Abstract
The focus of this review is a group of structures/organelles collectively known as extracellular vesicles (EVs) that are secreted by most, if not all, cells, varying from mammalian cells to protozoa and even bacteria. They vary in size: some are small (100–200 nm) and others are larger (> 200 nm). In protozoa, however, most of them are small or medium in size (200–400 nm). These include vesicles from different origins. We briefly review the biogenesis of this distinct group that includes (a) exosome, which originates from the multivesicular bodies, an important component of the endocytic pathway; (b) ectosome, formed from a budding process that takes place in the plasma membrane of the cells; (c) vesicles released from the cell surface following a process of patching and capping of ligand/receptor complexes; (d) other processes where tubules secreted by the parasite subsequently originate exosome-like structures. Here, special emphasis is given to EVs secreted by parasitic protozoa such as Leishmania, Trypanosoma, Plasmodium, Toxoplasma, Cryptosporidium, Trichomonas, and Giardia. Most of them have been characterized as exosomes that were isolated using several approaches and characterized by electron microscopy, proteomic analysis, and RNA sequencing. The results obtained show clearly that they present several proteins and different types of RNAs. From the functional point of view, it is now clear that the secreted exosomes can be incorporated by the parasite itself as well as by mammalian cells with which they interact. As a consequence, there is interference both with the parasite (induction of differentiation, changes in infectivity, etc.) and with the host cell. Therefore, the EVs constitute a new system of transference of signals among cells. On the other hand, there are suggestions that exosomes may constitute potential biotechnology tools and are important players of what has been designated as nanobiotechnology. They may constitute an important delivery system for gene therapy and molecular-displaying cell regulation capabilities when incorporated into other cells and even by interfering with the exosomal membrane during its biogenesis, targeting the vesicles via specific ligands to different cell types. These vesicles may reach the bloodstream, overflow through intercellular junctions, and even pass through the central nervous system blood barrier. There is evidence that it is possible to interfere with the composition of the exosomes by interfering with multivesicular body biogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early morphological studies based on observations made by light microscopy, and even the initial observations made with transmission electron microscopy, pointed to the existence of a large and continuously growing number of structures and organelles in eukaryotic cells. Most of the basic knowledge on these structures have been incorporated into the cell biology textbooks (see Alberts et al. 2015). However, advances in the techniques used to observe biological materials, especially in living eukaryotic cells, and the association of morphological with biochemical and molecular techniques have added new structures and organelles to the list of those already well characterized and described in all cell biology textbooks. Although structures such as exosomes, ectosomes, cytonemes, and nanotubes have been observed for many years, only more recently, they have been recognized as participating in cell-to-cell communication processes. Until recently, the participation in communication was usually considered to take place exclusively in gap junctions and in secretory processes in which the mediators are released in the vicinity of the receptors, such as those involved in neuron-to-neuron, neuron-to-muscle cell synapses or at long distances via the action of hormones. Structures that correspond to membrane-bounded vesicles of different sizes secreted by parasitic protozoa will be the focus of this review. Although vesicles can also be secreted by the host cells, especially in those infected by parasites that live within the cell, here, we will emphasize more the vesicles secreted by the protozoa. However, a few examples of secretory activity of the host cells will be presented.
Early studies carried out by Pan working group (Pan and Johnstone 1983, Pan et al. 1985) using reticulocytes demonstrated that inward budding of endosomal membranes resulted in the accumulation of intraluminal vesicles within large multivesicular bodies (Fig. 1). Some of these bodies migrate to the periphery of the cell followed by subsequent fusion with the plasma membrane and release of the vesicles into the extracellular space; these vesicles were then designated as exocytic multivesicular bodies and later on as exosomes (review in Mathivanan et al. 2010). Subsequently, it has been shown that vesicles with different diameters (varying from 80 to 400 nm and even more) can be found in the extracellular space and it was recently suggested to designate them as extracellular vesicles (EVs) (Witwer and Théry 2019). They were found around cells as well as in all biological fluids (Nieuwland et al. 1997). Based on studies carried out by several groups, it is now clear that different types of membrane-bounded vesicles exist in different cell types, probably formed by different mechanisms.
