Abstract
We have cloned the Aspergillus niger dapB gene. Analysis of its nucleotide sequence and the corresponding protein sequence indicates that the gene encodes a type IV dipeptidyl aminopeptidase (DPP IV). Based upon its deduced sequence we predict the presence of a transmembrane domain in the protein. Furthermore, dapB-overexpressing transformants display an increase in intracellular DPP IV activity. This is the first reported characterisation of a dipeptidyl aminopeptidase with a transmembrane domain from a filamentous fungus. Using the dapB sequence as a query, we were able to identify 14 DPP IV-encoding genes, and 12 additional DPPIV proteases in public genomic databases. Phylogenetic analysis reveals that in yeasts there are two clades of genes that encode DPP IV proteases with a transmembrane domain. In this study we demonstrate that, as in yeasts, two classes of DPP IV-encoding genes exist in filamentous fungi. However, only one of these codes for DPP IV proteases with a transmembrane domain. The second type present in filamentous fungi encodes extracellular DPP IV proteases. The dapB gene belongs to the first cluster. We propose that DapB plays a role in the proteolytic maturation of enzymes produced by A. niger.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dipeptidyl aminopeptidases are proteases that cleave a dipeptidyl moiety from the N-terminus of a polypeptide (Kreil 1990). They have been classified according to their substrate specificity. Dipeptidyl aminopeptidases of type IV (DPP IV) cleave immediately C-terminal to an Ala or a Pro residue, releasing N-terminal X-Ala or X-Pro dipeptides. Due to their unique structural features, proline residues often protect proteins from degradation, and in some cases even play a role in the regulation of their activation. The DPP IV belongs to a small set of specialised proteases that are capable of cleaving bonds adjacent to prolines (Cunningham and O’Connor 1997).
The DPP IV proteases have been characterised from a variety of organisms, such as yeasts, insects, frogs and humans, and found to perform many different functions. For instance, dipeptidyl aminopeptidase A (DPAP A), which is localised in the Golgi apparatus, is responsible for the processing of α-factor in the yeast Saccharomyces cerevisiae and also plays an important role in the maturation of proteins (Anna-Arriola and Herskowitz 1994). The second DPP IV found in S. cerevisiae, designated DPAP B, is bound to the vacuolar membrane (Roberts et al. 1989), but its in vivo function is unknown. The DPAP B is processed in the endoplasmic reticulum and the Golgi before it reaches the vacuole. Overexpression of DPAP B in yeast mutants that lack DPAP A results in complementation of the defect in α-factor maturation.
In filamentous fungi, only one extracellular DPP IV, designated dipeptidyl-peptidase IV, has been characterised from several Aspergillus species. The A. fumigatus protein is able to bind to collagen and could therefore play a role in the process of lung invasion by this pathogenic fungus (Beauvais et al. 1997), while the extracellular DPP IV present in A. oryzae is thought to be involved in the degradation of wheat gluten during koji fermentation (Doumas et al. 1998). Activity measurements have indicated that this extracellular DPP IV is absent in A. niger, but an intracellular DPP IV activity has been detected in this species.
In this study, we have cloned and characterised a gene, designated dapB, that codes for a novel type IV dipeptidyl aminopeptidase in A. niger. We propose that DapB is involved in protein maturation.
Materials and methods
Strains, plasmids and growth conditions
The A. niger strain NW219 (cspA1, pyrA6, leuA1 and nicA1) was used for transformation essentially as described by Kusters-van Someren et al. (1991). For Southern analysis, Northern analysis and the determination of DPP IV activity in cell-free extracts, the transformants were grown overnight at 37°C in 250-ml Erlenmeyer flasks containing 70 ml of complete medium (Pontecorvo et al. 1953) with 1% glucose (w/v) as carbon source. The flasks were shaken at 250 rpm in an Innova incubator.
