Abstract
The Rf3 gene restores the pollen fertility disturbed by S male sterile cytoplasm. In order to develop molecular markers tightly linked to Rf3, we used amplified fragment length polymorphism (AFLP) technique with near isogenic lines (NILs) and bulk segregant analysis (BSA). A BC1F1 population from a pair of NILs with different Rf3 locus was constructed and 528 primer combinations was screened. A linkage map was constructed around the Rf3 locus, which was mapped on the distal region of chromosome 2 long arm with the help of SSR marker UMC2184. The closest marker E7P6 was 0.9 cM away from Rf3. Marker E3P1, 2.4 cM from Rf3, and E12M7, 1.8 cM from Rf3, were converted into a codominant CAPS and a dominant SCAR marker, and designated as CAPSE3P1 and SCARE12M7, respectively. These markers are useful for marker-assisted selection and map-based cloning of the Rf3 gene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant cytoplasmic male sterility (CMS) is a maternally inherited trait, characterized by its inability to produce viable pollen without affecting the female fertility. It is often associated with novel mitochondrial open reading frames (ORFs) that are chimerical in structure and frequently co-transcribed with conventional mitochondrial genes (Schnable and Wise 1998). Nuclear genes designated fertility restorer (Rf) restore fertility to plant carrying CMS by suppressing the expression of deleterious mitochondrial ORFs or reducing CMS-associated proteins (Schnable and Wise 1998; Wise and Pring 2002). CMS/Rf system greatly facilitates hybrid seed production because it eliminates the need for tedious hand emasculation. Besides this practical exploitation, the isolation and characterization of ORFs associated with CMS and corresponding Rf genes also offer opportunity to examine the regulation of mitochondrial genes by nuclear genes in multicellular organisms (Maureen and Bentolila 2004).
In all, four restorer genes have been cloned and functionally characterized. The Rf2 gene in T-CMS maize encodes a mitochondrial aldehyde dehydrogenase, which compensates the metabolic defect caused by mitochondrial URF13 protein associated with CMS (Cui et al. 1996). Other three Rf genes including Rf in petunia (Bentolila et al. 2002), Rfk1/Rfo in radish (Koizuka et al. 2003; Brown et al. 2003), and Rf1 in rice (Kazama and Toriyama 2003; komori et al. 2004; Akagi et al. 2004) encode pentatricopeptide repeat (PPR) proteins, which are targeted to mitochondria and disrupt the accumulation of CMS-associated proteins. Except Rf1 in rice which functions gametophytically, other cloned Rf genes work sporophytically. In CMS-S maize, male sterility and fertility are also determined by mitochondrial–nuclear interactions in the haploid male gametophyte (Buchert 1961). It was discovered that S cytoplasmic male sterility in maize was due to the expression of a chimeric mitochondrial gene region designated orf355–orf77 (Zabala et al. 1997). The nuclear allele capable of restoring fertility to CMS-S plants is designated as Rf3 (Duvick 1965), and the nonrestoring allele (rf3) does not transmit through the pollen. Several attempts have been made to tag Rf3 with molecular markers. Rf3 has been located on the long arm of maize chromosome 2 (2L) by translocation and inversion heterozygotes (Laughnan and Gabay 1978). Two RFLP markers, whp and bnl7.14 in 2.09bin, were shown to be at a distance of 4.3 cM proximal and 6.4 cM distal to Rf3 locus, respectively (Kamps and Chase 1997). Shi et al. (1997) and Tie (2000) found a RAPD and a SSR marker linked with Rf3 locus, the distance between them and Rf3 is 2.7 and 2.29 cM, respectively.
Marker-assisted selection (MAS) can improve the breeding process by selecting molecular marker linked with genes controlling the target traits (Francia et al. 2005). AFLP markers (Vos et al. 1995) combine the strengths of RFLP and RAPD markers and overcome their problems. This approach can generate complex band patterns in each reaction and cover the whole genome. It requires no probe or sequence information as needed by RFLP, and is reliable due to its high stringent polymorphase chain reaction (PCR) in contrast to RAPD (Zhu et al. 1998). Combination of AFLP and BSA has been shown to be an efficient way to generate a number of markers closely linked with important agronomic traits (Negi et al. 2000; von Stackelberg et al. 2003; Hagihara et al. 2005; Feng et al. 2005). However, AFLP has limitations with large-scale, locus-specific application because of its intensity of labor, high costs and their dominant type of inheritance. Therefore, conversation of AFLP markers into sequence-specific PCR-based markers is required to facilitate large-scale population selection.
