Abstract
We searched for genetic linkage between DNA markers and quantitative trait loci (QTLs) for innate immunity, response to stress, biochemical parameters of blood, and fish size in an F2 population derived from an interspecific tilapia hybrid ( Oreochromis mossambicus × O. aureus). A family of 114 fish was scanned for 40 polymorphic microsatellite DNA markers and two polymorphic genes, covering ~80% of the tilapia genome. These fish had previously been phenotyped for seven immune-response traits and six blood parameters. Critical values for significance were P <0.05 with the false discovery rate (FDR) controlled at 40%. The genome-scan analysis resulted in 35 significant marker-trait associations, involving 26 markers in 16 linkage groups. In a second experiment, nine markers were re-sampled in a second family of 79 fish of the same species hybrid. Seven markers ( GM180, GM553 , MHC-I , UNH848 , UNH868 , UNH898 and UNH925) in five linkage groups (LG 1, 3, 4, 22 and 23) were associated with stress response traits. An additional six markers ( GM47, GM552 , UNH208 , UNH881 , UNH952 , UNH998) in five linkage groups (LG 4, 16, 19, 20 and 23) were verified for their associations with immune response traits, by linkage to several different traits. The portion of variance explained by each QTL was 11% on average, with a maximum of 29%. The average additive effect of QTLs was 0.2 standard deviation units of stress response traits and fish size, with a maximum of 0.33. In three linkage groups (LG 1, 3 and 23) markers were associated with stress response, body weight and sex determination, confirming the location of QTLs reported by several other studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tilapias (Cichlidae: Oreochromis sp.) are among the most important food fishes cultured in tropical and subtropical countries (Beveridge and McAndrew 2000). Aquaculture production systems and stock management methods expose fish to environmental factors that differ from those they encounter in their natural habitats. Changes in the environment result in disturbances of homeostasis in the fish. The maintenance of internal homeostatic equilibrium is essential for the normal function of the animal and, in case of disturbance, the fish will try to establish a new equilibrium. These behavioral and physiological reactions are commonly referred to as a stress response. Cultured fish, especially under conditions of intensive culture, are under prolonged stress, and their physiological responses under these circumstances affect energy dependent processes like growth, maturation and disease resistance (Pickering 1993). The strong link between stress and susceptibility to disease has long been acknowledged, and parameters of the innate immune response, such as respiratory burst activity, spontaneous hemolytic activity, lysozyme activity, complement concentration and total IgM, have been found to be associated with disease resistance in fish (Ellis 2001). Indeed, stress response traits, such as lysozyme activity and cortisol levels, have been used as selection criteria for disease resistance and immune system parameters in rainbow trout (Roed et al. 2002).
Interspecific crossing has been used in several fish species to increase genetic and phenotypic variation for both commercial and research purposes (reviewed by Bartley et al. 2000). Strain and species differences in disease resistance and stress response have previously been demonstrated in several fish species. In tilapias, differences were found between strains and species for several stress responses and non-specific immunity parameters (Balfry et al. 1997; Palti et al. 1999b; Cnaani et al. 2000, 2004). Strain differences in disease resistance were found to be associated with the non-specific immune system (Balfry et al. 1997).
Loci affecting traits of economic value have been detected and mapped in a variety of livestock species (reviewed by Andersson 2001). Genome scans using anonymous molecular markers have proved to be effective for the detection of quantitative trait loci (QTLs) in organisms with dense linkage maps (e.g. dairy cattle, Heyen et al. 1999; chicken, Van Kaam et al. 1999; pig, Nezer et al. 2002). Genome scans are often performed by crossing genetically diverse strains, and then following the co-inheritance of phenotypic traits and anonymous markers (Mackay 2001). Candidate gene markers can be used similarly to anonymous markers, but they are more precisely targeted and can detect variation in, or close to, genes of known function that relates directly or indirectly to the trait of interest. Markers are usually chosen in genes known to regulate physiological networks controlling a particular quantitative trait (Tabor et al. 2002).