Escherichia coli strains LE392 and DH5α (Promega, MI, USA) were used for phage propagation and plasmid transformation, respectively. For cloning purposes the plasmid pUC19 (GibcoBRL, MD, USA) and the phagemid pBluescript (Stratagene, CA, USA) were used. Plasmid pIM4007 was constructed as described below.
Molecular biological techniques
Standard DNA manipulations were carried out essentially as described by Sambrook et al. (1989). The DNA fragments were sequenced using the Thermo Sequenase fluorescence-labelled primer cycle sequencing kit with 7-deaza-dGTP (Amersham Pharmacia Biotech., Chalfont, UK) and the ALF automated sequencer (Amersham Pharmacia Biotech.). For Southern analysis of genomic DNA, total DNA from Aspergillus strains was isolated as described by de Graaff et al. (1988). For Northern analysis RNA was extracted with Trizol reagent. As a loading control, Northern blots were hybridised with A. niger DNA encoding 18S ribosomal RNA. All hybridisations were done in standard hybridisation buffer (Sambrook et al. 1989).
The degenerate forward primer 5′-GTNTAYACIGARMGNTAYATG-3′ and degenerate reverse primer 5′-TGRAARTGNACRTTRTCRTC-3′ were used to obtain a 144-bp fragment from A. niger genomic DNA by PCR. The PCR was performed using an annealing temperature of 45°C. The complete dapB gene was obtained by screening a genomic library of A. niger, constructed in λ EMBL4, with this PCR fragment.
Comparative molecular analysis
For structural analysis of the dapB gene and its predicted protein product, the BLAST search tools (Altschul et al. 1990) were used to search public databases. The programs SOSUI (Hirokawa et al. 1998) and TMHMM (Krogh et al. 2001) were used to identify putative transmembrane regions. Signal sequences for secretion were identified with SignalP (Nielsen et al. 1997). For the phylogenetic analysis the DapB protein sequence was used to query publicly available databases by BLASTP. Protein sequences with e-values of ≤e−100 were retrieved, together with the corresponding nucleic acid sequences. The protein sequences were used separately to query the yeast and fungal genomic databases by TBLASTN. Genomic sequences, which gave BLAST e-values ≤e−100 in any of these searches, and did not contain sequences identical to any of the otherwise known DPP IV sequences, were retrieved, and the putative DPP IVs were annotated using the BLAST homology. Protein sequences were aligned using ClustalX (Thompson et al. 1994). A bootstrap analysis (1000 replicates) was performed on the resulting alignments using the PHYLIP Package Version 3.57c (Felsenstein 1995). ProtDist, part of PHYLIP, was used with the standard Dayhoff PAM 001 matrix. Fitch, also part of PHYLIP, was used to generate the phylogenetic tree. Treeview (Page 1996) was used to view the resulting phylogram.
Overexpression and assay of DPP IV activity
After overnight growth on complete medium (CM; Pontecorvo et al. 1953), cultures of DapB-overproducing transformants were harvested by filtration over nylon gauze. The mycelium was rapidly frozen with liquid nitrogen and stored at −80°C.
To obtain cell-free extracts, harvested mycelium was ground and resuspended in 1 ml of extraction buffer (0.1 M HEPES–TRIS pH 7.0). After 30 s on ice, the extracts were centrifuged for 15 min at 14,000 rpm, and the supernatant was collected. The DPP IV activity was measured in a 500-μl assay mix (including 25 μl of enzyme solution) containing 0.3 mM Ala-Pro-pNA (Bachem) and 0.2 M HEPES–TRIS (pH 7.0). The assay mix was incubated at 37°C and the increase in hydrolysed pNA product formed was followed by measuring the absorbance at 400 nm (A400) in a spectrophotometer.