First observations of extracellular vesicles and multivesicular bodies used for externalization of transferrin receptors in reticulocytes. a A vesicular appearance of released antibody-labeled reticulocyte magnification material: 123000g (Pan and Johnstone 1983). b Intracellular localization of multivesicular body magnification × 85,000 (Pan et al. 1985). c Fusion of MVEs with the plasma membrane magnification: × 98,625 (Pan et al. 1985)
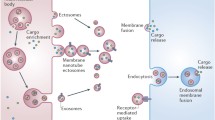
One of them is formed when vesicles are released by cytoplasmic multivesicular-like bodies (MVB) that fuse with the plasma membrane and discharge their vesicular content into the extracellular space. This type of vesicle, whose diameter is in the range of 30 to 130 nm, is presently known as an exosome. It is important to point out that the word “exosome” is also used to designate a large multi-subunit complex that post-transcriptionally regulates gene expression via degradation of mRNAs (Lorentzen and Conti 2006). On the other hand, some vesicles are formed by association of some macromolecular complexes with the inner face of the plasma membrane that leads to membrane budding and vesicular release outside the cell via a mechanism resembling the process by which viral budding occurs. These vesicles have been designated as ectosomes. Another type corresponds to vesicles that are shedded as a final step of the process of capping of surface ligands, that occurs in free cells such as lymphocytes and other cells, including parasitic protozoa such as Entamoeba histolytica (Trissl et al. 1977) and Trypanosoma cruzi (Schmunis et al. 1980) following ligand-receptor binding and mobility.
Structures similar to exosomes and shedding vesicles are also found in Gram-negative bacteria, eukaryotic parasites, and fungi (reviewed in Silverman and Reiner 2011). The released vesicles seem to play some role in the process these organisms interact with the host.
All of the abovementioned vesicles are represented in schematic Fig. 2.
Schematic of the major types of secretory extracellular vesicles. a Transmission electron microscopy (MET) of the extracellular release of exosomes (Pan et al., 1981). b TEM of multivesicular body (note vesicles in the interior). c TEM of the initial stage of microvesicle budding. d TEM of the intermediate stage of budding microvesicles. e TEM of the late stage of microvesicle budding
The Ectosomes
The existence of extracellular vesicles found in human plasma and derived from the plasma membrane of platelets was initially reported by Wolf in 1967 and since then has been reported in several other biological systems (Review in Frey and Gaipl 2011). The term ectocytosis was coined by Stein and Luzio (1991) to describe a complement-triggered shedding of right-side-out plasma membrane vesicles derived from the surface of polymorphonuclear leukocytes. The formation of these vesicles is in some way similar to that reported for the outer membrane of Gram-negative bacteria, a process that seems to be involved in the mechanism by which virulence factors can be released and transmitted to other bacteria (Vidakovics et al. 2010). Ectosome formation may involve participation of cytoskeletal components associated with specific regions of the plasma membrane’s cytoplasmic face. Upon stimulation, a protrusion of the membrane forms in a process resembling viral particle budding with concurrent enrichment of cholesterol and diacyglycerol on the ectosome membrane, thus indicating a specific sorting of lipids (review in Sadallah et al. 2011). The released vesicles may contain physiologically active effectors that play important roles in a variety of biological processes such as inflammation, hemostasis, thrombosis, angiogenesis, cancer, and vascular reactivity (review in Sadallah et al. 2011). Theoretically, these vesicles may vary significantly in size from 200 to 1 μm.