Several linkage maps based on DNA markers have been constructed for tilapia in recent years (Kocher et al. 1998; Agresti et al. 2000; McConnell et al. 2000). The recent development of hundreds of microsatellite DNA markers (Carleton et al. 2002) enables coverage of the tilapia genome at 4-cM intervals on average (B.-Y. Lee, W. J. Lee, J. T. Streelman, G. Hulata, K. L. Carleton, A. Howe, A. Slettan, T. D. Kocher, manuscript in preparation), thus providing the infrastructure for systematic genome scans for detection of QTLs. The ability of tilapiine fishes to yield viable interspecific hybrids makes them ideal organisms for genetic studies, using backcrosses or F2 intercrosses as a segregating population, as suggested by Poompuang and Hallerman (1997) and demonstrated by Cnaani et al. (2003a). In this study, we used an F2 hybrid population, derived from two species of tilapia, Oreochromis aureus and O. mossambicus, which differ in their innate immune response to stress and in several biochemical blood components. O. aureus seems to be more tolerant to air exposure stress, at least as reflected in the parameters measured so far (Palti et al. 1999b; Cnaani et al. 2004). These two species are commonly used in aquaculture and differ in a variety of traits. O. aureus is usually bigger, more cold tolerant, has a lighter body color and reaches sexual maturity later, while O. mossambicus shows high salinity tolerance, is easier to reproduce and has mutations for red body color. O. aureus and its hybrids are cultured mostly in the Middle East, China, Taiwan and the USA, while O. mossambicus is cultured mostly in Southern Africa and South-East Asia (Wohlfarth et al. 1983; Beveridge and McAndrew 2000).
Most QTL detection studies have used either maximum likelihood or regression methods to test for segregating QTLs. Generally, the statistical analysis is computed for each marker on the basis of the null hypothesis that there is no linkage between a segregating locus affecting the trait of interest and the genetic marker. The null hypothesis is rejected when the probability of the test statistic is below a predetermined level, usually 5% or 1%. However, if many markers and traits are tested, several null hypotheses will meet the rejection criteria by chance (Lander and Kruglyak 1995; Weller et al. 1998). Thus, a major problem with systematic genome scans is determining the appropriate type I error rate to declare significance. Various solutions have been proposed to overcome this problem. Weller et al. (1998) proposed applying the false discovery rate (FDR) of Benjamini and Hochberg (1995), and several recent studies have used this approach (Heyen et al. 1999; Mosig et al. 2001). Lander and Kruglyak (1995) suggested that marker effects that display statistical significance in two independent populations should be considered as confirmation of a QTL.
In this study we used 42 DNA markers, covering ~80% of the tilapia genome, for QTL detection. A genome scan was performed in one F2 family, and markers that were highly associated with loci affecting quantitative traits were re-analyzed in a second F2 family for validation. This study is a further step towards the goal of detecting and cloning genes that influence economically valuable traits in tilapia.
Materials and methods
Experimental design
This was a two-step study. First, a genome scan was conducted in an F2 family that had previously been characterized for segregation for stress response traits, in order to identify putative QTLs. The second step involved confirmation of those QTLs by re-analysis of the same markers in another interspecific F2 family.
Genetic materials and phenotypic characterization
The fish used for this study were full-sib F2 families of 114 and 79 offspring, obtained by mating of F1 hybrids derived from a cross between an O. mossambicus female and an O. aureus male. Each fish was measured for innate immunity response to stress, as reflected in lysozyme activity (Lys), respiratory burst activity (NBT), ceruloplasmin activity (Cerul), glucose concentration (Gluc), IgM levels (IgM), hematocrit (Hct) and leukocrit (Lct), and for six biochemical blood parameters: total protein (Prot), albumin (Alb), globulin (Glob), triglyceride (Trig), cholesterol (Chol) and calcium (Ca), which are known to be correlated with fish health (Maita et al. 1998) and differed significantly between the two species (Cnaani et al. 2004). Blood samples were taken from each fish to measure pre-stress values. One month later, air exposure stress was induced by removal of fish from water for 5 min, and blood samples were taken from the caudal vein 2–3 h after stress. Sampling protocols and methods of immunological and biochemical assays have been described by Palti et al. (1999b) and Cnaani et al. (2004). The innate immune parameters were analyzed as “post-stress”, which is the value that was measured after inducing stress, and as “stress-increment”, which is the post-stress value minus the pre-stress value for each fish (Table 1). Both parameters serve as indicators for stress response, as one shows the actual levels of the immune parameters in the fish and the other shows the changes that occurred in the fish following exposure to the stressor.
Genome scan using DNA markers
DNA was extracted from fin tissue by the salting-out procedure (Miller et al. 1988). Seventy DNA markers that were scattered along an early version of the tilapia genome map in 40-cM intervals were tested for the genome scan. Forty microsatellite DNA markers and two genes that were polymorphic in the experimental population were used for genotyping. These markers covered ~80% of the tilapia genome (Lee et al., in preparation; http://hcgs.unh.edu/comp/).