The nucleotide sequence reported in this paper has been deposited in the EMBL database under the Accession No. AJ278532
Results and discussion
Cloning and analysis of dapB
Two degenerate primers were designed based on the sequences of regions that are conserved between type IV dipeptidyl aminopeptidases from yeast, rat and human (see Materials and methods section). Using these primers a 144-bp fragment was amplified by PCR from genomic DNA of A. niger. The 144-bp fragment was then used as a probe to screen a genomic library of A. niger constructed in bacteriophage λ. Two positive clones were identified and subsequently purified. Southern analysis of the DNAs from these phages with the 144-bp fragment resulted in the identification of a 6-kb EcoRI fragment. Sequence analysis of this fragment indicated the presence of a 2.7-kb ORF with only 500 bp of upstream sequence. In order to extend the fragment in the desired direction, SalI and EcoRI−SstI subfragments of the initially isolated EcoRI insert were used for Southern analysis of the insert from other positive phage, and three adjacent SalI fragments of 1.3, 1.5 and 2.6 kb, respectively, were identified.
These SalI fragments were isolated and sequenced, allowing us to assemble a 3989-bp sequence made up of a 2706-bp ORF starting at bp 817 of the first SalI fragment (Fig. 1a). The ORF is interrupted by two introns located at positions 3134–3192 and 3463–3544, respectively. The presence of both introns was confirmed by comparison of the genomic sequence with EST sequences from A. niger, which were obtained by BLASTN analysis of publicly available EST databases. A BLASTX analysis of the sequence was performed using publicly available protein sequence databases, and similarities were found to several members of the DPP IV protease family. The best hit (69% identity over 775 amino acids) was the hypothetical protein AN2946.2 of A. nidulans . The next best hits were hypothetical proteins from other filamentous fungi. The characterised protein with the highest similarity was the vacuolar DPP IV protease from S. cerevisiae, DPAP B (39% identity over 674 amino acids). Identity scores of 37 and 31% were found for the extracellular DPP IV of A. oryzae and DPAP A, the Golgi-specific DPP IV of S. cerevisiae, respectively. The DPAP A is known to function in the proteolytic maturation of secreted proteins. The extracellular A. oryzae DPP IV is believed to play a role in the degradation of proteins for consumption by the fungus. The in vivo functions of the other DPP IV proteases are not known. Therefore, the results of the BLAST analysis indicate that DapB is a member of the DPP IV protease family, but they do not provide any insights into the functional role of the protease in A. niger.
a, b Schematic views of the dapB genomic region (a) and the domain structure of the DapB protein (b). a The dapB gene is indicated by the grey box; the two black boxes represent the introns. b Structural organisation of the DapB protein, and comparison with four other type IV dipeptidyl peptidases. The three boxes indicate (From left to right) the transmembrane domain, the dipeptidyl peptidase IV N-terminal region (pfam00930.8) and the peptidase S9 domain (pfam00326.8)
The predicted amino acid sequence of DapB was analysed for the presence of possible structural domains (Fig. 1b). RPS-BLAST analysis resulted in recognition of two typical protease domains, the dipeptidyl peptidase IV (DPP IV) N-terminal region (pfam00930.8; http://pfam.wustl.edu/index.html) and the prolyl oligopeptidase domain (pfam00326.8). SOSUI identified a putative transmembrane domain, starting at amino acid 77 and ending at amino acid 99. Therefore, the topology of DapB resembles that of a type II membrane protein, with the N-terminus located in the cytosol, followed by a single membrane-spanning region, with the remaining portion located in the extracellular (lumen) compartment. Further sequence comparisons with the functional domains of other DPP IVs resulted in the identification of a putative catalytic triad, which consists of Ser739, Asp816 and His849. The sequence DWVYEEE, which is highly conserved among the different DPP IVs (Abbott et al. 1999), is also present in the DAPB sequence (residues 305–311). This motif contains two glutamic acid residues that are necessary for proteolytic activity. Therefore, DapB has all the characteristics of a type IV dipeptidyl peptidase, and it has a putative membrane-spanning domain.