The Exosomes
These are extracellular vesicles, usually with a uniform size (Kastelowitz and Yin 2014) originated from the endocytic pathway of the cell. The biogenesis of an exosome consists of four stages: (1) initiation (selection of molecules such as nucleic acids, proteins, and others); (2) endocytosis (invagination of plasma membrane); (3) MVB formation (formed through early endosome maturation); and (4) secretion (Théry et al. 2002). With respect to this process, it has been well established that in addition to the classical secretory pathway that is constitutive and regulated upon appropriate stimuli, the endocytic network also presents an alternative secretory pathway (reviewed in Van Niel et al. 2006; Schorey and Bhatnagar 2008). There is always involvement of MVBs, considered as intermediate and well-defined compartments that are formed when invagination of the membrane lining the endosome takes place. The inward budding of the endosome membrane generates the accumulation of nanovesicles (30–150-nm diameter) inside the late endosome subset of MVB (Huotari and Helenius 2011). This process happens in at least two situations: (1) when ubiquitination of endosomal proteins occurs (Katzmann et al. 2004) or (2) when membrane proteins undergo agglutination induced by binding to some ligands (Vidal et al. 1997; reviewed in Piper and Katzmann 2007). In both cases, the participation of the endosomal sorting complexes required for transport (ESCRT) machinery is important for endosomal membrane invagination (Hurley 2008; Hurley and Hanson 2010). Recently, an ESCRT-independent pathway involving ceramides and the neutral sphingomyelinase 2 has also been characterized (Bissig et al. 2013). Remarkably, another sphingomyelinase, the acid sphingomyelinase, is involved in exosome formation following translocation of the enzyme to the plasma membrane outer leaflet at which point it generates ceramides that trigger microvesicular budding (Bianco et al. 2009). It is important to point out that in most of the cases, the MVBs fuse with the lysosomes and thus, an MVB’s cargo is then degraded. In other situations, however, the MVB fuses with the plasma membrane, thus releasing the exosomes (Denzer et al. 2000). The fusion process may be triggered by calcium ionophores (review in Lakkaraju and Rodriguez-Boulan 2008), may be regulated by p53, and may be under the control of the cytoskeletal activation pathway. The MVBs move along the microtubules via a dynamic cholesterol-regulated process (Huotari and Helenius 2011). Although exosomes are formed from the MVBs’ intraluminal vesicles, there are some differences between them. Intraluminal vesicles are generated in an acidic pH environment (pH 5.5) inside the MVB, and they are then released into a neutral pH environment to become exosomes. Such a pH variation might affect their membrane organization because it has been shown that the membrane rigidity of exosomes increases between pH 5 and 7 (Laulagnier et al. 2004).
The available data indicate that exosomes are secreted by a large number of cells into culture medium in vitro, interstitial fluids, urine, milk, and even in snake venom (Ogawa et al. 2008a, b).
In relation to the strategies applied for detecting and isolating exosomes, a lot of techniques are utilized: (1) transmission electron microscopy, (2) ultracentrifugation, (3) density-gradient separation, (4) immunoaffinity capture (Greening et al. 2015), and (5) microfluidic systems (Yang et al. 2017). Based on the small size and low density of exosomes, ultracentrifugation is the most developed and commonly used method for their isolation. This technique employs an exceedingly high centrifugal force, which can reach 100,000g, in order to precipitate subcellular components or even macromolecules. However, it is very time-consuming, and the resulting exosome purity is poor (Momen-Heravi et al. 2013). As technology improves, new separation techniques such as sequential filtration have emerged (Heinemann and Vykoukal 2017). Considering the importance of exosomes, a low-cost, hypersensitive, and simple detection method is desirable. Relatively new stochastic techniques for exosome detection include photoactivated localization microscopy (PALM) or stochastic optical reconstruction microscopy (STORM). PALM and STORM are based on single-molecule localization to track exosomes, which can be observed down to the nanometer level and allow for the visualization of intracellularly incorporated exosomes (Chen et al. 2016).
Exosomal protein and nucleic acid contents have been studied in detail. Isolated extracellular vesicles have been analyzed using different techniques, including mass spectrometry. Several proteins such as tetraspanin 29 and 30, Rabs, annexins, adhesion molecules, cytoskeletal proteins, and many others have been identified and compiled in the ExoCarta consensus database (Keerthikumar et al. 2016). Rab 27A and B guanosine triphosphatases (GTPases) have been involved as exosome effector proteins (reviewed in Pfeffer 2010). More importantly, it has been shown that exosomes produced by mast cells contain about 1300 mRNAs and 121 microRNAs but are without DNA or rRNA (Valadi et al. 2007). Circular RNAs, a type of non-coding RNAs with a closed annular structure, have also been found in exosomes. There are more than 3000 kinds of such RNAs that are located in the cytoplasm and the nucleus (Hou et al. 2018).
In relation to lipid composition, it is known that the membrane of the exosomes is rich in cholesterol, phosphatidylserine, and ceramide (Yáñez-Mó et al. 2015). The exosomes also present a defined repertoire of glycans, including sialic acid residues that play some role in their recognition by the cells (reviewed in Williams et al. 2018).