PCR was performed in a 10-μl volume containing 1×PCR buffer containing 2 mM MgCl2, 1 U Taq DNA polymerase (JMR, London, UK), each dNTP (Fermentas, Hanover, MD, USA) at 125 μM, each primer at 5 μM and 50 ng of genomic DNA as template. The amplification conditions were: 92°C for 40 s, 50–62°C for 40 s and 72°C for 1 min, for a total of 30 cycles. After calibrating PCR conditions, up to four different primer pairs were combined for multiplex reactions. The DNA markers and their annealing temperatures are listed in Table 3. Nine markers ( GM131, GM180 , GM553 , MHC-1 , UNH208 , UNH848 , UNH868 , UNH898 and UNH925) highly associated with stress response, were genotyped in a second family.
PCR products from up to five different reactions were combined according to differences in either size or fluorescent label. A 1-μl aliquot of the mixture was added to 1.5 μl of formamide-loading buffer and 0.5 μl of MapMarker 400 TMR standard ladder (Bio Ventures, Murfreesboro, TN, USA). After denaturation at 92°C for 2 min, 1 μl of the solution was loaded onto an acrylamide gel (4%) in an ABI-377 DNA sequencer (Applied Biosystems). The DNA fragments were separated by electrophoresis and automatically sized by comparison with the internal standard using Genescan software (version 3.1). Genotypes of individual samples were determined using Genotyper software (version 2.0) and automatically exported to a database. For the data analyses genotypes were determined according to the grandparental origin of the alleles. Thus, for each marker there were three classes of genotypes in the F2 population: AA when the two alleles originated from O. aureus (grand paternal homozygous), MM when the two alleles originated from O. mossambicus (grand maternal homozygous), and AM when the two alleles were inherited from different species (heterozygous).
Statistical analysis
Tests for associations between DNA markers and loci affecting phenotypic traits were conducted separately for each marker-trait combination using one-way analysis of variance (ANOVA), with the three F2 genotypes as classes. The R2 values of the tests are an indication of the amount of within-family variation explained by each effect. The Shapiro-Wilk W test was used to test for normal distribution of the phenotypic traits (Table 1). Associations of markers with traits that were not normally distributed were analyzed using the Kruskal-Wallis non-parametric test. Additive and dominant effects were calculated as the deviations of the homozygous (additive effect) and of the heterozygous (dominant effect) from the midpoint of the two homozygous, divided by the remainder root mean square provided by ANOVA. Thus the effects are given in units of standard deviation. The Pearson χ2 test was conducted to test for linkage between sex and the three genotypes in the F2 family and to test for departures from the expected Mendelian segregation. Critical values for significance were determined by the FDR method (Benjamini and Hochberg 1995) as proposed by Weller et al. (1998) for QTL detection. The objective in controlling the FDR is to try to minimize the FDR, while at the same time maximizing the expected number of true effects. The appropriate value for controlling the FDR was therefore determined by inspecting the number of rejected null hypotheses, and the highest comparison-wise probability of the rejected hypotheses with the FDR controlled over the range from zero to 0.5. The FDR was computed jointly for all seven stress-related traits, analyzed for 42 markers. Correlations between different traits were analyzed using least-squares regression.
Results
Detection of QTLs for stress responses
Forty-eight out of the seventy DNA markers yielded adequate amplification and were thus included in the calibration phase of this study. Six of the markers ( GM284, GM633 , UNH738 , UNH843 , UNH849 and UNH961) were homozygous in the grandparental species, and were therefore non-informative and excluded from further analysis. The other 42 informative markers were used for the genome scan. With five markers ( GM263, UNH130 , UNH132 , UNH431 and UNH907) significant ( P <0.01) departures from the expected Mendelian segregation were observed in the genotypes of the F2 hybrid family.
The phenotypic distribution of stress response traits in the first F2 family is presented in Table 1. Half of the traits showed a skewed distribution. Correlations between the different traits in the first F2 family are presented in Table 2. The seven immune response traits measured in this study were not correlated with fish size or sex or with each other, while high correlations were observed between post-stress and stress-increment values of the same trait. Correlations between post-stress and stress increment values were high, ranging from 0.58 (NBT) to 0.92 (Lys), and therefore they were not considered as independent trials in the FDR analysis.
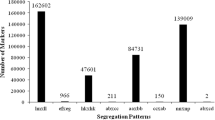
FDR was calculated for seven non-related traits analyzed for 42 markers. The FDR values (up to 0.45) computed with all traits analyzed jointly are plotted in Fig. 1, as a function of the comparison-wise probabilities. There were 35 marker-trait combinations for health condition indicators in tilapia with FDR controlled at 0.41. Thus, it is expected that approximately 20 of these are true effects.