Construction of a DapB-overproducing strain
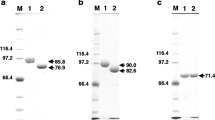
To confirm the DPP IV activity of DapB, an expression plasmid, pIM4007, was constructed, which contains the complete dapB gene, 810 bp of upstream and about 900 bp of downstream sequence (Fig. 2a). A. niger transformants bearing this construct were identified by selection for the restoration of uridine auxotrophy. Subsequently, five selected transformants and a pyrA-complemented control strain were grown overnight, and mycelium was harvested for the preparation of genomic DNA, RNA and cell-free extracts. Southern analysis of the genomic DNA (Fig. 2b) and Northern analysis of isolated RNA (Fig. 3a) were performed to determine if extra dapB copies were present in the genome and, if so, whether they were actively transcribed in the mycelium of the transformants. In addition to Northern and Southern analyses, cell free extracts prepared from the transformants were assayed for DPP IV activity (Fig. 3b). A five to sevenfold increase in DPP IV activity was measured in the cell-free extracts of the DapB transformants compared to the control strain. This increase is clearly significant and shows that increased dapB mRNA levels correlate with higher DPP IV activity. Measurements of DPP IV activity in the culture supernatant of the transformants did not detect any significant activity (data not shown). However, while we were unable to measure significant DPP IV activity in the culture fluid of any of the A. niger strains that we tested, we were able to detect such an activity in the supernatants of A. nidulans cultures, as reported by Doumas et al. (1998). The increased activity found in the cell free extracts of the A. niger transformants, and the lack of DPP IV activity in culture fluid is compatible with the idea that the protein indeed possesses a transmembrane domain.
a, b Construction of the transforming plasmid pIM4007 and analysis of A. niger transformants. a The dapB gene is indicated by the grey box. The following construction steps are indicated: I. Cloning of the upstream dapB fragment (EcoRI-SstII) distal to the SalI-EcoRI dapB promoter fragment, resulting in the 5′ portion of the construct. II. Cloning of the BamHI-SstI terminator region distal to the NcoI-BamHI downstream dapB fragment, resulting in the downstream part of the construct. III. Linkage of the downstream portion of the construct (NcoI-SstI fragment) to the upstream portion (SalI-NcoI), resulting in the dapB overexpression construct. b Southern analysis of dapB multicopy transformants. Genomic DNAs of the transformants (numbers are indicated above the lanes), the negative control [A. niger transformed with pyrA plasmid (C)] and the wild-type (W) strain were digested with XhoI+ HindIII. The molecular weights of hybridising bandsare indicated. The upper arrow indicates tandem insertions of the construct; the lower arrow indicates the endogenous dapB gene. A 1.2 kb EcoRI-PstI fragment of the dapB gene was used as the probe
a, b Analysis of the dapB transformants. a Northern analysis of dapB multicopy transformants. Transformant numbers are indicated above the lanes. The arrow indicates the position of the dapB RNA. A 1.2-kb EcoRI-PstI fragment of the dapB gene was used as the probe. As a loading control, the membrane was rehybridized with DNA encoding the 18S ribosomal RNA (lower panel). b Specific activity of DPP IV in cell-free extracts of the transformants. The numbers below the bars correspond to the transformant numbers; transformant C is A. niger transformed with the pyrA construct. Ala-Pro-pNA was used as substrate
Phylogenetic analysis of DPP IV proteases in ascomycetes
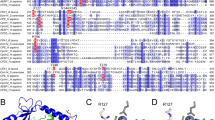
To clarify the phylogenetic relationship between DapB and other DPP IV proteases, we analysed publicly available DNA and protein sequence databases using BLAST. We identified 15 new DPP IV-like genes in the publicly available, but as yet poorly annotated, genomic sequences of A. fumigatus, A. nidulans, Coccidioides posadasii, Podospora anserina, Saccharomyces bayanus, S. castellii, S. kluyveri, S. mikatae and S. paradoxus. In addition, we obtained high similarity scores with 12 hypothetical protein sequences from Candida albicans, C. glabrata, Debaryomyces hansenii, Eremothecium gossypii, Gibberella zeae and kluyveromyces lactis.
