Abstract
Photosystem (PSII) is a supramolecular polypeptide complex found in oxygenic photosynthetic membranes, which is capable of extracting electrons from water for the reduction of plastoquinone. An intriguing feature of this assembly is the fact that it includes more than a dozen low-mass polypeptides of generally unknown function. Using a transplastomic approach, we have individually disrupted the genes of the psbEFLJ operon in Nicotiana tabacum, which encode four such polypeptides, without impairing expression of downstream loci of the operon. All four mutants exhibited distinct phenotypes; none of them was capable of photoautotrophic growth. All mutants bleached rapidly in the light. Disruption of psbE and psbF, which code for the α and β apoproteins of cytochrome b 559 , abolished PSII activity, as expected; ΔpsbL and ΔpsbJ plants displayed residual PSII activity in young leaves. Controlled partial solubilisation of thylakoid membranes uncovered surprisingly severe impairment of PSII structure, with subunit and assembly patterns varying depending on the mutant considered. In the ΔpsbL mutant PSII was assembled primarily in a monomeric form, the homodimeric form was preponderant in ΔpsbJ, and, unlike the case in ΔpsbZ, the thylakoids of both mutants released some PSII supercomplexes. On the other hand, Photosystem I (PSI), the cytochrome b6f complex, ATP synthase, LHCII, and CP24/CP26/CP29 antennae were present in near wild-type levels. The data are discussed in terms of their implications for structural, biogenetic and functional aspects of PSII.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photosystem II (PSII) is a unique molecular machine that houses the catalytic components that mediate light-driven oxidation of water to molecular oxygen, release of protons and electrons being coupled to the reduction of plastoquinone during photosynthesis. It is the most complex supramolecular polypeptide assembly found in oxygenic photosynthetic membranes, is predominantly located in the grana regions of the thylakoid membrane system, and consists of more than two dozen intrinsic and peripheral subunit species. Controlled partial solubilization and fractionation of photosynthetic membranes has demonstrated that PSII is made up of various subcomplexes which can complement each other functionally. These include an innermost reaction-center core complex, made up of the polypeptide species D1 (encoded by psbA), D2 ( psbD), cytochrome b 559 ( psbE and psbF) and PsbI, which carries out the primary charge separation. Associated with the core is an ensemble of chlorophyll a and chlorophyll a/b assemblies which cooperate in light collection, energy transfer to the core complex and its thermal dissipation. The 51-kDa and 43-kDa chlorophyll a -binding proteins (CP47, CP43; PsbB and PsbC) act as the light-capturing antenna for the core. They are intimately associated with the reaction center. The minor chlorophyll a/b antenna comprising CP24, CP26 and CP29, and the light-harvesting chlorophyll a/b -containing antenna LHCII, which is present in both mobile and bound forms at the periphery (Sandona et al. 1998; Andersson et al. 2001) are external to the core and chlorophyll a antenna, and are not essential for the primary photochemical charge-separation step, although they serve to enlarge the photochemically active cross-section. Recent structural data, obtained by X-ray crystallography and high-resolution electron microscopy, are basically consistent with this topography deduced from biochemical and biophysical work (Barber and Kühlbrandt 1999; Boekema et al. 1999; Hankamer et al. 1999; Nield et al. 2000a, 2000b; Zouni et al. 2001).
One of the most intriguing findings in PSII biology was the detection of a relatively large number (16) of intrinsic or peripheral polypeptide species of low mass (summarized in Hankamer et al. 2001; see also Baena-Gonzalez et al. 2001; Swiatek et al. 2001), which are not found in bacterial, non-oxygenic, quinone-reducing photosystems (Debus 2000). It is conceivable that an increased number of subunit species is required to regulate the electron flow in PSII, which is more complex than the cyclic flow that operates during anaerobic photosynthesis in bacterial cells. However, the functions of these polypeptides are not well understood, and even their association with, and/or localization within, PSII has not been rigorously demonstrated in all instances. Moreover, various transmembrane helices uncovered by structural studies could not be assigned to distinct polypeptides at the level of resolution presently available (Hankamer et al. 2001; Zouni et al. 2001). Low mass polypeptides such as PsbY (Gau et al. 1998) or PsbZ, a possible link between PSII and antenna (Baena-Gonzalez et al. 2001; Swiatek et al. 2001), obviously represent potential candidates to fill these gaps. Another set of such small polypeptides is encoded on most plastid chromosomes reported to date by the psbEFLJ operon. psbE and psbF encode the apoproteins α and β of cytochrome b 559 , the only two-chain cytochrome known in nature (Herrmann et al. 1984). Cytochrome b 559 is found in the minimal PSII subcomplex that is capable of light-induced charge separation (Nanba and Satoh 1987). Deletion of the corresponding genes, psbE and F, abolishes PSII activity (Pakrasi et al. 1990; Morais et al. 1998). Although the cytochrome can undergo light-induced redox reactions, it is not involved in the primary electron transfer reactions of PSII, leaving its role in the photosynthetic process uncertain. Recent proposals have focused on alternative electron transfer pathways involving cytochrome b 559 that protect PSII against light-induced damage, either by dissipating excess energy through a cyclic electron flow within PSII or by deactivating potentially damaging redox states (Barry et al. 1994; Stewart and Brudvig 1998). Structural data deduced from a cyanobacterial PSII assembly support the idea that cytochrome b 559 exists as an α/β heterodimeric structure (Zouni et al. 2001), with the heme molecule bound by single His residues in each of the chains, as predicted earlier from sequence and spectroscopic data (Herrmann et al. 1984; Babcock et al. 1985). The roles of PsbL and PsbJ remain unclear. Synechocystis 6803 cells in which psbL has been inactivated lack a functional PSII (Anbudurai and Pakrasi 1993; Kitamura et al. 1994; Ozawa et al. 1997); cyanobacterial ΔpsbJ cells display altered oxygen evolution and the data suggest that the polypeptide is membrane associated (Lind et al. 1993). Little information is available on these components in thylakoids of vascular plants, which may differ in some respects from their cyanobacterial equivalents (Hager et al. 2002; Regel et al. 2001).
It is obvious that the low-mass polypeptides play an important role in photosynthesis, and hence knowledge of their structural and functional impact is essential for a full understanding of the photosynthetic process that occurs in PSII, and its biogenesis. This, together with the fact that the function and crucial structural details of cytochrome b 559 are still enigmatic, has provided the incentive to use a transplastomic approach to disrupt and modify the genes of the psbEFLJ operon in the plastid chromosome of tobacco. The genes all encode small polypeptides of 38–83 amino acid residues ( psbL, 38; psbF, 39; psbJ, 40; psbE, 83) that appear to share a common topology. They are known (E, F) or predicted (L, J) to be bitopic, i.e. each with a single central transmembrane segment of 18–22 residues flanked by relatively short hydrophilic stretches protruding from either side of the polypeptide chain into organelle stroma and thylakoid lumen, specifically by 18/39 (PsbE), 13/6 (PsbF), 17/1 (PsbL) and 9/9 (PsbJ) N-/C-terminal amino acid residues. In this communication we describe the phenotypes of mutants with defects in the genes for each of these proteins.