The objective of this study was to identify AFLP markers more closely linked with the Rf3 of S-CMS in maize, and to convert them into PCR-based markers for the benefit of MAS and map-based cloning of Rf3. Furthermore, SSR markers on chromosome 2L reported previously were conducted to provide anchor points.
Materials and methods
Plant materials
A backcross population was set up using a cross among S-Mo17rf3rf3, a S type cytoplasmic male sterile line, and a S-Mo17Rf3Rf3, the near isogenic line (NIL) related to S-Mo17rf3rf3 (Xia and Zheng 2002). They share similar genetic background with only different Rf3 locus and its adjacent region. Simultaneously, N-Mo17rf3rf3, a sterility maintainer performed as recurrent male parent. Materials mentioned above and their deriving BC1F1 population, 343 plants of (S-Mo17rf3rf3 × S-Mo17Rf3Rf3) × N-Mo17rf3rf3, were grown on the farm of Huangzhong Agricultural University, Wuhan, China.
Male fertility
The BC1F1 plants of genotype (Rf3/rf3) produce 50% functional and 50% aborted pollen due to the gametophytic nature of fertility restoration, whereas the rf3/rf3 plants are completely sterile. According to their ability in shedding pollen and stainability of pollen grains by 1% I-KI (Xia and Zheng 2002), each plant was scored as male fertile or sterile.
Isolation of genomic DNA and construction of DNA bulks
Genome DNA was prepared from parents and BC1F1 individuals by a CTAB procedure described by Zhang et al (1994) with minor modifications. 5 g leaf tissues were ground into powder in liquid nitrogen, then extracted at 65°C, de-proteinized with chloroform/isoamyl alcohol, and then precipitated with isopropanol prior to treatment with RNase. Briefly dried DNA was solubilized in 0.5 ml TE (10 mM Tris/HCl pH 8.0, 1 mM EDTA). Equivalent amounts of DNA from ten fertile plants and ten sterile plants were used to construct fertile (BF) and sterile (BS) DNA bulks in order to find markers linked to Rf3.
SSR and AFLP analysis
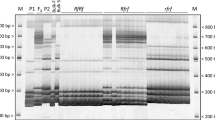
Thirteen microsatellite primers on maize chromosome 2.09bin were screened by parents and DNA bulks, their sequences were available on the maize genetic and genomics database (http://www.maizegdb.org). Special primers were used for linkage analysis as anchor points to distinguish Rf3/rf3 parents and BF/BS bulks. The temperature and time profile is 30 cycles of 94°C for 30 s, 54–60°C for 30 s (modified when needed), and 72°C for 1 min. Electrophoresis was performed with 6% denaturing polyacrylamide gels. The DNA was visualized by silver staining following the protocol of Bassam et al. (1991).
AFLP marker analysis was performed as described by Vos et al. (1995) with some minor modifications, such as using three restriction enzyme combinations, EcoRI/MseI (E/M), PstI/MseI (P/M) and EcoRI/PstI (P/E). A total of 250 ng genomic DNA was digested and ligated with corresponding adaptors. Pre-amplification was performed with primers containing a single selective nucleotide. And selective amplification was carried out with primers containing two (only in the PstI primers) or three additional selective nucleotides. The electrophoresis and silver staining were performed as method in SSR.
Conversion of AFLP markers into PCR-based markers
Gel with polymorphic DNA fragments was excised with a scalpel blade and transferred into a microcentrifuge tube. Distilled water (50 ul) was added, each piece was crushed with a pipette tip and the mixture was boiled for 20 min. The solution was centrifuged and the supernatant was used as template for PCR amplification with corresponding selective AFLP primers for 30 cycles at 94°C for 30 s, 58°C for 1 min and 72°C for 1 min. The product was electrophoresed on 1.5% agarose gel, and the appropriate size of fragment was inserted into a pGEM-T easy vector (Promega) and sequenced (Shanghai Sangon). A search for sequences homologous with the AFLP fragments was conducted in Genbank (http://www.ncbi.nlm.nih.gov/BLAST) and Clustal W (http://www.ebi.ac.uk/clustalW) was used to compare marker sequences for homology.