Introns were identified in the A. fumigatus, A. nidulans, C. posadasii, G. zeae and P. anserina genes by comparison with either the A. niger dapB DNA sequence or the DNA sequence encoding the extracellular DPP IV of A. fumigatus. The positions of the introns in the A. nidulans and N. crassa genes were confirmed by comparison with EST sequences. We identified one additional intron in the annotated A. nidulans gene for a DPAP A-like protein (BK001296); this results in both a larger ORF and a longer protein sequence. Interestingly, the sequences of the genes for putative secreted DPP IVs in G. zeae and N. crassa lack the intron at the start of the gene which is present in the other genes that encode extracellular DPP IVs. The protein sequences, 28 in total, were deduced from the DNA sequences, or retrieved from protein databases, and subsequently analysed for the presence of putative domains (Fig. 1b) and aligned with the A. niger DapB sequence. The resulting alignment was tested by bootstrap analysis (Fig. 4). Sequences with a low bootstrap value were removed from the analysis, without affecting the structure of the resulting phylogram. The sequences used in the phylogenetic analysis are listed in Table 1. The phylogram comprises four well-defined clusters of protein sequences, each of which contains at least one characterised protein (underlined in Fig. 4). Thus clusters 1 and 2 contain the maturase DPAP A found in the Golgi and the vacuolar DPAP B, respectively, and similar protein sequences from yeasts. All the protein sequences in cluster 3 originate from filamentous fungi. Their domain structures resemble that of DapB, and a putative transmembrane domain was identified at a similar position in each. Cluster 4 is also made up of sequences from filamentous fungi, but its members are similar to the extracellular DPP IV of A. fumigatus. All the protein sequences in this last cluster are predicted by TMHMM analysis to lack a transmembrane domain, while the same method predicts the presence of a transmembrane domain in all the protein sequences found in the DapB cluster (the TMHMM predictions for all the protein sequences are included in the Electronic Supplementary Material). We should mention here that SOSUI predicts the presence of a transmembrane domain in the members of the extracellular protease group of this cluster. However, the predicted domain is located in the first 25 amino acid residues and, according to SignalP, it resembles a signal sequence which is removed from the protein before secretion. In contrast to SOSUI, TMHMM masks possible signal sequences before analysis. These results therefore indicate that most of the filamentous fungi tested have one extracellular DPP IV protease and one intracellular DPP IV, containing a transmembrane domain.
Phylogenetic analysis of yeast and fungal type IV dipeptidyl peptidases. The tree comprises the following DPP IV subgroups: yeast DPAP A-like (cluster 1), yeast DPAP B-like (cluster 2), DapB-like from the filamentous fungi (cluster 3), and the extracellular DPP IV of filamentous fungi (cluster 4). The human DPP IV was taken as outgroup and does not fit in any of the four categories. The bootstrap values are indicated at each node. The branch lengths indicate evolutionary distances
We established that in both A. niger (results not shown) and N. crassa only one DPP IV gene is present in the genome. In both cases this gene encodes the DapB-like intracellular DPP IV protease. In N. crassa, as in several other species of Aspergilli, an α-factor homolog has been identified (Bobrowicz et al. 2002). It contains the same DPP IV cleavable dipeptide stretches as its yeast counterparts. Furthermore, it has been shown that inactivation of this pheromone leads to a defect in mating-type phenotype in N. crassa. If processing of α-factor in N. crassa is as essential as it is in yeast, the N. crassa DPP IV protease is likely to be responsible for this step. We therefore propose that the DPP IV proteases in the DapB cluster (cluster 3) are involved in protein maturation.