Twenty-six markers on 16 linkage groups were associated with loci affecting health condition indicators in tilapia. Twenty of these markers, on 12 linkage groups, were found to be associated with post-stress immune parameters, while nine of them were associated with more than one trait (Table 3). Eleven markers on seven linkage groups were associated with stress-increment immune parameters (Table 3). Seven markers were associated with both post-stress and stress-increment immune parameters. Nine markers, on six linkage groups, were associated with fish size (see below). UNH848 on LG23 was highly significantly ( P <0.0001) associated with both weight and length, and explained 19% of the variation for each trait. Five markers were associated with the sex of the fish. The most significant markers, UNH868 and UNH925, explained 34% of the variation (see below).
Confirmation of marker-trait associations in a second F2 family
In order to validate some of these putative QTLs, nine markers that were associated with several traits, or highly associated with a single trait ( P <0.01), were genotyped in another F2 family. Seven of the markers were found to be associated with stress response traits also in the second family (Table 4). Overall, 14 markers on nine linkage groups were verified for their associations with stress response traits (post-stress or stress-increment values), either by re-sampling in a second family or by association with several different traits. Table 4 summarizes the extent of each effect as a percentage of the within-family variance it explained, and the additive and dominant effects in each marker-trait association. These 14 markers explained 6–29% of variance, with the majority of markers having intermediate values of 9–13%. None of these markers showed segregation distortion in the F2 generation.
The grandparental origin of the increasing allele (GOIA) is presented in Table 4. A minus sign indicates that the difference between parental marker genotypes was opposite in sign to the overall difference between the parental strains. MHC class I, IgM ( UNH881) and markers on LG23 ( GM47, UNH848 and UNH898) had a consistent but opposite effect from that expected in 14 out of 15 marker-trait associations. For six other markers ( GM131, GM180 , GM553 , UNH208 , UNH868 and UNH925) the expected origin of the increasing allele was observed in 16 out of 21 marker-trait associations. GM131, associated with two traits, was inconsistent for GOIA, and GM180 was consistent for three traits in the first family but not for six traits in the second family (Table 4). GM552 and UNH998 were associated with four biochemical parameters of plasma, with the expected inheritance of the increasing allele from O. aureus. UNH868 was associated with two plasma parameters, for which the increasing allele was inherited from O. mossambicus (Table 4).
Discussion
Few QTLs have been detected in domesticated lines of fish (Clark 2003). Some of the QTLs are for disease resistance (Palti et al. 1999a; Ozaki et al. 2001; Grimholt et al. 2003) and tolerance of thermal stress (Jackson et al. 1998; Perry et al. 2001; Cnaani et al. 2003a). In all of these studies the measured parameter was fish survival, which is the tertiary response to stress (Barton 2002). The objective of this study was to detect QTLs affecting metabolic and immune responses to stress. These are secondary responses to stress, which control disease resistance and thermal tolerance (Barton 2002).
Our understanding of the biology of the stress response is still limited. Studies in rainbow trout have shown that fish strains selected for high cortisol and lysozyme levels in response to stress may be more resistant to certain pathogens but more susceptible to others (Roed et al. 2002). Thus, there is genetic variation for stress response but it is difficult to determine a superior strain for aquacultural purposes. These differences may reflect the different challenges to which fish have been exposed in their native habitats. The two tilapia species used in this study have been spread from their areas of origin, in the Middle East and Southern Africa, throughout the world, and are facing new stressors in aquaculture systems. In this study, both the actual value of stress response (post-stress) and the physiological changes from baseline levels (stress-increment) were used to represent immune parameters. The high but incomplete correlations between the two measures may indicate that they represent different aspects of stress response and consequently contribute to disease resistance.
The tilapia linkage map is still in a developmental stage. Over the last 5 years some markers have been removed or assigned to different locations within or between linkage groups, and the total length of the map has been reduced (Kocher et al. 1998; Lee et al., in preparation). The microsatellites used in this study provided ~80% coverage of the ~1200-cM tilapia genome, with a maximum spacing of 50 cM between markers. There are some gaps in this genome scan, as markers were chosen on the basis of an early version of the map and some markers were not polymorphic in the tested family. Three linkage groups (LG 10, 19 and 23) were densely covered, with markers every 10–20 cM, while in other linkage groups there were markers only every 20–40 cM, and coverage of some linkage groups was incomplete. Genetic markers in four linkage groups (LG 5, 12, 14 and 24) were not analyzed at all (Table 3).
Genome-scan studies, in which dozens of markers are analyzed for multiple traits, are prone to false discovery (Lander and Kruglyak 1995; Weller et al. 1998). In all, 294 marker-trait combinations were analyzed in this study, with the expectation that approximately 15 comparisons would achieve statistical significance at P <0.05 purely by chance. The results of this study demonstrate 35 significant marker-trait associations, involving 26 markers in 16 linkage groups, with QTLs influencing health condition indicators in tilapia. By controlling the FDR at 0.41 to determine significance, it is clear that several of the significant effects are in fact false positives. Further evidence to support the results presented can be obtained by analysis on independent families and comparison to other QTL analyses in tilapia.