Primers were designed with Primer Premier 5.0 and synthesized commercially. The primers based on the original sequences of AFLP fragments were used to amplify the corresponding region from S-Mo17Rf3Rf3 and S-Mo17rf3rf3 to detect polymorphism. Sequences whose polymorphism was not obtained in this process were used to design external primers according to their flanking sequences. Restriction endonucleases required for digesting PCR products to get CAPS markers were purchased from Promega.
Linkage analysis
Codominant markers or dominant markers with fragments present in S-Mo17Rf3Rf3 and BF pool but not in S-Mo17rf3rf3 and BS pool were applied for linkage analysis in 343 BC1F1 plants. Mapmaker/Exp, version3.0 software (Lander et al. 1987) was employed to calculate genetic distance on a minimum LOD score of 3.0 between Rf3 and associated markers, and to draw the linkage map.
Results
Segregation analysis of male fertility
The BC1F1 generation yielded 178 semi-fertile (having 50% or more stainable pollen) and 165 sterile plants. This segregation ratio fits a monogenic Mendelian inheritance model of 1 fertile (Rf3rf3):1 sterile (rf3rf3) (χ2 = 0.419, P > 0.95) in this population, it confirmed that fertility restoration was conditioned by one dominant restorer gene in these maize materials.
Identification of molecular markers linked to rf3
The fertile parent, bulk and sterile parent, bulk were compared to screen SSR and AFLP markers. One of 13 SSRs on chromosome 2.09bin, UMC2184, was detected to show codominant polymorphism between parents and bulks. No differences were revealed between our study materials with other 12 SSRs, thus UMC2184 should be employed to analyze linkage relationship between itself and rf3 and perform anchor point to other markers.
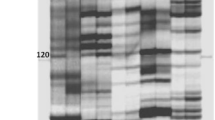
The total 528 (144 of E/M, 192 of P/M, 192 of P/E) AFLP primer combinations (PC) were screened for their polymorphism between parents and bulks. Approximately 29,000 fragments were scored, with an average of 55 fragments per PC. Each P/E (both are hexa-nucleotide-required endonucleases) PC generated an average of 40 bands, while 62 bands were revealed by the other PC, P/M and E/M (MseI recognizes tetra-nucleotide site) on average. The number of bands was consistent with expected results with respect to the site length recognized by restriction enzyme. What’s more, P/E PC provided clearer bands than others. We totally identified 31 polymorphic fragments which were present in the fertile parent and bulk while absent from the sterile parent and bulk. About 21 were shown by the P/E PC. Polymorphic bands were named as the PC where they originated from, and were further confirmed whether they were linked to Rf3 by amplifying them from the individuals composing the bulks. Only five polymorphic fragments from E3P1, E4P6, E7P6, E10P7 and E12M7 were present in ten fertile individuals and absent from ten sterile individuals. A representative gel from PC E10P7 is shown in Fig. 1. Its linkage relationship with Rf3 was analyzed subsequently using the BC1F1 population.
Conversion of AFLP markers into PCR-based markers
In order to convert AFLP markers into simple single-locus PCR-based markers, the AFLP markers, confirmed to be linked to rf3, were gel-purified, re-amplified, cloned and sequenced. Eighty percent fragments except one from E10P7 were re-amplified successfully. Homologous analysis among other four marker sequences show that sequences from E7P6 and E4P6 were homologous with only one nucleotide difference, originated from EcoRI selective base. Therefore, only AFLP markers from PC E3P1, E7P6, E12M7 were selected to conduct conversion. The locus-specific primers were designed based on their sequences for amplifying parents and bulks to detect polymorphism. A summary of every converted marker is shown in the Table 1.