Materials and methods
Vector construction and transformation of tobacco chloroplasts
A 3675-bp SalI- Eco 47III fragment of the plastid chromosome of Nicotiana tabacum cv. Bright Yellow 4 (nucleotide positions 65,310–68,985; EMBL Accession No. Z00044) containing the entire psbEFLJ operon and the species-specific orf103 was inserted into the SmaI+SalI-digested Bluescript KS- vector, and cloned in E. coli XL1-Blue. The four genes of the operon were inactivated individually by the insertion (in the correct polarity) of a terminator-less chimeric aadA gene fused to the homologous psbA promoter (Koop et al. 1996) into internal restriction sites for HindIII ( psbE and L) or PstI ( psbF and J). These restriction sites had been introduced by site-specific mutagenesis (Kunkel 1985) using the synthetic oligonucleotides 5´-CTGGAAGCACAGGAGAAGCTTCGTTTGCTG-3´ ( psbE), 5´-GGAGGCCCTAATGACTGCAGATCGAACCTATCC-3´ ( psbF), 5´-CCGGAACGAACAAAAGCTTGAATTGAATCGTACC-3´ ( psbL) and 5´-GGGGTAAATGGCCGATACTGCAGGAAGGATTCCTC-3´ ( psbJ). The aadA cassette with a heterologous ( Chlamydomonas reinhardtii) rbcL terminator (Koop et al. 1996) was also placed in an EcoRV site located 3´ within the untranslated region of the operon (nucleotide position 66,053). The transplastomic line that carries this insertion is hereafter referred to as the RV line.
Chloroplasts of N. tabacum cv. Petit Havanna were transformed in one of two ways, by PEG treatment of protoplasts as described by Koop and Kofer (1995) or by particle bombardment of leaves (Svab and Maliga 1993). Selection and culture of transformed material, as well as the assessment of plastome segregation and of the homoplastomic state, were performed essentially as described by De Santis-Maciossek et al. (1999) and Swiatek et al. (2003). The material was grown on agar plates of MS medium (Murashige and Skoog 1962) containing 2% sucrose, and grown on 12 h dark/light cycles at 25°C with 20 μmol of photons/m2 per s and, to maintain selective conditions, 500 μg/ml spectinomycin.
DNA and RNA analysis, protein overexpression and antisera
Nucleotide sequences were determined by the dideoxy chain-termination method (Sanger et al. 1992). Both labeling with energy-transfer fluorochrome dideoxynucleotide (Rosenblum et al. 1997) with the ABI 377 system (Applied Biosystems) and fluorochrome primer labeling with the LI-COR 4200IR2 two-laser system (MWG Biotech) were used for detection of sequencing products (Hupfer et al. 2000).
Total RNA was isolated from leaves using the TRIazol reagent (Gibco/BRL), electrophoretically fractionated in 1% agarose-MOPS-formaldehyde gels (5 μg/lane), and transferred onto Hybond membranes (Pall Biodyne) for hybridization as outlined in Sambrook et al. (1989).
The individual genes of the psbEFLJ operon were amplified by PCR from the 3675-bp SalI-Eco47III fragment mentioned above, using gene-specific primer pairs extended by XhoI and XbaI restriction sites at their 5´ and 3´ ends, respectively: 5´-GACTCGAGTCTGGAAGCACAGGAGAACG-3´ and 5´-GATCTAGACTAAAACGATCTACTAAATTCATCG-3´ for psbE; 5´-TCCTCGAGACTATAGATCGAACCTATCC-3´ and 5´-ATTCTAGATTATCGTTGGATGAACTGC-3´ for psbF; 5´-GACTCGAGACACAATCAAACCCGAACG-3´ and 5´-GATCTAGATTAATTGAAGAAATAATTGG-3´ for psbL; and 5´-GACTCGAGGCCGATACTACTGGAAGGATTCC-3´ and 5´-GATCTAGACTAGAGGGATGAACCCAATCC-3´ for psbJ. The resulting fragments were double-digested with XhoI and XbaI, inserted into Bluescript SK- or pThioHisA,band C vectors (Invitrogen), and cloned in Escherihia coli DH5α or BL21, respectively. Expression of the fusion products in the pThiolHis system was performed according to the manusfacturer's recommendations. The PsbF, PsbL and PsbJ fusion proteins were electrophoretically purified from inclusion bodies and used to elicit antisera by immunizing rabbits in the presence of TiterMax adjuvant (CytRX Corporation) according to Harlow and Lane (1988). Initial attempts to overexpress the small, bitopic proteins themselves in E. coli using the customary vectors outlined in Haehnel et al. (1994) failed. None of the proteins could be obtained in sufficient amounts. Antisera were stored at −20°C after addition of 0.01% sodium azide. Where titers were low, IgG fractions were purified on HiTrap rProtein A columns (Amersham Pharmacia Biotech) according to the manufacturer's protocol. The fractions were desalted on PD-10 columns (Amersham Pharmacia Biotech) and subsequently concentrated on Urifil-10 concentrators (Millipore).
Strand-specific riboprobes were prepared by in vitro transcription and [α-32P]dUTP labeling of PCR products using the T7 and T3 promoters of XbaI- or XhoI-digested recombinant Bluescript vectors, respectively. Hybridization with double-stranded and single-stranded probes was carried out overnight at 65°C in Rapid Hybridization Buffer (Amersham).
Protein extraction, polyacrylamide gel electrophoresis and immunoblotting
Total leaf proteins were isolated and sucrose-gradient fractionation of partial thylakoid lysates was performed as described in Swiatek et al. (2001). Thylakoid membranes were prepared according to Zer et al. (1999) from leaves of 4-week-old, axenically grown plants. Aliquots were suspended in 50 mM HEPES-KOH (pH 7.5), 5 mM MgCl2, 50% glycerol and stored at −70°C until use. Proteins were separated in 14% (w/v) polyacrylamide gels (Schägger and von Jagow 1987) in the presence of 6 M urea, or in the gel systems described in Westhoff et al. (1985). Samples were heated for 5 min at 80°C, mixed with 1/10 vol of glycerol/dye solution, immediately applied to the gel, and electrophoresed at 30 mA for 20 h at room temperature. Two-dimensional electrophoresis was performed using non-denaturing polyacrylamide gels (6–12% "blue native" gradient gels; Schägger and von Jagow 1991) for the first dimension and a 10–16% denaturing gradient gel in the second (Laemmli 1970). Individual bands in one-dimensional gels, and spots in 2-D gels were identified by immunodetection, after electrophoretic transfer to nitrocellulose filters (Schleicher and Schuell) or PVDF membranes (Pall Biodyne) and probing with monospecific, polyclonal antisera. Antibody binding was detected with horseradish peroxidase-conjugated α-rabbit IgG (Boehringer, Mannheim), and peroxidase activity was visualized by reaction with luminol and H2O2 (the enhanced chemiluminescence assay; ECL, Amersham). The antisera used were raised in rabbits against polypeptides from spinach, Arabidopsis or tobacco: D1 (psbA), D2 (psbD), CP47 (psbB), CP43 (psbC), α and β subunits of cytochrome b 559 (psbE, psbF), PsbH (psbH), PsbL (psbL), PsbJ (psbJ), PsbS (psbS), PsbW (psbW), PsbZ (psbZ), the extrinsic 33-, 23- and 16-kDa polypeptides of the oxygen-evolving complex (psbO, psbP and psbQ) of PSII, CP24 (lhcb4), CP26 (lhcb5), CP29 (lhcb6), LHCII (lhcb1-3), LHCI (lhca), subunits Ia and b (psaA and B), II (psaD) and III (psaF) of PSI, cytochrome f (petA), cytochrome b 6 (petB) and Rieske FeS protein (petC) of the cytochrome b6f complex, and subunits α (atpA) and b´ (atpG) of the thylakoid ATP synthase, plastocyanin (petE) and lumenal polyphenol oxidase (ppo).