It has been shown that once released into the extracellular space, the exosomes may bind to the surface of cells and fuse with it (Raposo et al. 1996; Morelli et al. 2004) with subsequent opening of the vesicle inside the target cell or degradation of the exosome membrane (review in Record et al. 2011). Using exosomes containing CD63 that was fused to either green fluorescent protein (GFP) or the red fluorophore mCherry, it has been shown that they are quickly incorporated by endocytosis that occurs at the base of filopodia (Heusermann et al. 2016). This incorporation was preceded by surfing on the filopodia. Costa-Verdera et al. (2017) demonstrated that exosomes are incorporated by endocytosis, using predominantly clathrin-independent endocytosis pathways and macropinocytosis. After internalization, exosomes are transported as intact vesicles within endosomes and then move toward the lysosomes. They may also fuse with the plasma membrane releasing their contents into the cytosol. They may also move along the ER before reaching the lysosomal compartments. It has even been considered that exosome traffic shares mechanisms similar to those presented by some viruses. This possibility demonstrates a novel mode of communication between cells in addition to other well-characterized structures such as synapses and gap junctions. For instance, it was shown that incubation of human cells with exosomes from mouse cells led to synthesis of mouse proteins from the mRNAs present in the exosomes. This indicates a process of horizontal gene transfer (Valadi et al. 2007; review in Lakkaraju and Rodriguez-Boulan 2008; Izquierdo-Useros et al. 2011). It has also been shown that exosomes content can be modified by the cell response to stimuli. Tumor virus Karposi’s sarcoma–associated herpesvirus hijacks the signaling pathway to induce cell proliferation, migration, and transcriptome reprogramming in cells not infected with the virus through the release of exosomes with different content (McNamara et al. 2019). In relation to metabolic regulation, exosomes contribute to cell–cell and inter-organ communication, and the transfer of miRNAs has been particularly highlighted in cellular signaling. Exosomes have been strongly implicated in the communication between key metabolic organs during metabolic homoeostasis, and dysregulation seems to be important for the cross-talk between adipocytes and insulin resistance regulation (reviewed by Samuelson and Vidal-Puig 2018). It has been shown that viruses, prions, and other infectious materials can be transmitted from one cell to the other via the exosomes (Van Niel et al. 2006; Février and Raposo 2004; Pelchen-Matthews et al. 2004; Meckes Jr et al. 2010). They also act as vectors for mRNAs, miRNAs, virulence factors, and different types of mediators (reviews in Record et al. 2011; Silverman and Reiner 2011). One good example of the role played by exosomes in RNA transfer was reported in a recent study by Bukong et al. (2014). They demonstrated that exosomes isolated from the sera of chronic hepatitis C virus (HCV)-infected patients or supernatants of HCV-infected cells containing HCV RNA could mediate viral receptor-independent HCV transmission to hepatocytes. These authors also showed that HCV transmission was suppressed by some cellular proteins such as heat shock protein 90 (Hsp90) that inhibited proteins and vacuolar H+-ATPases, both of which significantly suppressed exosome-mediated HCV transmission to naïve cells. More recently, Zhou et al. (2018) showed that flavivirus RNA and proteins can be transferred via exosomes from tick arthropods to human-skin keratinocytes and blood endothelial cells. The available data indicate that virtually all cells can produce exosomes. A good example is what happens in the male reproductive system where studies carried out from insects to man show the presence of exosomes in the prostate secretion. For instance, it has been shown that prostate-derived exosomes promote motility during sperm capacitation (see Alberts et al. 2015) and maturation (Sullivan R and Saez 2013). More importantly in the context of this short review, it has been shown that the exosomes fuse specifically to the head–neck region of the sperm. In the case of Drosophila, secreted exosomes fuse with sperm in vivo in females after mating and also interact with female reproductive tract epithelial cells (Corrigan et al. 2014). Fu et al. (2017) showed that exosomes secreted by virally infected cells selectively packaged high-level miR-146a. This miR can be functionally transfected to and facilitate exosomal RNA replication in the recipient cells by suppressing interferon response. Since exosomes play a pivotal role in mediating intercellular communications and package delivery and can act as diagnostic biomarkers, some research groups have discussed the possibility of these exosomes acting as possible drug delivery vehicles based on their nanometer size range and capability to transfer biological materials to recipient cells. This is because the unique biocompatibility, high stability, and adjustable targeting efficiency can make exosomes an attractive and potentially effective tool for drug delivery mainly in cancer therapy (reviewed Kibria et al. 2018).