Using primers derived from internal sequences of E12M7 markers, we amplified a 170 bp fragment from fertile parent and bulk, but no product from sterile parent and bulk. Further confirmation of this marker linked to rf3 was accomplished by examining two individual bulks. The same result as originated AFLP marker E12M7 was obtained (Fig. 2a). This marker was then designated as SCARE12M7, and employed for linkage analysis in BC1F1 population.
a BSA analysis of SCAR marker converted from E12M7, arrow indicates a 170 bp band specific for fertile parent, bulk, and individuals. b BSA analysis of CAPs marker converted from E3P1, arrow indicates a 347 bp band specific for fertile parent, bulk, and individuals, this band is cut into 238 and 109 bp bands in sterile parent and individuals, all the three bands are present in fertile bulk and individuals due to their heterozygosity in Rf3 locus. Marker is DNA ladder 2000 from Takara (Rf3, BF: fertile parent and bulk; rf3, BF: sterile parent and bulk)
The AFLP marker E3P1 is 271 bp with AT-rich (60%), the internal primers were not designed successfully. To acquire its flanking sequences, the convenient homologous search was applied. A maize GSS sequence from Genbank (accession No.: CL993366) is high homologous with our query (E value = 3e−127) and long enough to cover the sequence studied. According to the flanking sequence, the appropriate external primers were designed and a 359 bp fragment was produced from both fertile and sterile parents. The fragments were cloned into pGEM-T easy vector and sequenced. Sequences comparing analysis indicated that a SNP resided in the site recognized by MseI. Polymorphism distinguishing Rf3 and rf3 alleles were displayed by cutting this 359 bp fragments from fertile parent once into a 347 bp fragment and a 12 bp invisible piece while from sterile parent twice into 238 and 109 bp fragments and a 12 bp invisible piece. This difference can be displayed distinctly through electrophoresis in 2% agarose gel, as indicated in Fig. 2b. Further confirmation in the individuals of bulks indicated the undoubted linked character to the rf3 locus (Fig. 2b). This marker was thus converted into a codominant CAPS marker and named as CAPSE3P1 for subsequent linkage analysis in BC1F1 population.
Linkage analysis
In our study, one SSR marker, two AFLP marker and two PCR-based markers, converted from AFLP markers, were chosen to analyze all members in the segregated population. Linkage analysis revealed a single linkage group with a total size of 11.1 cM and demonstrated that this group was located on the maize chromosome 2L anchored by marker UMC2184 lying on 2.09bin (http://www.maizegdb.org). Markers E7P6 and SCARE12M7, flanking Rf3 most closely, delimited a 2.7 cM window with 0.9 and 1.8 cM from Rf3, respectively, and the codominant marker CAPSE3P1 was 2.4 cM from Rf3 on the same side with E7P6 (Fig. 3).
Discussion
Near isogenic lines were successfully applied in screening molecular markers tightly linked to interested trait (Komori et al. 2003). Generally, fertile NIL plants are generated by transferring corresponding Rf gene from exotic germplasm into an elite CMS line through multi-generation backcross. Theoretically, selected fertile NILs plants will share probably uniform genetic background with original sterile plants other than the restorer gene and its vicinity. When molecular markers are screened in the NILs materials by BSA method, only those differing in the Rf locus and its adjacent region will exhibit polymorphism. In our study, we totally identified 31 polymorphic fragments in the fertile parent and bulk while absent from the sterile parent and bulk. According to present and absent fragments oppositely, approximately 62 polymorphic fragments from total 29,000 were revealed by AFLP analysis. The low polymorphism was expected because we employed NILs and BSA methods. The markers, RAPD Eo8-1.2, and SSR UMC1525, bnlg1520, previously showed to link to rf3 (Shi et al. 1997; Tie 2000), displayed no polymorphisms (data not shown). This can be explained by the low polymorphism between NILs.