Kinetic analysis of fluorescence and light absorption
Changes in chlorophyll fluorescence yield were measured using a commercial pulse-modulated fluorimeter (PAM-101, Walz, Effeltrich, Germany); the light guide that delivers the measuring and saturating light was held 5 mm from the upper surface of selected leaves. Leaves were dark adapted for 15–30 min prior to measurement of the induction fluorescence, and kept at 20°C. After recording the minimum fluorescence (Fo) induced by the measuring beam, saturating light flashes were provided in order to completely reduce the PSII acceptor side QA - and to measure the maximum fluorescence yield (Fm). The intensity of the saturating light flash (800 ms) used for the measurements of Fm was 6000 µmol of photons/m2 per s. The variable fluorescence (Fv) was calculated as Fm−Fo. The ratio Fv/Fm reflects the potential yield of the photochemical reaction of PSII (Krause and Weis 1991). Reoxidation of QA - was recorded for 2 min. The actinic light intensity used for fluorescence induction was 20 µmol of photons/m2 per s. The application of additional consecutive light pulses over a 5-min induction period served to calculate the amount of photochemical and non-photochemical quenching.
PSI activity in intact leaves was measured by detecting absorption changes resulting from P700 oxido-reduction using the dedicated PSI attachment of the PAM-101 instrument. For thylakoid preparations, 8–12 leaves exhibiting variable fluorescence were selected from different plants. Oxygen evolution by isolated thylakoid membranes was measured polarographically using dimethylbenzoquinone (DMBQ) or dichlorophenylindophenone as electron acceptors (Keren et al. 1997).
Other methods
Chlorophyll contents were measured in 80% acetone according to Porra et al. (1989). Samples for ultrastructural analysis and electron microscopy were prepared as described by De Santis-Maciossek et al. (1999).
Results
Disruption of the genes psbE, psbF, psbJ and psbL
For gene disruption, a terminator-less chimeric aadA cassette which confers spectinomycin resistance on chloroplasts was inserted in sense orientation into each of the four genes separately, using as targets restriction sites that had been generated by site-directed mutagenesis of each gene (Fig. 1). The vectors were designed to interrupt the ORFs near the N-terminus: between the codons for Glu7 and Arg8 in psbE, Thr3 and Ile4 in psbF, Asn10 and Val11 in psbL, and the two threonine residues at positions 4 and 5 in psbJ. Except in psbL, the insertions also caused a shift in the reading frame that prevents translational reinitiation in the interrupted gene but did not interfere with the decoding of the preceding and downstream genes in the operon. As controls, and to assess possible secondary effects of cassette insertion, the wild-type tobacco line and the RV line carrying the aadA gene in the transcribed intercistronic region downstream of psbJ were used. The latter insertion was previously shown to be phenotypically neutral (Bock et al. 1994), i.e. the transformed strain is functionally and ultrastructurally indistinguishable from wild-type.
Construction of Nicotiana tabacum psbE -, psbF -, psbL -, and psbJ - plastome mutants. Each of the four genes was individually mutated by insertion of the aadA cassette into artifically generated, unique restriction sites within the respective reading frames of the genes, as described in Materials and methods. Only the disruption of psbE is shown in detail. The arrows mark the sites of insertion of the cassette into the other genes
The first resistant colonies or shoots formed approximately 3 weeks after transformation in all cases. The transformation efficiency varied between 18 and 74 independent lines per 106 treated protoplasts or 5 bombarded leaves, respectively. Correct integration of the aadA cassette, plastome segregation and the homoplastomic state were monitored by Southern analysis, nucleotide sequence analysis, and pulsed-field electrophoresis followed by PCR analysis of the band containing monomer plastid chromosomes (De Santis-Maciossek et al. 1999; Swiatek et al. 2003). The PFGE/PCR approach provides an efficient tool with which heteroplastomic lines can be recognized. Three cycles of shoot regeneration from leaf disks on selective medium were found to be sufficient for complete segregation of wild-type and mutant plastomes. In the case of ΔpsbE and ΔpsbF, which segregated relatively slowly, a clonal propagation step was included to speed up plastome separation, employing an efficient protocol for regeneration of plants from protoplasts, which allows shoot formation to occur within approximately 2 weeks (Dovzhenko et al. 1998). The mutant lines were maintained for several months on medium that induced the formation of multiple shoots. Individual plants were grown in jars for 4–6 weeks. With the exception of ΔpsbE, all lines grew quite well and resembled the wild type morphologically (apart from the fact that leaves were occasionally smaller than normal).
Phenotypic characterization of the mutants
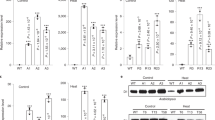
The material selected for analysis was homoplastomic (Swiatek et al. 2003) and displayed distinct phenotypes in all instances. All mutants, with the exception of ΔpsbL and, to some extent, ΔpsbJ., remained fairly green in dim light (Fig. 2), but bleached rapidly when exposed to light intensities of 100 μmol photons/m2 per s or higher. The psbJ and psbL mutants displayed an unusual developmental phenotype in that older leaves senesced rapidly and turned off-white even at 10 μmol photons/m2 per s (Fig. 2). None of the lines grew photoautotrophically, i.e. the plantlets were not capable of surviving on soil or growing axenically in the absence of sucrose, despite the fact that residual PSII activity (20–30%) could be detected in the ΔpsbJ (Regel et al. 2001) and ΔpsbL lines (see below).
The plastids of all four mutants were similar in size to those of the wild-type, but differed ultrastructurally from the typical lens-shaped wild-type and RV mutant chloroplasts, which show normal grana stacks interconnected by stroma thalykoids (Fig. 3A). The mutants ΔpsbE and ΔpsbF contained only stacked tylakoid membranes with 2–6 apposed membranes, which were unusually sectored and sometimes locally swollen (Fig. 3B). Generally, these membranes extended throughout the entire cross-section of the organelle. Similarly, ΔpsbL and ΔpsbJ were alike ultrastructurally, in that they exhibited densely stacked thylakoids, with bundles containing up to 20–30 stacked membranes in their central regions, and little, if any, stroma lamellae (Fig. 3C and D). All four mutants expressed normal levels of transcripts of the genes under study, and exhibited Northern patterns as expected. The general pattern of processing of the primary polycistronic transcript was unchanged, except for those RNA species that included the respective disrupted genes, which were enlarged by 906 bp (without terminator) and 1356 bp (with terminator) due to cassette insertion (data not shown).
Electron micrographs of ultra-thin sections of leaf tissue from wild-type tobacco and various PSII mutants. A Typical lens-shaped RV (and wild-type) chloroplast with well established grana and stroma thylakoids. The matrix is densely packed, except at nucleoids which are transparent. bTypical ΔpsbF mutant plastid. Note the bundles of thylakoid layers without discernible stroma lamellae. Mutant ΔpsbE plastids exhibit a similar phenotype. C and D Typical ΔpsbL and ΔpsbJ plastids, respectively, with disk-like, often locally swollen membrane stacks. Bar=1 μm
Effects of gene disruption on the structure and function of PSII
Gel electrophoresis of total cellular proteins or of thylakoid proteins, as well as two-dimensional electrophoresis and high-resolution sucrose gradient centrifugation of dodecyl maltoside lysates of thylakoid fractions (Swiatek et al. 2001), combined with immunolabeling with specific antibodies, were performed in order to evaluate the effect of each of the mutations upon the status of the thylakoid system in general and of PSII in particular. Since the numerous low mass polypeptides of the thylakoid membrane system were difficult to resolve electrophoretically in a single gel system, high resolution electrophoresis on various gel systems, including that of Schägger and von Jagow (1987) and systems with and without urea (Westhoff et al. 1985), were employed to identify polypeptides in the low-molecular weight range. However, Western analysis with polyclonal, monospecific antisera raised against each of the components encoded by the psbEFLJ operon, as well as against PsbZ (Swiatek et al. 2001) and PsbW, was required to definitively identify the subunits of interest and clearly distinguish them from other comigrating thylakoid components (Fig. 4). No signals were found in stroma fractions that would have indicated unincorporated polypeptides or their degradation products (Fig. 4). It is worth noting that the relative positions of the six low-molecular weight proteins (including PsbZ and PsbW) in the different gel systems did not coincide (see also Westhoff et al. 1985) nor did the respective molecular masses calculated from the sequence agree with those estimated from their electrophoretic migration velocities. Without the use of specific antisera, no unequivocal assignment of bands or meaningful interpretation of the data would have been possible.
