressor-mutator transposon insertions in Arabidopsis: a tool for functional genomics. Plant Cell 11:1841–1852
Tsugeki R, Kochieva EZ, Fedoroff NV (1996) A transposon insertion in the Arabidopsis SSR16 gene causes an embryo-defective lethal mutation. Plant J 10:479–489
Wilson K, Long D, Swinburne J, Coupland G (1996) A Dissociation insertion causes a semidominant mutation that increases expression of TINY, an Arabidopsis gene related to APETALA2. Plant Cell 8(4):659–671
Acknowledgments
We thank Dr. V. Sundaresan for the gift of constructs pWS32 and pAJ6, the low copy vector pMuCBs and the transposase line Ac2; Dr. R. Schmidt for providing the transposase line sAc; Dr. Monika Frey for the transposase line Spm404; Dr. J.D.G. Jones for the construct pSLJ532 harboring the 35S-spec-pA cassette and Dr. R. Raina for the construct pEMBL118-dSpm used for generating the dSpm borders used in the present study. We are grateful to Dr. Sanjay Phogat for useful discussions and suggestions. We thank Shankar for technical assistance in screening for transpositions and maintenance of the growth rooms and Prasad Rao and UmaShanker for assistance in infiltration experiments. This work was supported by grant-in-aid from CSIR (Council of Scientific and Industrial Research) under the NMITLI (New Millennium Indian Technology Leadership Initiative) programme. Priya Panjabi was supported by a CSIR research fellowship and a post-doctoral research fellowship by MAHYCO (Maharashtra Hybrid Seeds Company Ltd.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M.-A. Grandbastien.
Rights and permissions
About this article
Cite this article
Panjabi, P., Burma, P.K. & Pental, D. Use of the transposable element Ac/Ds in conjunction with Spm/dSpm for gene tagging allows extensive genome coverage with a limited number of starter lines: functional analysis of a four-element system in Arabidopsis thaliana . Mol Genet Genomics 276, 533–543 (2006). https://doi.org/10.1007/s00438-006-0158-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-006-0158-0