AFLP is an efficient way to screen markers linked closely to the target trait because of its high volume and no sequence information needed (Bentolila and Hanson 2001; Touzet et al. 2004; Hagihara et al. 2005). Previous reports introduced the conversion of AFLP markers into single-locus markers (Meksem et al. 2001; Brugmans et al. 2003; kusterer et al. 2005). In the study, we identified five AFLP markers linked to rf3 locus, and converted markers E3P1 and E12M7 into a codominant CAPS marker and a dominant SCAR marker, respectively. During converting E3P1 into CAPSE3P1, flanking sequence for external primers designing were derived from GenBank in NCBI instead of using inverse PCR method in other studies (Wen et al. 2002). Making the best use of available database recourses could save significant labor in laboratory. Unfortunately, the flanking sequence of E7P6 is not available in the database, and thus, the experimental means such as inverse PCR is required for studying unknown sequences in non-modern organism without the whole genomic sequence information, e.g. maize. Additionally, we cannot re-amplify the fragments from marker E10P7, the rate of re-amplification success was 80%, and the corresponding study was nearly 90% (Nicod and Largiadèr 2003). Furthermore, we unpresedentedly employed AFLP analysis with two hexa-nucleotide-required endonucleases in cutting maize genome DNA. The present data indicated that this modifications displayed less and clearer bands and higher polymorphism than combination of tetra- and hexa-nucleotide-required endonucleases.
Conventional breeding of restorer lines is laborious and time-consuming, because selected plants should be tested for their ability to restore fertility. The single-locus markers in the study are suitable for efficiently selecting maintainer and restorer plants for S-CMS in maize early at the seedling stage. Especially, CAPSE3P1 can discriminate homozygotes and heterozygotes. Additionally, blastX analysis showed that the sequence of marker SCARE12M7 was high homologous with resistance protein T10rga2-1A in Triticum aestivum (E value = 4e−06). Gene containing this marker segment may encode a resistant protein because pathogen resistant genes have high sequence homology in local region (Hammond-Kosack and Parker 2003). Recently, Shi et al. (2005) identified a QTL resistant to head smut, adjacent to SSR marker bnlg1520 in maize chromosome 2.09bin, on which the rf3 gene exactly locates (Fig. 4c). It is coincident that marker bnlg1520 is 8.9 cM from rf3 reported by Tie (2000) (Fig. 4b). Hence, we assumed that marker SCARE12M7 was tightly linked to gene resistant to head smut as well as rf3, even cosegregant with this resistant gene. If so, marker SCARE12M7 can be applied to assess and select plants for Rf3 gene and gene resistant to head smut, simultaneously. However, it is very difficulty to select two traits simultaneously using conventional breeding method because both head smut outbreaking and pollen dispersing are at the florescence (Lindsey et al. 1999).
Comparative linkage relationship of Rf3 and associated locus. a Linkage relationship of Rf3 and marker SCARE12M7 from the present study; b Linkage relationship of Rf3 and marker bnlg1520 (from Tie 2000); c mapping of a putative QTL resistant to head smut in maize. Black broad line indicates putative QTL resistant to head smut mapped on the chromosome 2L of maize (from Shi et al. 2005)
Heretofore, all of the cloned restorer genes, except Rf2 in T-CMS maize, encode PPR proteins (Bentolila et al. 2002; Koizuka et al. 2003; Brown et al. 2003; Kazama and Toriyama 2003; komori et al. 2004; Akagi et al. 2004). Due to its functioning gametophytically and large genome size as non-modern organism, the cloning of Rf3 in S-CMS maize would further demonstrate whether PPR-containing protein is prevalent in fertility restorer. Additionally, the Rf3 locus segregated with the presence of shorter transcripts of both the CMS-associated orf355/orf77 region and smaller transcripts of cob and atp6 (Wen and Chase 1999a). Wen et al. (2003) showed that a nuclear fertility restorer mutation disrupts the accumulation of mitochondrial ATP synthase subunit α in the developing pollen of S male-sterile maize. According to the above effects on multiple transcripts, the cloning of Rf3 will permit detailed explanation on the interaction of mitochondrial and nuclear genomes, which results in the restoration of pollen fertility. This study provides the foundation for map-based cloning of the Rf3 gene.