Immunoblot analysis of low-mass thylakoid membrane polypeptides of wild-type tobacco and psbE, F, L, J and Z mutants. A Lines were grown at 25°C under 10 μE/m2 s, and thylakoid extracts were analyzed using the indicated monospecific polyclonal antisera. Since sera raised against PsbF, L and J had low titers, antibodies were purified and concentrated before use as described in Materials and methods. The blot with anti-F was qualitatively comparable to that of anti-E and is not shown. b Summary of the low-mass PSII subunits present in, and the PSII activity of, the mutants under study
One of the striking findings of this work was the variation in subunit patterns in the individual mutants, due to co-operative polypeptide loss or instability (Fig. 4). Probing thylakoid proteins of developing young leaves with anti-PsbE revealed that the α subunit of cytochrome b 559 was missing, as expected, in the ΔpsbE material, but it was lacking also in ΔpsbF. In the absence of PsbF, PsbE obviously does not stably insert into the membrane. It is important to note that PsbZ was also undetectable in both mutants, but present in ΔpsbL and ΔpsbJ, albeit in reduced quantities. On the other hand, PsbL was only absent in the corresponding mutant, but detectable in all others, including PsbJ. PsbJ, missing in the corresponding mutant, was present in ΔpsbE, ΔpsbF, and ΔpsbZ, but—strikingly—absent in ΔpsbL. PsbW and PsbH were present in all mutants (Fig. 4). The findings just outlined were substantiated and refined in two ways.
Approximately 20 additional antisera, directed against plastid- and nucleus-encoded subunits of all major thylakoid assemblies, were chosen for Western analysis of the thylakoid systems of the mutant material under study. All subunits of PSI, ATP synthase, the cytochrome complex checked, and the lumenal proteins plastocyanin and polyphenoloxidase were present in isolated thylakoid membranes in amounts (per g fresh weight) comparable to those in the wild type (Fig. 5). In contrast, the pattern of PSII polypeptides differed markedly depending on the mutant and developmental stage of the leaves studied. Disruption of psbE and psbF caused the most serious effects (see below). Nevertheless a residual subunit spectrum of the PSII components was clearly present. In both mutants, the steady-state concentrations of D1 (psbA), D2 (psbD) and CP47 (psbB) were reduced to less than 5% of the wild-type level. On the other hand, approximately 15–30% of wild-type PSII polypeptide levels were found in ΔpsbL, and up to 50% in ΔpsbJ lines (Fig. 5). The detection of components of the oxygen-evolving complex, PsbO, PsbP and PsbQ, was strongly dependent upon the stage of leaf development examined. All three subunits were present in near-wild-type quantities in very young leaves, but greatly reduced or absent in older—but still green—leaf material (data not shown). Virtually no change was found in any of the mutants for PsbW, PsbH, and PsbS, for LHCII apoproteins and the minor antenna CP24, CP26 and CP29. All proteins of nuclear/cytosolic origin were terminally processed, including those located in the thylakoid lumen.
Controlled partial solubilization of thylakoid membranes with dodecyl maltoside (Westhoff et al. 1983) was chosen to evaluate the status of the principal membrane assemblies in the mutant thylakoids. The major photosynthetic complexes released were separated either by sucrose-gradient centrifugation or by electrophoresis in two-dimensional gels (data not shown; see Suorsa et al., submitted), and checked visually, photometrically and serologically for their assembly status and polypeptide composition, respectively. Figure 6 shows an example of solubilized ΔpsbL and ΔpsbJ thylakoids fractionated by the former approach, followed by electrophoretic separation of the polypeptides in relevant gradient fractions in a denaturing polyacrylamide gel, and immunological analysis for PSII components. This approach demonstrated that the subunits tested were assembled into dodecylmaltoside-stable PSII complexes in the mutants. Unlike the case in the wild-type, the monomer form of PSII was dominant in ΔpsbL, but the dimer form was dominant in the ΔpsbJ thylakoids, as in the wild type. Small amounts of PSII dimers were noted in ΔpsbL. PSII supercomplexes (Boekema et al. 1995; Hankamer et al. 1997) could be detected as well, but their levels were extremely low in both mutants, and they were usually apparent in overloaded gradients only. In mutant thylakoid lysates, a minor fraction of core components, such as D1 or the α subunit of cytochrome b 559 , was consistently found in the soluble protein fraction at the top of the gradients (data not shown). PSI, the cytochrome complex, ATP synthase, trimeric and monomeric LHCII and antenna with CP24, CP26 and CP29 (which comigrated with the LHC monomer) displayed banding and gel patterns that were essentially indistinguishable from those seen in solubilized wild-type membranes (Fig. 6).
Chlorophyll-protein complexes in wild-type, ΔpsbL and ΔpsbJ mutant thylakoids. Right panel Sucrose gradient fractionation of partially solubilized thylakoids obtained by treatment with β-dodecylmaltoside (gradient not overloaded; see Materials and methods). Left panel Immunodetection of various PSII assemblies with antiserum against D1 protein. Note that the higher-order PSII assemblies that occur in low amounts in the ΔpsbL and ΔpsbJ mutants can only be visualized in overloaded gradients. An equivalent pattern was obtained with anti-PsbE (not shown). The fraction numbers given at the top correspond to those given for the gradient on the left
No variable fluorescence nor oxygen evolution could be detected in ΔpsbE and ΔpsbF material (not shown). In contrast, ΔpsbL and ΔpsbJ displayed impaired though detectable PSII activity (for a more detailed analysis of PSII activity in ΔpsbJ material, see Regel et al. 2001). Like ΔpsbJ, the ΔpsbL mutant exhibited PSII activity characterized by a relatively high Fo level and slow reoxidation of QA -, compared to wild-type and RV control plants, after the application of saturating light pulses (Fig. 7). Unlike ΔpsbJ (Regel et al. 2001), ΔpsbL also showed significant fluorescence quenching, which consists of photochemical and non-photochemical components. The Fv/Fm values were in the range of 0.74–0.76 for wild-type and RV controls, 0.25 (±0.1) for ΔpsbL, and 0.37 (±0.1) for ΔpsbJ. The large variation in the Fv/Fm parameter for the mutant leaves was due to progressive senescence in these leaves (Fig. 7, see also Fig. 2). This feature was not seen in the controls maintained under the same conditions for up to 6 weeks. Whereas the light pulse-induced fluorescence of ΔpsbL returned to the Fo level within about 1 min., in Δ psbJ leaves QA - exhibited a significantly longer half-life. The PQ pool was reduced by PSII in the psbL mutant, as indicated by the fluorescence kinetics (Fig. 7). The oxygen evolution activity of the control plants was in the range of 60–120 mmol of oxygen/mg chlorophyll per h, as compared to 20–35 µmol of oxygen/mg chlorophyll per h when DMBQ was used as an electron acceptor for ΔpsbJ thylakoids (Regel et al. 2001).