Seven out of the nine markers that were re-analyzed in a second F2 family were verified for association with immune traits ( GM180, GM553 , MHC-1 , UNH848 , UNH868 , UNH898 and UNH925). The exact marker-trait associations were repeated in only two markers, GM180 and MHC-I, that were associated with stress-increment of glucose levels and lysozyme activity, respectively (Table 4). However, the repeated associations of the same markers with parameters of the physiological response to stress may imply association with factors modifying the stress response. A possible explanation for the association of the same markers with different traits in the two tested populations could be that these two families were phenotyped at different times and life stages of the fish; thus, a stress response could be observed in different traits. Seven other markers ( GM47, GM131 , GM552 , UNH208 , UNH881 , UNH952 , UNH998) were associated with more than one trait, reducing to some extent the possibility of association resulting from type I statistical error.
Jackson et al. (1998) searched among 24 polymorphic loci for linkage with temperature tolerance in trout, and detected two QTLs. In our study, 1–4 QTLs were detected for each of the stress-response traits. The extents of variance explained by the stress response QTLs were similar to those observed for QTLs for temperature tolerance in trout, ranging from 9% to 13% (Jackson et al. 1998). In a trout genome-scan, O’Malley et al. (2003) detected QTLs for body weight in seven different linkage groups, while in this study QTLs for body weight were detected in six linkage groups. The proportion of variance explained by QTLs for body weight varied from 7% to 19%, which is somewhat lower than the 11–25% that was found in trout (O’Malley et al. 2003). Additive effects varied in their magnitude, between 0.05 and 0.33 standard deviations, but most of them were close to 0.2 standard deviations. Dominant effects showed greater variation in their magnitude, between 0.01 and 0.67 standard deviation units of the traits. Most of the additive and dominant effects are relatively small, and the small F2 family sizes used here did not provide sufficient statistical power to detect the same effects in repeated experiments, as reflected by the high FDR of 40%.
Significant differences in the response to stress have been observed between O. mossambicus and O. aureus. The latter strain had higher levels of hematocrit, lysozyme, ceruloplasmin, respiratory burst activity and IgM, and lower levels of glucose and leukocrit (Palti et al. 1999b; Cnaani et al. 2004). Thus these strains were used to construct an F2 segregating population in which to search for QTLs for immune response traits. The grandparental origin of the increasing allele (GOIA) was tested for each significant QTL (Table 4). Consistent patterns of inheritance of the GOIA for several traits and in related families may support the detection of true QTLs. Many QTLs showed highly significant effects of opposite sign to the overall difference between the parental strains. It appears that, in spite of the overall difference between the two strains, they both harbor favorable alleles in different genes for the traits analyzed. This has been denoted as “cryptic” genetic variation that can be uncovered by marker-linkage analysis (Weller et al. 1988).
Two out of the 42 markers utilized in this study were polymorphic genes, for MHC class I ( MHC-I) and transferrin ( TF), which are related to the immune system, and were previously mapped in the tilapia genome (Cnaani et al. 2002b, 2003b). In addition, two of the microsatellite markers, UNH881 and UNH208, were previously found to be part of genes coding for the IgM light chain and attractin, respectively (Cnaani et al. 2002a). The MHC genes are likely candidates for identifying genetic variation associated with immune response in all vertebrate species (Van der Zijpp and Egberts 1989). Thus, associations between the MHC and infectious diseases have been reported for non-mammalian species, notably the chicken (Zhou and Lamont2003). Association between polymorphism in the MHC genes and disease resistance was found in trout (Palti et al. 2001) and salmon (Langefors et al. 2001; Lohm et al. 2002; Grimholt et al. 2003), but, to the best of our knowledge, association studies between polymorphism in the MHC and stress response have not been published previously. The polymorphism in the variable region of the IgM light chain ( UNH881) was found to be associated with post-stress levels of hematocrit and with stress-increment levels of lysozyme. The polymorphism in the attractin gene ( UNH208) was found to be associated with post-stress levels of ceruloplasmin and stress-increment levels of both ceruloplasmin and respiratory burst activity (measured as NBT).