Although the markers tightly linked to Rf3 are necessary for high-efficiency map-based cloning, the relationship between genetic and physical distance is of predominant importance concerning the success of a map-based cloning approach. Recent reports showed that genes are hot spots for recombination event. It is suggested that in gene-rich regions the relation of basepair to cM, with less than 200 kbp/cM, is similar in all plants, regardless of their overall genome size (Künzel et al. 2000). In maize, Fu et al. (2001) demonstrated that unusually high rate of recombination in bronze locus is related to very high gene density of the region. A recent paper reported that 42 Rf alleles for S-CMS in Mexican maize and Teosinte were mapped to the long arm of chromosome 2 (2L), and 5 of these were further mapped to the whp1–rf3 region (Susan et al. 2004). It was supported that the rf3 region of 2L potentially encodes a complex of linked rf genes, which have functions in normal mitochondrial gene expression and restore male fertility in S cytoplasm by chance (Susan et al. 2004). Although fertility restoration is controlled by one dominant restorer gene in the present segregating population, it is assumed that the rf3 region of the materials here may encode a complex of linked rf genes which play a role on normal mitochondrial gene expression and display no polymorphism between the parental NILs. However, all of them is not enough to deduce that high rate of recombination exists in rf3 region. A detailed analysis of genome structure in this region is needed to study the relationship between genetic and physical distance. Additionally, higher recombination rates in distal than in proximal chromosome regions have been demonstrated (Künzel et al. 2000). Therefore, recombination may occur with high frequency since rf3 gene is located on the distal region of the chromosome 2 long arm (2.09bin) (Kamps and Chase 1997; Shi et al. 1997; Tie 2000). If so, we can attempt to use present linked markers, especially E7P6, 0.9 cM from Rf3 locus, as landmarks to screen S-Mo17Rf3Rf3 BAC library for genome walking to identify candidates of Rf3 gene.
In this study, we identified markers closely linked to the Rf3 gene of S-CMS in maize. We also converted markers E3P1 and E12M7 into a codominant CAPS marker and a dominant SCAR marker, respectively. Such information will provide a basis for a map-based cloning approach and marker-assisted breeding.
Abbreviations
- AFLP:
-
Amplified fragment length polymorphism
- BF:
-
Fertile DNA bulks
- BS:
-
Sterile DNA bulks
- BSA:
-
Bulk segregant analysis
- CAPS:
-
Cleaved amplified polymorphic sequence
- CMS:
-
Cytoplasmic male sterility
- E/M :
-
EcoRI/MseI
- P/M :
-
PstI/MseI
- P/E :
-
EcoRI/PstI
- MAS:
-
Marker-assisted selection
- NILs:
-
Near isogenic lines
- ORF:
-
Open reading frames
- PC:
-
Primer combinations
- PPR:
-
Pentatricopeptide repeat
- RAPD:
-
Random amplified polymorphic DNA
- RFLP:
-
Restriction fragment length polymorphism
- SCAR:
-
Sequence characterized amplified regions
- SNP:
-
Singe nucleotide polymorphism
- SSR:
-
Simple sequence repeat, microsatellite
References
Akagi H, Nakamura A, Yokozeki-Misono Y, Inagaki A, Takahashi H, Mori K, Fujimura T (2004) Positional cloning of the rice Rf-1 gene, a restorer of BT-type cytoplasmic male sterility that encodes a mitochondria-targeting PPR protein. Theor Appl Genet 108:1449–1457
Bassam BJ, Caetano Anoll SG, Gresshoff PM (1991) Fast and sensitive silver staining of DNA in polyacrylamide gels. Ann Biochem 196:80–83
Bentolila S, Hanson MR (2001) Identification of a BIBAC clone that co-segregates with the petunia restorer of fertility (Rf) gene. Mol Genet Genomics 266:223–230
Bentolila S, Alfonso AA, Hanson MR (2002) A pentatricopeptide repeat-containing gene restores fertility to cytoplasmic malesterile plants. Proc Natl Acad Sci USA 99:10887–10892