Photosynthetic activity of the RV line (WT control) and representative ΔpsbL and ΔpsbJ mutants. Fluorescence emission traces were recorded by pulse-modulated fluorimetry as described in detail in Materials and methods. Saturating light pulses were administered at the points indicated by the asterisks. The respective levels of Fo and Fm are indicated. Reoxidation of QA - was recorded after the application of saturating flashes to dark-adapted leaves. Actinic light was administered over a period of 5 min
All mutants exhibited P700 oxido-reduction activity, as measured with the PAM-101 PS I attachment. Under steady state conditions the oxidation state of P700, expressed as ΔA/ΔAmax (Harbinson and Woodward 1987), was nearly at its maximal level (1) in both ΔpsbL and ΔpsbJ, whereas in wild-type it was 10 times lower (~0.1). Multiple turnover flashes, which are known to reduce the plastoquinone pool completely, induced complete reduction of P700 in background far-red light in wild-type, but led to only ~50% reduction in ΔpsbL leaves.
Discussion
Photosystem II (PSII) is the most complex and most intriguing multisubunit assembly in the thylakoid system. Although, in recent years, the polypeptide complex has been dissected both biochemically and biophysically, crucial aspects of its structure, function, biogenesis and evolution have remained enigmatic—including the roles of the numerous low-mass subunits it contains (Herrmann 1996; Wollman et al. 1999; Hankamer et al. 2001).
Here, we demonstrate that all translation products of the psbEFLJ operon are present, and play a crucial role, in the assembly and activity of PSII, as well as contributing to the general structure of the thylakoids in tobacco. The complete or partial absence of PSII complexes in the mutants described here may perturb the segregation of thylakoid membrane complexes, and this may explain why stacking of membranes increases relative to the appearance of stroma lamellae (Kretzer et al. 1976). Northern and Western analysis of subthylakoid fractions with 30, generally monospecific, polyclonal antisera elicited against thylakoid proteins (including 19 components of PSII and LHCII) verified that the disruption of individual genes led to a monogenic lesion in each instance. It is important to note that the products of genes located downstream of a disrupted locus of the operon could be detected in the mutants, although the absence of one of them can interfere with the stability of others. This confirms that the individual lesions were monocistronic, and indicates that the remaining subunits are made but are rapidly degraded in the absence of others (see below), rather than arguing that epigenetic effects suppress the expression of their genes. Under non-destructive light regimes, the other major polypeptide complexes of the thylakoid system, Photosystem I, the cytochrome complex, ATP synthase, and even the formation of the LHCII trimer or CP24/CP26/CP29-containing LHC complexes remained largely unaffected (Fig. 5), substantiating the notion that there is no substantial reciprocal control between individual thylakoid assemblies during overall biogenesis.
Disruption of each of the four genes of the psbEFLJ operon caused quite serious effects. All four subunits are indispensable for the function of PSII, and essential for photoautotrophic growth. This is at variance with other low-mass components of PSII, such as PsbY1 and PsbY2 (Gau et al. 1998), PsbW (Thidholm et al. 2002), and PsbZ (Baena-Gonzalez et al. 2001; Swiatek et al. 2001), which are dispensable for basic photoautotrophic growth; the requirement for these components only becomes manifest when plants are cultivated under particular growth regimes. While disruption of the genes for the two-chain cytochrome b 559 , psbE and psbF, abolishes PSII activity (Pakrasi et al. 1990; Morais et al. 2001), ΔpsbJ and ΔpsbL lines displayed residual activity of PSII. The increase in long-lived fluorescence in ΔpsbJ could reflect an accumulation of QA - due to alteration of the QA/QB potential (Regel et al. 2001). The decay kinetics of QA - in ΔpsbL following the excitation light pulse also follows a slower course than in ΔpsbJ. However, the slow oxidation of QA - in that case is due to changes in the accessibility of PSII to back electron flow from reduced plastoquinone (Chad et al., submitted).
PSII assemblies appear to exist as homodimers, which probably represent the functional form in vivo. In the supramolecular organization of PSII, the Lhcb proteins of the antenna dock with hydrophobic domains of the PSII core to form higher order PSII-antenna complexes with varying antenna composition (Boekema et al. 1995; Hankamer et al. 1997; Baena-Gonzalez et al. 2001; Swiatek et al. 2001). During the past few years, the composition and gross structures of several isolated PSII particles have been reported, including those of highly active dimeric PSII-LHCII supercomplexes. The loss of any of the low-mass subunits PsbE, F, L or J destabilizes PSII structures to a surprising extent, but at least the lack of the latter two does not destroy them. These are therefore the first mutants reported in higher plants in which PSII subunits have been lost entirely without complete loss of the ability to assemble appreciable amounts of relatively stable entities with residual photochemical activity. Assembled non-functional or impaired PSIIs have also been described recently from site-specific mutants of cytochrome b 559 (Morais et al. 2001). Mutants of this sort will therefore not only allow the study of functions, but also of hitherto inaccessible aspects of structure and assembly during the biogenesis or repair of photosynthetic activity following light stress. However, determination of the details of the processes and mechanisms by which PSII is formed, modified, and able to drive the highly oxidizing photochemical reactions will require substantially refined structural models. In this context, the data presented here have implications in three basic areas.
Aspects of biogenesis
Circumstantial evidence suggests that assembly and repair of PSII are highly regulated and occur gradually. So far, the sequence of subunit integration during PSII biogenesis has been studied only with the compounds that constitute the innermost reaction-center core, notably D1, D2, CP47 and CP43 and cytochrome b 559 (e.g. Müller and Eichacker 1999; Zhang et al. 2001). No information is available on other, especially low mass, subunits, their relationships or impact on assembly kinetics. Also, it is not known whether free monomers exist in vivo, at what stage in assembly the unit dimerizes, whether this process depends upon the availability of new subunits, at what stage and how antenna complexes are added, whether the LHC trimer and minor antenna are made independently. Moreover, little is known about PSII dynamics and the components involved in the decomposition of the complexes, for instance, in the absence of their complete assembly. The results presented here indicate both a sequence, perhaps a hierarchy, of events, and co-operativity of subunit insertion/loss in the course of PSII assembly. The results presented in Figs. 4 and 5 demonstrate that the absence of the low-mass subunits studied does not prevent the core components and a few other subunits of unknown location within PSII from inserting into the membrane. Furthermore, three kinds of defect patterns for low molecular weight subunits became apparent from Western analyses and thylakoid fractionation data: PsbE, PsbF and PsbZ form one group, and PsbJ and PsbL a second that appears to integrate independently of the former. PsbW and PsbH represent a third group of small subunits (Fig. 4). Like PsbS, they are present in near wild-type amounts in all mutants.