Although sex is a binary trait with only two possible phenotypes, the determination of sex in tilapia may be considered as a quantitative trait, which is controlled by several genes on different chromosomes and is influenced also by environmental factors (Devlin and Nagahama 2002; Shirak et al. 2002; Lee et al., 2004). In three linkage groups (LG 1, 3 and 23), markers were associated with loci affecting several traits: sex determination, fish size, response to stress and biochemical blood components (Table 5). The discovery of three regions in the genome that are associated with a variety of traits implies the existence of genes which control major physiological or developmental pathways. DNA markers in these linkage groups were previously associated with loci affecting sex determination (Shirak et al. 2002; Cnaani et al. 2003a; Lee et al. 2003, 2004), deleterious alleles (Palti et al. 2002), cold tolerance and fish size (Cnaani et al. 2003a; Moen et al. 2004). The marker GM553 on LG4 that was associated with post-stress levels of glucose and ceruloplasmin was also associated with cold tolerance in another study on tilapia hybrids (Moen et al. 2004).
The associations of markers located in LG23 with response to stress, as reflected in levels of glucose and IgM, and with body weight, provide evidence for a major role of one or more genes on this linkage group in fish fitness. Cnaani et al. (2003a) detected two QTLs, for cold tolerance and body weight, in this linkage group, and Moen et al. (2004) verified these QTLs in a different tilapia hybrid population. Palti et al. (2002) and Shirak et al. (2002) found an association between loci with deleterious alleles and distorted sex ratio in this linkage group in a gynogenetic line of O. aureus. DNA markers on LG23 associated with fish size in the current study had similar effects to those previously detected by Cnaani et al. (2003a), with a decreasing over-dominant effect and a small additive effect in the QTL (Table 5). Notably, decreasing dominant effects were found also for the stress related traits associated with markers in LG23 (Table 4). The markers UNH130 and UNH907, which are located on this linkage group, were significantly distorted from the expected Mendelian segregation, and showed the same effect in another F2 hybrid family (Cnaani et al. 2003a). The QTLs found in previous studies were located at positions 25, 29 and 39 cM along this 51-cM linkage group. The results of the current study imply the presence of QTLs at positions 1, 16 and 23 cM. Therefore, it seems that at least three QTL regions were detected in this linkage group.
Searching for QTLs for several traits with many markers, as in genome scans, increases the chance of finding false positive associations. In order to control false discovery of QTLs, a validation experiment was performed by genotyping a second F2 family only for markers that were highly significant for immune response in the genome scan. In addition, the grandparental source of the QTL alleles was compared between traits for a given marker, and between the two F2 families for the same marker and trait. Thus, the QTLs in LG 1, 3, 4 and 23 for immune response were confirmed by supporting evidence. Putative QTLs for blood components on LG 16 and 20, and association of the MHC class I and IgM genes with stress related traits, were also demonstrated.
Several studies have characterized the phenotypic variance and inheritance of production traits such as growth, reproduction and cold tolerance (e.g. Wohlfarth et al. 1983; Cnaani et al. 2000). The QTL mapping in this study is only the first step towards the detection of genes that influence traits of economic importance in tilapia. Additional studies in fine mapping of the QTLs and subsequent positional cloning are required in order to turn molecular knowledge into selective breeding plans for disease resistance and sex control in populations of tilapia.
References
Agresti JJ, Seki S, Cnaani A, Poompuang S, Hallerman EM, Umiel N, Hulata G, Gall GAE, May B (2000) Breeding new strains of tilapia: development of an artificial center of origin and linkage map based on AFLP and microsatellite loci. Aquaculture 185:43–56
Andersson L (2001) Genetic dissection of phenotypic diversity in farm animals. Nat Rev Genet 2:130–138
Balfry SK, Shariff M, Iwama GK (1997) Strain differences in non-specific immunity of tilapia ( Oreochromis niloticus) following challenge with Vibrio parahemolyticus. Dis Aquat Org 30:77–80