We can conclude that the A. niger DPP IV protease identified and analysed in this study is an intracellular DPP IV protease. Furthermore, by analysing partially sequenced genomes, we have identified two distinct DPP IV-like protease clades in yeasts and two distinct DPP IV-like protease clusters in filamentous fungi. Of the DPP IV-like proteases in filamentous fungi, only the DapB-like protein sequences contain a putative transmembrane domain. A possible DPP IV-like maturase would need a membrane-spanning domain to ensure its retention in the secretory pathway, where it can perform its maturation function. We therefore propose that DapB of A. niger plays a role in the proteolytic maturation of enzymes produced by this fungus.
References
Abbott CA, McCaughan GW, Gorrell MD (1999) Two highly conserved glutamic acid residues in the predicted β propeller domain of dipeptidyl peptidase IV are required for its enzyme activity. FEBS Lett 458:278–284
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Anna-Arriola SS, Herskowitz I (1994) Isolation and DNA sequence of the STE13 gene encoding dipeptidyl aminopeptidase. Yeast 10:801–810
Beauvais A, Monod M, Wyniger J, Debeaupuis JP, Grouzmann E, Brakch N, Svab J, Hovanessian AG, Latgé JP (1997) Dipeptidyl-peptidase IV secreted by Aspergillus fumigatus, a fungus pathogenic to humans. Infect Immun 65:3042–3047
Bobrowicz P, Pawlak R, Correa A, Bell-Pedersen D, Ebbole DJ (2002) The Neurospora crassa pheromone precursor genes are regulated by the mating type locus and the circadian clock. Mol Microbiol 45:795–804
Cunningham DF, O’Connor B (1997) Proline specific peptidases. Biochim Biophys Acta 1343:160–186
De Graaff L, van den Broek H, Visser J (1988) Isolation and expression of the Aspergillus nidulans pyruvate kinase gene. Curr Genet 13:315–321
Doumas A, van den Broek P, Affolter M, Monod M (1998) Characterization of the prolyl dipeptidyl peptidase gene (dpplV) from the koji mold Aspergillus oryzae. Appl Environ Microbiol 64:4809–4815
Felsenstein J (1995) PHYLIP: Phylogeny Inference Package (Version 3.57c). University of Washington, Seattle, Wash
Hirokawa T, Boon-Chieng S, Mitaku S (1998) SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics 14:378–379
Kreil G (1990) Processing of precursors by dipeptidylaminopeptidases: a case of molecular ticketing. Trends Biochem Sci 15:23–26
Krogh A, Larsson B, Von Heijne G, Sonnhammer ELL (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305:567–580
Kusters-van Someren MA, Harmsen JAM, Kester HCM, Visser J (1991) Structure of the Aspergillus niger pelA gene and its expression in Aspergillus niger and Aspergillus nidulans. Curr Genet 20:293–299
Nielsen H, Engelbrecht J, Brunak S, von Heijne G (1997) Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng 10:1–6
Page RD (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Pontecorvo G, Roper JA, Hemmons LJ, MacDonald KD, Bufton AWJ (1953) The genetics of Aspergillus nidulans. Adv Genet 5:141–238
Roberts CJ, Pohlig G, Rothman JH, Stevens TH (1989) Structure, biosynthesis, and localisation of dipeptidyl aminopeptidase B, an integral membrane glycoprotein of the yeast vacuole. J Cell Biol 108:1363–1373
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Acknowledgements
This work was supported by Grant No. WBI 4100 from the Dutch Technology Foundation (STW).
Author information
Authors and Affiliations
Corresponding author
Additional information
P. Punt
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Jalving, R., Godefrooij, J., Veen, W.J.t. et al. Characterisation of the Aspergillus niger dapB gene, which encodes a novel fungal type IV dipeptidyl aminopeptidase. Mol Genet Genomics 273, 319–325 (2005). https://doi.org/10.1007/s00438-005-1134-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-005-1134-9