PsbJ and PsbL, like PsbZ (Swiatek et al. 2001), severely influence the stability of higher-order PSII assemblies, but the generation of such assemblies is not hindered by the inactivation of PsbJ or PsbL. The patterns of subunit loss are consistent with the mutant phenotypes. Absence of PsbL, which also destabilizes PsbJ (but not conversely) has a more severe effect on PSII structure than the lack of PsbJ alone, which, in turn, is more deleterious than loss of PsbZ. Only the ΔpsbZ mutant can grow photoautotrophically, albeit with reduced efficiency when compared to wild-type (Baena-Gonzalez et al. 2001; Swiatek et al. 2001). This is consistent both with the finding that all six low-mass subunits investigated assemble into PSII in ΔpsbZ thylakoids and with the idea that this subunit is required relatively late in PSII biogenesis. In the absence of PsbJ, or of PsbZ, a relatively stable PSII dimer can be formed; thylakoids of the ΔpsbJ and ΔpsbZ mutants release this structure as the preponderant form. The ΔpsbL mutant influences dimer stability more than the former, without notably affecting PsbE, F or Z. In that mutant, the PSII monomer is the dominant structure in sucrose gradients. It is important to note that overloaded gradients revealed minute amounts of both dimer (~2%) and supercomplex (~0.6%) in ΔpsbL, and supercomplex in ΔpsbJ, implying that even the loss of both subunits does not prevent the association of the mutant dimer complex with LHCII (Fig. 6). The release of core components, e.g. PsbE or D1 protein, that are found in soluble form in the top fractions of the gradient even after mild thylakoid lysis at low temperatures suggests that the different monomer, dimer and supercomplex ratios observed in ΔpsbL and ΔpsbJ thylakoids are due in large part to the instability of the higher-order assemblies. These polypeptides are not detectable in lysates from wild-type thylakoids nor in stroma fractions (Fig. 4), and hence must originate in the mutant membranes. It is therefore likely that even in ΔpsbL the dimer is preponderant in vivo, but is not stable under the conditions of lysis used here. This differs from ΔpsbZ, in which supercomplexes could not be detected, and indicates that both (stable) dimer formation and the presence of PsbZ are prerequisites for stable LHC attachment. Comparison of the ΔpsbL, ΔpsbJ and ΔpsbZ phenotypes rules out the possibility that secondary effects in ΔpsbZ result in the failure of LHCII and core to assemble, and argues that it is indeed the Z subunit, and not any of the other low-molecular-mass subunits studied, that is the real linker molecule between core complex, minor antenna and LHCII (Swiatek et al. 2001).
Western analyses of ΔpsbL and ΔpsbJ material show that the composition and assembly status of PSII can change with leaf maturation, premature senescence, and environmental factors, especially the light regime (Fig. 2). Of particular note are changes in composition of the extrinsic lumenal 33-kDa (PsbO), 23-kDa (PsbP) and 16-kDa (PsbQ) polypeptides of the oxygen-evolving system (see also Hager et al. 2002) which accompany the loss of PSII activity and are probably caused by secondary effects. These three polypeptides are easily detectable in very young leaves (Fig. 6), but are greatly reduced or even absent in older, still green leaves, notably PsbP and PsbP/PsbQ in ΔpsbL and ΔpsbJ, respectively. Although these findings correlate well with the assumed asymmetric arrangement of the extrinsic subunits in PSII (Nield et al. 2000a), the lack of effect on two other lumenal proteins (plastocyanin, polyphenoloxidase; Fig. 5), and PSII activity in leaves, it is not clear whether this instability reflects structural relationships or whether it is caused by a general release and degradation of these subunits from the ageing or photobleaching membrane. Substantially shorter half-lives have been noted for detached PsbO when compared to its membrane-associated form (Palomares et al. 1993). The developmental and environmental effects on protein stability, the co-operative instability of small subunits and observations such as the finding that approximately 50% of cytochrome b 559 or of the extrinsic subunits of the oxygen-evolving system may accumulate and persist in etiolated material or in off-white Δrpo mutants, without any functional PSII assembly (Herrmann et al. 1992; DeSantis-Maciossek et al. 1999) reflect the operation of a quality control system, which is not just a mere scavenging system that recognizes and removes unassembled proteins, misassembled or incomplete structures (Herrmann 1996). A differentially operating quality control system renders unequivocal interpretations of the data difficult, until differences in intrinsic protein stability, and subtle aspects of proteolytic regulation and their impact for PSII are understood.
Structural aspects
A minimum of 36 (34) transmembrane helices that constitute PSII have been localized in the 3-D structure of a cyanobacterial PSII core preparation by X-ray crystallography (Zouni et al. 2001) and high-resolution electron microscopy with 2-D crystal diffraction (Barber und Kühlbrandt 1999; Hankamer et al. 1999), single-particle averaging (Boekema et al. 1999) and cryoelectron microscopy (Nield et al. 2000b). Of these, 24 have been assigned to D1, D2, CP43, CP47, PsbE and PsbF, 7 have been tentatively assigned (PsbH, I, K, L, W, Tc and X), and 5 helices could not be correlated with individual subunits (Hankamer et al. 2001; Zouni et al. 2001). It is difficult to relate low-molecular-mass subunits to structural elements, since the presently deduced structures display less helices than would be expected from known PSII subunits; assignments of PsbL and PsbK, for instance, rest on reasonable, but unproven assumptions, and PsbJ, PsbZ, PsbY1/Y2 and PsbS have not yet been considered or allocated to a PSII structure at all.
If co-operative subunit instability is structurally relevant, the finding that PsbH, PsbW and PsbS are present in near wild-type quantities suggests that they are not in direct spatial relation with the four low-mass subunits of PSII studied in this work. Due to the instability of CP26 in the ΔpsbZ mutant, this subunit has been placed in the vicinity of CP26 (Swiatek et al. 2001). We tentatively assign PsbZ to either helix XII (which is conserved between cyanobacteria and higher plants) or XI according to Hankamer et al. (2001) which these authors have attributed to PsbH and/or X. The two helices reside close to cytochrome b 559 (helices IX and X), which has been unambiguously localized in PSII by virtue of the heme iron (Zuoni et al. 2001). It may not be fortuitous that PsbZ (but not PsbL and PsbJ) is missing in ΔpsbE and ΔpsbF material, or that these helices reside in a region that is structurally variable between cyanobacterial and chloroplast PSIIs—which operate with different antenna systems. In spite of the phenotypic similarities between their respective mutants, several observations suggest that the positions and roles of PsbJ and PsbZ in LHCII attachment must be different: (1) the influence of PsbL upon the stability of PsbJ, but not of PsbZ; (2) the fact that loss of PsbL and PsbJ has no obvious effect on cytochrome b 559 , (3) the differences in physiological and spectroscopic features between ΔpsbJ and ΔpsbZ mutants (Baena-Gonzalez et al. 2001; Hager et al. 2002; Regel et al. 2001; Swiatek et al. 2001), and (4) the finding that, unlike PsbZ, the stability of none of the three minor antenna apoproteins appears to be altered in ΔpsbJ. Our data are compatible with a spatial relationship of the L and J subunits. By analogy, subunits K and L (and perhaps H or X) have been proposed (Hankamer et al. 2001) to be constituents of adjacent triple helices that form symmetry-related antipodes to PsbE, PsbF, and PsbZ, and are situated centrally at the monomer/monomer interface in the PSII homodimer. If this is correct, PsbJ, and not PsbH or X, could well be the third element. However, at this point, a firm structural assignment of subunits L and J must remain tentative, since effects may be indirect, and hence, loss of a subunit with concomitant dimer instability does not necessarily imply that this subunit is involved in dimer formation nor that it resides in the dimer groove. Also, the reduced or transient stability of PSII supercomplexes in ΔpsbL and ΔpsbJ thylakoids is at variance with a simple core-PsbZ-CP26 docking model of antenna association. It suggests topologically multiple association sites or (indirect) long-distance effects, comparable to those that have been inferred for CP29 in ΔpsbZ (Swiatek et al. 2001).
Phylogenetic and ecophysiological aspects
It has been repeatedly noted that the loss of a particular gene can cause various, sometimes significantly different, phenotypes in different materials. That cyanobacterial ΔpsbJ, ΔpsbH or ΔpsbK mutants, but not the corresponding mutants in Chlamydomonas and higher plants, are capable of reduced photoautotrophic growth (Ikeuchi et al. 1991; Lind et al. 1993; Mayers et al. 1993; Takahashi et al. 1994; Hager et al. 2001; Regel et al. 2001) reinforces the view that results obtained with different models, such as cyanobacteria, algae, or higher plants, cannot necessarily be generalized, in spite of the overall similarity in the basic photosynthetic process and membrane design. Thylakoids of widely different organisms have different phylogenetic histories and structural/functional details have evolved differently. PSII is an appealing model in which to probe into phylogenetic as well as ecophysiological aspects, to evaluate differences and subtleties in the regulation of corresponding processes among and within prokaryotes and eukaryotes, and to unravel the relationships between gene dispersal and the sequence of subunit integration, if any, in the overall process of PSII biogenesis and maintenance. However, the substantial effects seen with only a few low mass subunits makes it obvious that a holistic approach will be indispensable if one wishes to derive a meaningful overall picture of PSII biology.