Brown GG, Formanova N, Jin H, Wargachuk R, Dendy C, Patil P, Laforest M, Zhang J, Cheung WY, Landry BS (2003) The radish Rfo restorer gene of Ogura cytoplasmic male sterility encodes a protein with multiple pentatricopeptide repeats. Plant J 35:262–272
Brugmans B, Ron GM, Van Der H, Visser GF, Richard P, Lindhout, van Eck Herman J (2003) A new and versatile method for the successful conversion of AFLP markers into simple single locus markers. Nucleic Acids Res 31:e55
Buchert JG (1961) The stage of the genome–plasmon interaction in the restoration of fertility to cytoplasmically pollen-sterile maize. Genetics 47:1436–1440
Cui X, Wise RP, Schnable PS (1996) The Rf2 nuclear restorer gene of male-sterile, T-cytoplasm maize. Science 272:1334–1336
Duvick DN (1965) Cytoplasmic pollen sterility in corn. Adv Genet 13:1–56
Feng CD, Stewart JMcD, Zhang JF (2005) STS markers linked to the rf1 fertility restorer gene of cotton. Theor Appl Genet 110:237–243
Francia E, Tacconi G, Crosatti C, Barabaschi D, Bulgarelli D, Dall’Aglio E, Valè G (2005) Marker assisted selection in crop plants. Plant Cell Tissue Organ Cult 82:317–342
Fu HH, Park W, Yan X, Zheng ZW, Shen BZ, Dooner HK (2001) The highly recombinogenic bz locus lies in an unusually gene-rich region of the maize genome. Proc Natl Acad Sci USA 98:8903–8908
Hagihara E, Itchoda N, Habu Y, Mikami T, Kubo T (2005) Molecular mapping of a fertility restorer gene for Owen cytoplasmic male sterility in sugar beet. Theor Appl Genet 111:250–255
Hammond-Kosack KP, Parker JE (2003) Deciphering plant–pathogen communication: fresh perspectives for molecular resistance breeding. Curr Opin Biotechnol 14:177–193
Kamps TL, Chase CD (1997) RFLP mapping of the maize gametophytic restorer of fertility locus (rf3) and aberrant pollen transmission of the nonrestoring rf3 allele. Theor Appl Genet 95:525–531
Kazama T, Toriyama K (2003) A pentatricopeptide repeat-containing gene that promotes the processing of aberrant atp6 RNA of cytoplasmic male-sterile rice. FEBS Lett 544:99–102
Koizuka N, Imai R, Fujimoto H, Hayakawa T, Kimura Y, Kohno Murase J, Sakai T, Kawasaki S, Imamura J (2003) Genetic characterization of a pentatricopeptide repeat protein gene, orf687, that restores fertility in the cytoplasmic male-sterile Kosena radish. Plant J 34:407–415
Komori T, Yamamoto T, Takemori N, Kashihara M, Matsushima H, Nitta N (2003) Fine genetic mapping of the nuclear gene, Rf-1, that restores the BT-type cytoplasmic male sterility in rice (Oryza sativa L.) by PCR-based markers. Euphytica 129:241–247
Komori T, Ohta S, Murai N, Takakura Y, Kuraya Y, Suzuki S, Hei Y, Imaseki H, Nitta N (2004) Map-based cloning of a fertility restorer gene, Rf-1, in rice (Oryza sativa L.). Plant J 37:315–325
Künzel G, Korzun L, Meister A (2000) Cytologically integrated physical restriction fragment length polymorphism maps for the barley genome based on translocation breakpoints. Genetics 154:397–412
Kusterer B, Horn R, Friedt W (2005) Molecular mapping of the fertility restoration locus Rf1 in sunflower and development of diagnostic markers for the restorer gene. Euphytica 143:35–42
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincole SE, Newburg L (1987) MAPMAKER: an interactive computer package for construction primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Laughnan JR, Gabay SJ (1978) Nuclear and cytoplasmic mutations to fertility in S male sterile maize. In: Walden DB (ed) Maize breeding and genetics. Wiley, New York, pp 427–447
Lindsey J, Toit D, Pataky JK (1999) Effects of silk maturity and pollination on infection of maize ears by ustilago maydis. Plant Dis 83:621–626
Maureen RH, Bentolila S (2004) Interactions of mitochondrial and nuclear genes that affect male gametophyte development. Plant Cell 16:S154–S169