Bartley DM, Rana K, Immink AJ (2000) The use of inter-specific hybrids in aquaculture and fisheries. Rev Fish Biol Fisher 10:325–337
Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integrative Comp Biol 42:517–525
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B Methods 57:289–300
Beveridge MCM, McAndrew BJ (eds) (2000) Tilapias: biology and exploitation. Kluwer Academic Publishers, Dordrecht
Carleton KL, Streelman JT, Lee B-Y, Garnhart N, Kidd M, Kocher TD (2002) Rapid isolation of CA microsatellites from the tilapia genome. Anim Genet 33:140–144
Clark MS (2003) Genomics and mapping of Teleostei (bony fish). Comp Funct Genom 4:182–193
Cnaani A, Gall GAE, Hulata G (2000) Cold tolerance of tilapia species and hybrids. Aquacul Int 8:289–298
Cnaani A, Ron M, Hulata G, Seroussi E (2002a) Fishing in silico: searching for tilapia genes using sequences of microsatellite DNA markers. Anim Genet 33:474–476
Cnaani A, Ron M, Lee B-Y, Hulata G, Kocher TD, Seroussi E (2002b) Mapping the transferrin gene in tilapia. Anim Genet 33:78–80
Cnaani A, Hallerman EM, Ron M, Weller JI, Indelman M, Kashi Y, Gall GAE, Hulata G (2003a) Detection of a chromosomal region with two quantitative trait loci, affecting cold tolerance and fish size, in an F2 tilapia hybrid. Aquaculture 223:117–128
Cnaani A, Lee B-Y, Ron M, Hulata G, Kocher TD, Seroussi E (2003b) Linkage mapping of major histocompatibilty complex class I loci in tilapia ( Oreochromis spp.). Anim Genet 34:390–391
Cnaani A, Tinman S, Avidar Y, Ron M, Hulata G (2004) Comparative study of biochemical parameters in response to stress in Oreochromis aureus, O. mossambicus and two strains of O. niloticus. Aquac Res, in press
Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208:191–365
Ellis AE (2001) Innate host defense mechanisms of fish against viruses and bacteria. Dev Comp Immunol 25:827–839
Grimholt U, Larsen S,·Nordmo R, Midtlyng P, Kjoeglum S, Storset A, Saebø S, Stet RJM (2003) MHC polymorphism and disease resistance in Atlantic salmon ( Salmo salar): facing pathogens with single expressed major histocompatibility class I and class II loci. Immunogenetics 55:210–219
Heyen DW, Weller JI, Ron M, Band M, Beever JE, Feldmesser E, Da Y, Wiggans GR, Van Raden PM, Lewin HA (1999) A genome scan for QTL influencing milk production and health traits in dairy cattle. Physiol Genomics 1:165–175
Jackson TR, Ferguson MM, Danzmann RG, Fishback AG, Ihssen PE, O’Connell M, Crease TJ (1998) Identification of two QTL influencing upper temperature tolerance in three rainbow trout ( Oncorhynchus mykiss) half-sib families. Heredity 80:143–151
Kocher TD, Lee WJ, Sobolewska H, Penman D, McAndrew B (1998) A genetic linkage map of a cichlid fish, the tilapia ( Oreochromis niloticus). Genetics 148:1225–1232
Lander E, Kruglyak L (1995) Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 11:241–247
Langefors A, Lohm J, Grahn M, Andersen O, von Schantz T (2001) Association between major histocompatibility complex class IIB alleles and resistance to Aeromonas salmonicida in atlantic salmon. P Roy Soc Lond B Biol 268:479–485
Lee B-Y, Penman DJ, Kocher TD (2003) Identification of the sex-determining region in tilapia ( Oreochromis niloticus) using bulked segregant analysis. Anim Genet 34:379–383
Lee B-Y, Hulata G, Kocher TD (2004) Two unlinked loci controlling the sex of blue tilapia ( Oreochronis aureus). Heredity 92:543–549
Lohm J, Grahn M, Langefors A, Andersen O, Storset A, von Schantz T (2002) Experimental evidence for major histocompatibility complex-allele-specific resistance to a bacterial infection. P Roy Soc Lond B Biol 269:2029–2033
Mackay TFC (2001) The genetic architecture of quantitative traits. Annu Rev Genet 35:303–339
Maita M, Satoh K, Fukuda Y, Lee HK, Winton JR, Okamoto N (1998) Correlation between plasma component levels of cultured fish and resistance to bacterial infection. Fish Pathol 33:129–133
McConnell SKJ, Beynon C, Leamon J, Skibinski DOF (2000) Microsatellite marker based genetic linkage maps of Oreochromis aureus and O. niloticus (Cichlidae): extensive linkage group segment homologies revealed. Anim Genet 31:214–218
Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215
Moen T, Agrest JJ, Cnaani A, Moses H, Famula TR, Hulata G, Gall GAE, May B (2004) A genome scan of a four-way tilapia cross supports the existence of a quantitative trait locus for cold tolerance on linkage group 23. Aquac Res, 35:893–904