References
Anbudurai PR, Pakrasi HB (1993) Mutational analysis of the PsbL protein of photosystem II in the cyanobacterium Synechocystis sp PCC 6803. Z Naturforsch 48:267–274
Andersson J, Walters RG, Horton P Jansson S (2001) Antisense inhibition of the photosynthetic antenna proteins CP29 and CP26: implications for the mechanism of protective energy dissipation. Plant Cell 13:1193–1204
Babcock GT, Widger WR, Cramer WA, Oertling WA, Metz JG (1985) Axial ligands of chloroplast cytochrome b-559: identification and requirement for a heme-cross-linked polypeptide structure. Biochemistry 24:3638–3645
Baena-Gonzalez E, Gray JC, Tyystjarvi E, Aro EM, Maenpaa P (2001) Abnormal regulation of photosynthetic electron transport in a chloroplast ycf9inactivation mutant. J Biol Chem 276:20795–20802
Barber J, Kühlbrandt W (1999) Photosystem II. Curr Opin Struct Biol 9:469–475
Barry BA, Boerner RJ, de Paula JC (1994) The use of cyanobacteria in the study of the structure and function of photosystem II. In: Bryant DA (ed) The Molecular biology of cyanobacteria. Kluwer Academic, Dordrecht, pp 217–257
Bock R, Kossel H, Maliga P (1994) Introduction of a heterologous editing site into the tobacco plastid genome: the lack of RNA editing leads to a mutant phenotype. EMBO J 13:4623–4628
Boekema EJ, Hankamer B, Bald D, Kruip J, Nield J, Boonstra AF, Barber J, Rögner M (1995) Supramolecular structure of the photosystem II complex from green plants and cyanobacteria. Proc Natl Acad Sci USA 92:175–179
Boekema EJ, Van Roon H, Van Breemen JF, Dekker JP (1999) Supramolecular organization of photosystem II and its light-harvesting antenna in partially solubilized photosystem II membranes. Eur J Biochem 266:444–452
Chad (2003)
De Santis-MaicIossek G, Kofer W, Bock A, Schoch S, Maier RM, Wanner G, Rudiger W, Koop HU, Herrmann RG (1999) Targeted disruption of the plastid RNA polymerase genes rpoA,band C1: molecular biology, biochemistry and ultrastructure. Plant J 18:477–89
Debus RJ (2000) The polypeptides of photosystem II and their influence on manganotyrosyl-based oxygen evolution. Met Ions Biol Syst 37:657–711
Dovzhenko A Bergen U, Koop HU (1998) Thin-alginate-layer technique for protoplast culture of tobacco leaf protoplasts: shoot formation in less than two weeks. Protoplasma 20:114–118
Gau AE, Thole HH, Sokolenko A, Altschmied L, Hermann RG, Pistorius EK (1998) PsbY, a novel manganese-binding, low-molecular-mass protein associated with photosystem II. Mol Gen Genet 260:56–68
Haehnel W, Jansen T, Gause K, Klösgen RB, Stahl B, Michl D, Huvermann B, Karas M, Herrmann RG (1994) Electron transfer from plastocyanin to photosystem I. EMBO J 13:1028–1038
Hager M, Hermann M, Biehler K, Krieger-Liszkay A, Bock R (2002) Lack of the small plastid-encoded PsbJ polypeptide results in a defective water-splitting apparatus of photosystem II, reduced photosystem I levels, and hypersensitivity to light. J Biol Chem 277:14031–14039
Hankamer B, Nield J, Zheleva D, Boekema E, Jansson S, Barber J (1997) Isolation and biochemical characterisation of monomeric and dimeric photosystem II complexes from spinach and their relevance to the organisation of photosystem II in vivo. Eur J Biochem 243:422–429
Hankamer B, Morris EP, Barber J (1999) Revealing the structure of the oxygen-evolving core dimer of photosystem II by cryoelectron crystallography. Nat Struct Biol 6:560–564
Hankamer B, Morris E, Nield J, Carne A, Barber J (2001) Subunit positioning and transmembrane helix organisation in the core dimer of photosystem II. FEBS Lett 504:142–151
Harbinson J, Woodward FI (1987) The use of light-induced absorbance changes at 820 nm to monitor the oxidation state of P700 in leaves. Plant Cell Environ 10:131–140
Harlow E, Lane D (1988) Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., pp 55–116
Herrmann RG (1996) Photosynthesis research: aspects and perspectives. In: Andersson B, Salter AH, Barber J (eds) Frontiers in molecular biology: molecular genetics in photosynthesis. Oxford Univ Press, pp 1–44
Herrmann RG, Alt J, Schiller B, Widger WR, Cramer WA (1984) Nucleotide sequence of the gene for apocytochrome b-559on the spinach plastid chromosome: implications for the structure of the membrane protein. FEBS Lett 176:239–244
Herrmann RG, Westhoff P, Link G (1992) Biogenesis of plastids in higher plants. In: Herrmann RG (ed) Plant gene research: cell organelles. Springer Verlag, Wien-New York, pp 275–349
Hupfer H, Swiatek M, Hornung S, Herrmann RG, Maier RM, Chiu WL, Searsb(2000) Complete nucleotide sequence of the Oenothera elata plastid chromosome, representing plastome I of the five distinguishable Euoenothera plastomes. Mol Gen Genet 263:581–585
Ikeuchi M, Eggers B, Shen GZ, Webber A, Yu JJ, Hirano A, Inoue Y, Vermaas W (1991) Cloning of the psbK gene from Synechocystis sp PCC 6803 and characterization of photosystem II in mutants lacking PSII-K. J Biol Chem 266:11111–11115
Keren N, Berg A, van Kann PJ, Levanon H, Ohad I (1997) Mechanism of photosystem II photoinactivation and D1 protein degradation at low light: the role of back electron flow. Proc Natl Acad Sci USA 94:1579–1584
Kitamura K, Ozawa S, Shiina T, Toyoshima Y (1994) L protein, encoded by psbL, restores normal functioning of the primary quinone acceptor, QA, in isolated D1/D2/CP47/Cytb-559/I photosystem II reaction center core complex. FEBS Lett 354:113–116
Koop H-U, Kofer W (1995) Plastid transformation by polyethylene glycol treatment of protoplasts and regeneration of transplastomic tobacco plants In: Potrykus I, Spangenberg G (eds) Gene transfer to plants. Springer Verlag, Berlin, pp 75–82
Koop H-U, Steinmüller K, Wagner H, Rossler C, Eibl C, Sacher L (1996) Integration of foreign sequences into the tobacco plastome via polyethylene glycol-mediated protoplast transformation. Planta 199:193–201
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Kretzer F, Ohad I, Bennoun P (1976) Ontogeny, insertion and activation of two thylakoid peptides required for photosystem II activity in the nuclear, temperature sensitive T4 mutant of Chlamydomonas reinhardtii. In: Bücher T (eds) Genetics and biogenesis of chloroplasts and mitochondria. Elsevier/North Holland Biomedical Press, Amsterdam, pp 25–32
Kunkel TA (1985) Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA 82:488–492
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage. T4 Nature 227:680–685
Lind LK, Shukla VK, Nyhus KJ, Pakrasi HB (1993) Genetic and immunological analyses of the cyanobacterium Synechocystis sp PCC 6803 show that the protein encoded by the psbJ gene regulates the number of photosystem II centers in thylakoid membranes. J Biol Chem 268:1575–1579
Mayers SR, Dubbs JM, Vass I, Hideg E, Nagy L, Barber J (1993) Further characterization of the psbH locus of Synechocystis sp PCC 6803: inactivation of psbH impairs QA to QB electron transport in photosystem 2. Biochemistry 32:1454–1465