Meksem K, Ruben E, Hyten D, Triwitayakorn K, Lightfoot DA (2001) Conversion of AFLP bands into high-throughput DNA markers. Mol Genet Genomics 265:207–214
Negi MS, Devic M, Delseny M, Lakshmikumaran M (2000) Identification of AFLP fragments linked to seed coat colour in Brassica juncea and conversion to a SCAR marker for rapid selection. Theor Appl Genet 101:146–152
Nicod JC, Largiadèr CR (2003) SNPs by AFLP (SBA): a rapid SNP isolation strategy for non-model organisms. Nucleic Acids Res 31:e19
Schnable PS, Wise RP (1998) The molecular basis of cytoplasmic male sterility and fertility restoration. Trends Plant Sci 3:175–180
Shi YG, Zheng YL, Li JS, Liu JL (1997) Mapping CMS-S restorers gene Rf3 with RFLP and RAPD. Acta Agron Sin 23:1–6
Shi HL, Jiang YX, Wang ZH, Li XH, Li MS, Zhang SH (2005) QTL identification of resistance to heat smut in maize. Acta Agron Sin 31:1449–1454
von Stackelberg M, Lindemann S, Menke M, Riesselmann S, Jacobse HJ (2003) Identification of AFLP and STS markers closely linked to the def locus in pea. Theor Appl Genet 106:1293–1299
Susan GL, Chase CD, Ortega VM, Zhao LM (2004) Molecular-genetic characterization of CMS-S restorer-of-fertility alleles identified in Mexican maize and teosinte. Genetics 166:959–970
Tie SG (2000) Genetic characterization and gene mapping of S-CMS in maize. Ph. D. Dissertation. Huazhong Agricultural University, Wuhan
Touzet P, Hueber N, Bürkholz A, Barnes S, Cuguen J (2004) Genetic analysis of male fertility restoration in wild cytoplasmic male sterility G of beet. Theor Appl Genet 109:240–247
Vos P, Hoigers R, Bleeker M, Reijans M, Van De Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M (1995) AFLP: a new concept for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Wen L, Chase CD (1999) Pleiotropic effects of a nuclear restorer-of-fertility locus on mitochondrial transcripts in male-sterile and S male-sterile maize. Curr Genet 35:521–526
Wen L, Tang HV, Chen W, Chang R, Pring DR, Klein PE, Childs KL, Klein RR (2002) Development and mapping of AFLP markers linked to the sorghum fertility restorer gene rf4. Theor Appl Genet 104:577–585
Wen LY, Ruesch KL, Ortega VM, Kamps TL, Laughnan SG, Chase CD (2003) A nuclear restorer-of-fertility mutation disrupts accumulation of mitochondrial ATP synthase subunit α in developing pollen of S male-sterile maize. Genetics 165:771–779
Wise RP, Pring DR (2002) Nuclear-mediated mitochondrial gene regulation and male fertility in higher plants: light at the end of the tunnel? Proc Natl Acad Sci USA 99:10240–10242
Xia JH, Zheng YL (2002) Molecular marker-assisted backcross breeding of maize Rf3 NIL and its efficient analysis. Acta Agron Sin 28:339–344
Zabala G, Laughnan SG, Laughnan JL (1997) The nuclear gene rf3 affects the mitochondrial chimeric sequence R implicated in S-type male sterility in maize. Genetics 147:847–860
Zhang Q, Gao YJ, Yang SH, Ragab RA, Saghai Maroof MA, Li ZB (1994) A diallel analysis of heterosis in elite hybrid rice based on RFLPs and microsatellites. Theor Appl Genet 89:185–192
Zhu J, Gale MD, Quarrie S, Jackson MT, Bryan GJ (1998) AFLP markers for the study of rice biodiversity. Theor Appl Genet 96:602–611
Acknowledgments
The authors thank Prof. Neil Forsberg for critical review of this manuscript. This research was supported by the National High Technique Foundation of China “863” (2004AA222160), “948” Fund in Ministry of Agronomy of China (201010), the State Key Basic Research and Development Plan of China “973” (2001CB1088).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Hagemann
Rights and permissions
About this article
Cite this article
Zhang, Z.F., Wang, Y. & Zheng, Y.L. AFLP and PCR-based markers linked to Rf3, a fertility restorer gene for S cytoplasmic male sterility in maize. Mol Genet Genomics 276, 162–169 (2006). https://doi.org/10.1007/s00438-006-0131-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-006-0131-y