Mosig MO, Lipkin E, Khutoreskaya G, Tchourzyna E, Soller M, Friedmann A (2001) A whole genome scan for quantitative trait loci affecting milk protein percentage in Israeli-Holstein cattle, by means of selective milk DNA pooling in a daughter design, using an adjusted false discovery rate criterion. Genetics 157:1683–1698
Nezer C, Moreau L, Wagenaar D, Georges M (2002) Results of a whole genome scan targeting QTL for growth and carcass traits in a Pietrain × Large White intercross. Genet Sel Evol 34:371–387
O’Malley KG, Sakamoto T, Danzmann RG, Ferguson MM (2003) Quantitative trait loci for spawning date and body weight in rainbow trout: testing for conserved effects across ancestrally duplicated chromosomes. J Hered 94:273–284
Ozaki A, Sakamoto T, Khoo S, Nakamura K, Coimbra MRM, Akutsu T, Okamoto N (2001) Quantitative trait loci (QTLs) associated with resistance/susceptibility to infectious pancreatic necrosis virus (IPNV) in rainbow trout ( Oncorhynchus mykiss). Mol Genet Genomics 265:23–31
Palti Y, Parsons JE, Thorgaard GH (1999a) Identification of candidate DNA markers associated with IHN virus resistance in backcrosses of rainbow ( Oncorhynchus mykiss) and cutthroat trout ( O. clarki). Aquaculture 173:81–94
Palti Y, Tinman S, Cnaani A, Avidar Y, Ron M, Hulata G (1999b) Comparative study of biochemical and nonspecific immunological parameters in two tilapia species ( Oreochromis aureus and O. mossambicus). Isr J Aquacult-Bamid 51:148–156
Palti Y, Nichols KM, Waller KI, Parsons JE, Thorgaard GH (2001) Association between DNA polymorphisms tightly linked to MHC class II genes and IHN virus resistance in backcrosses of rainbow and cutthroat trout. Aquaculture 194:283–289
Palti Y, Shirak A, Cnaani A, Hulata G, Avtalion RR, Ron M (2002) Detection of genes with deleterious alleles in an inbred line of tilapia ( Oreochromis aureus). Aquaculture 206:151–164
Perry GML, Danzmann RG, Ferguson MM, Gibson JP (2001) Quantitative trait loci for upper thermal tolerance in outbred strains of rainbow trout ( Oncorhynchus mykiss). Heredity 86:333–341
Pickering AD (1993) Growth and stress in fish production. Aquaculture 111:51–63
Poompuang S, Hallerman EM (1997) Towards detection of quantitative trait loci and marker-assisted selection in fish. Rev Fisher Sci 5:253–277
Roed KH, Fevolden SE, Fjalestad KT (2002) Disease resistance and immune characteristic in rainbow trout ( Oncorhynchus mykiss) selected for lysozyme activity. Aquaculture 209:91–101
Shirak A, Palti Y, Cnaani A, Korol A, Hulata G, Ron M, Avtalion RR (2002) Association between loci with deleterious alleles and distorted sex ratios in an inbred line of tilapia ( Oreochromis aureus). J Hered 93:270–276
Tabor HK, Risch NJ, Myers RM (2002) Candidate-gene approaches for studying complex genetic traits: practical considerations. Nat Rev Genet 3:391–396
Van der Zijpp AJ, Egberts E (1989) The major histocompatibility complex and diseases in farm animals. Immunol Today 10:109–111
Van Kaam JBCHM, Groenen MAM, Bovenhuis H, Veenendaal A, Vereijken ALJ, van Arendonk JAM (1999) Whole genome scan in chickens for quantitative trait loci affecting carcass traits. Poultry Sci 78:1091–1099
Weller JI, Soller M, Brody T (1988) Linkage analysis of quantitative traits in interspecific cross of tomato ( Lycopersicon esculentum × Lycopersicon pimpinellifolium) by means of genetic markers. Genetics 118:329–339
Weller JI, Song JZ, Heyen DW, Lewin HA, Ron M (1998) A new approach to the problem of multiple comparisons in the genetic dissection of complex traits. Genetics 150:1699–1706
Wohlfarth GW, Hulata G, Rothbard S, Itzkowich J, Halevy A (1983) Comparison between interspecific tilapia hybrids for some production traits. In: Fishelson L, Yaron Z (eds) International Symposium on Tilapia in Aquaculture. Tel Aviv University, Israel, pp 559–569
Zhou HJ, Lamont SJ (2003) Chicken MHC class I and II gene effects on antibody response kinetics in adult chickens. Immunogenetics 55:133–140
Acknowledgements
The authors thank Thomas D. Kocher for allowing the use of data on the tilapia linkage map and microsatellite sequences before they were published. This study was supported by the Israel Science Foundation (Grant no. 418/99-1), and has been carried out in compliance with the current laws governing genetic experimentation in Israel
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Reuter
Rights and permissions
About this article
Cite this article
Cnaani, A., Zilberman, N., Tinman, S. et al. Genome-scan analysis for quantitative trait loci in an F2 tilapia hybrid. Mol Genet Genomics 272, 162–172 (2004). https://doi.org/10.1007/s00438-004-1045-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-004-1045-1