Morais F, Barber J, Nixon PJ (1998) The chloroplast-encoded alpha subunit of cytochrome b-559 is required for assembly of the photosystem two complex in both the light and the dark in Chlamydomonas reinhardtii. J Biol Chem 273:29315–29320
Morais F, Kuhn K, Stewart DH, Barber J, Brudvig GW, Nixon PJ (2001) Photosynthetic water oxidation in cytochrome b(559) mutants containing a disrupted heme-binding pocket. J Biol Chem 276:31986–31993
Müller B, Eichacker LA (1999) Assembly of the D1 precursor in monomeric photosystem II reaction center precomplexes precedes chlorophyll a-triggered accumulation of reaction center II in barley etioplasts. Plant Cell 11:2365–2377
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nanba O, Satoh K (1987) Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome. Proc Natl Acad Sci USA 84:109–112
Nield J, Funk C, Barber J (2000a) Supermolecular structure of photosystem II and location of the PsbS protein. Philos Trans R Soc LondbBiol Sci 355:1337–1344
Nield J, Kruse O, Ruprecht J, da Fonseca P, Büchel C, Barber J (2000b) Three-dimensional structure of Chlamydomonas reinhardtii and Synechococcus elongatus photosystem II complexes allows for comparison of their oxygen-evolving complex organization. J Biol Chem 275:27940–27946
Ozawa S, Kobayashi T, Sugiyama R, Hoshida H, Shiina T, Toyoshima Y (1997) Role of PSII-L protein ( psbL gene product) in the electron transfer in photosystem II complex 1. Over-production of wild-type and mutant versions of PSII-L protein and reconstitution into the PSII core complex. Plant Mol Biol 34:151–161
Pakrasi HB, Nyhus KJ, Granok H (1990) Targeted deletion mutagenesis of the beta subunit of cytochrome b559 protein destabilizes the reaction center of photosystem II. Z Naturforsch 45:423–429
Palomares R, Herrmann RG, Oelmüller R (1993) Post-transcriptional and post-translational regulatory steps are crucial in controlling the appearance and stability of thylakoid polypeptides during the transition of etiolated tobacco seedlings to white light. Eur J Biochem 217:345–352
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a andbwith four different solvents: verification of the concentration of chlorophyll by atomic absorption spectroscopy. Biochem Biophys Acta 975, 385–394
Regel RE, Ivleva NB, Zer H, Meurer J, Shestakov SV, Herrmann RG, Pakrasi HB, Ohad I (2001) Deregulation of electron flow within photosystem II in the absence of the PsbJ protein. J Biol Chem 276:41473–41478
Rosenblum BB, Lee LG, Spurgeon SL, Khan SH, Menchen SM, Heiner CR, Chen SM (1997) New dye-labeled terminators for improved DNA sequencing patterns. Nucleic Acids Res 25:4500–4504
Sambrook J, Fritsch EF, Maniatis T (1989): Molecular cloning: a laboratory manual (2nd edn). Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Sandona D, Croce R, Pagano A, Crimi M, Bassi R (1998) Higher plants light harvesting proteins. Structure and function as revealed by mutation analysis of either protein or chromophore moieties. Biochim Biophys Acta 1365:207–214
Sanger F, Nicklen S, Coulson AR (1992) DNA sequencing with chain-terminating inhibitors 1997. Biotechnology 24:104–108
Schägger H, von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368–379
Schägger H, von Jagow G (1991) Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem 199:223–231
Stewart DH, Brudvig GW (1998) Cytochrome b559of photosystem II. Biochim Biophys Acta 1367, 63–87
Svab Z, Maliga P (1993) High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci USA 90:913–917
Swiatek M, Kuras R, Sokolenko A, Higgs D, Olive J, Cinque G, Müller B, Eichacker LA, Stern DB, Bassi R, Herrmann RG, Wollman FA (2001) The chloroplast gene ycf9encodes a photosystem II (PSII) core subunit, PsbZ, that participates in PSII supramolecular architecture. Plant Cell 13:1347–1367
Swiatek M, Greiner S, Kemp S, Drescher A, Koop H-U, Herrmann RG, Maier RM (2003) PCR analysis of pulse-field gelelectrophoresis purified plastid DNA, a sensitive tool to judge the hetero- or homoplastomic status of plastid transformation. Curr Genet, in press
Takahashi Y, Matsumoto H, Goldschmidt-Clermont M, Rochaix JD (1994) Directed disruption of the Chlamydomonas chloroplast psbK gene destabilizes the photosystem II reaction center complex. Plant Mol Biol 24:779–788
Thidholm E, Lindstrom V, Tissier C, Robinson C, Schröder WP, Funk C (2002) Novel approach reveals localisation and assembly pathway of the PsbS and PsbW proteins into the photosystem II dimer. FEBS Lett 513:217–222
Westhoff P, Alt J, Herrmann RG (1983) Localization of the genes for the two chlorophyll a-conjugated polypeptides (mol wt 51 and 44 kd) of the photosystem II reaction center on the spinach plastid chromosome. EMBO J 2:2229–2237
Westhoff P, Alt J, Nelson N, Herrmann RG (1985) Genes and transcripts of the ATP synthase CF0 subunits I and II from spinach thylakoid membranes. Mol Gen Genet 199:290–299
Wollman FA, Minai L, Nechushtai R (1999) The biogenesis and assembly of photosynthetic proteins in thylakoid membranes. Biochim Biophys Acta 1411:21–85
Zer H, Vink M, Keren N, Dilly-Hartwig HG, Paulsen H, Herrmann RG, Anderssonband Ohad I (1999) Regulation of thylakoid protein phosphorylation at the substrate level: reversible light-induced conformational changes expose the phosphorylation site of the light-harvesting complex II. Proc Natl Acad Sci USA 96:8277–8282
Zhang L, Paakkarinen V, Suorsa M, Aro EM (2001) A SecY homolog is involved in chloroplast-encoded D1 protein biogenesis. J Biol Chem 276:37809–37814
Zouni A, Witt HT, Kern J, Fromme P, Krauss N, Saenger W, Orth P (2001) Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution. Nature 409:739–743
Acknowledgements
We are most grateful to Ms. Martina Reymers and Ms. Claudia Nickel for expert technical assistence, to Dr. Roberto Barbato for providing antisera against D1, D2, CP43 and CP47, to Dr. Peter Westhoff and Dr. Wolfgang Schröder for antisera against PsbH and PsbW, respectively. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 184, SFB-TR1), the Human Frontier Science Programme (HFSP), the Fonds der Chemischen Industrie, the Department of Energy, USA (DOE), the National Institutes of Health (NIH) and the Israeli Science Foundation (ISF)
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Hagemann
The first two authors contributed equally to this work
Rights and permissions
About this article
Cite this article
Swiatek, M., Regel, R.E., Meurer, J. et al. Effects of selective inactivation of individual genes for low-molecular-mass subunits on the assembly of photosystem II, as revealed by chloroplast transformation: the psbEFLJ operon in Nicotiana tabacum . Mol Gen Genomics 268, 699–710 (2003). https://doi.org/10.1007/s00438-002-0791-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-002-0791-1