Exosomes can be released from different regions of the cell surface, including the basolateral region, as has recently been shown in lymphatic endothelial cells (Brown et al. 2018). The extent of exosome secretion can be stimulated in the presence of inflammatory cytokines. Exosomes from both dendritic and B cells express both major histocompatibility class (MHC) class I and II molecules in addition to costimulatory molecules that can promote T cell activation (Chaput et al. 2006; Bhatnagar et al. 2007).
Recent studies have suggested that exosomes are potentially interesting in regenerative medicine and may constitute a platform for membrane-associated therapeutics protein delivery (Keshtkar et al. 2018; Yang et al. 2017). It has been shown that mesenchymal stem cell-derived exosomes show renoprotective effects in kidney injury models probably due to activation of tubular cell proliferative pathway via horizontal transfer of mRNAs (Bruno et al. 2016). The same trend has been reported for cardiovascular and neurological diseases (reviewed in Keshtkar et al. 2018).
Another interesting approach is the expression of membrane proteins on the surface of exosomes during their biogenesis. Examples include expression of epidermal growth factor receptor (EGFR) on vesicles that allow transfer of oncogenic activity by activating signaling pathways (reviewed in Yang et al. 2017).
EVS in parasitic protozoa
The first data inferring the release of microvesicles by protozoan parasites date from 1912 (Schepilewsky 1912) where “appendices” were observed in T. brucei. Posteriorly, Silveira et al. (1979) demonstrated the secretion of plasma membrane vesicles by T. cruzi epimastigote forms. However, up to the moment of these descriptions, these vesicles were not known as exosomes and so few functions were assigned to them. A landmark paper on the presence of exosomes in parasites was published by Silverman and Reiner (2011) with Leishmania donovani. Posteriorly, a lot of papers demonstrate the same biological event with another parasitic protozoa. Below, the main findings regarding the discovery and studies in the most distinct disease caused by protozoa are reported (Table 1).
Leishmania sp.
Currently, it is known that exosomes from L. mexicana, L. donovani, L. major, and L. braziliensis have been shown to play a crucial role in host–pathogen interactions inducing modifications in non-infected neighboring cells or acting as antigen presenters for the immune system (Schorey et al., 2015). However, as mentioned above, studies demonstrating the participation of EVs in this protozoan date back to 2010 (Silverman et al. 2010). In some way, previous studies carried out with the pathogenic fungus Cryptococus neoformans (Rodrigues et al. 2008) and the free living nematode Caenorhabditis elegans (Liégeois et al. 2006) inspired Silverman and co-workers to further explore the initial finding that only 14% of the proteins detected in the secretome of Leishmania donovani contained an N-terminal classical secretion signal peptide (Silverman et al. 2008). Proteomic analysis of the secretome showed that all proteins for which there was a Leishmania ortholog resembled those previously identified in B lymphocyte-secreted exosomes (Silverman et al. 2008). Using differential centrifugation, they isolated a fraction containing exosomes with a diameter of 30 to 70 nm, specific density of 1.08 to 1.15 g/ml, and exosomal markers such as HSP70 and 90 and elongation factor-1α. Over 400 proteins were identified by liquid chromatography-tandem mass spectrometry and around 52% of them were mammalian exosomal protein orthologs. Analysis of the temperature and pH effects on exosomal release showed that exosomal release increased significantly in response to temperature elevation. However, the acidic environment did not influence bulk exosomes released at 37 °C but affected vesicular cargo. It is important to point out that the proteins found in the exosomes account for about 52% of the total proteins found in the protozoan secretome, thus indicating the relevance of the process in the case of Leishmania. The seminal work by Silverman et al. (2010) also analyzed potential functional exosome roles during Leishmania interactions with host cells. First, using parasites expressing GFP, it was found that they secrete labeled exosomes. Incubation of labeled parasites with macrophages showed the presence of parasites within the cells in addition to exosome incorporation by non-infected macrophages. A ring of actin was observed by fluorescence microscopy around the ingested exosomes. Labelling of non-infected cells gradually disappeared probably due to protein degradation. Based on electron microscopy images, it was suggested that vesicles presented the same morphology as budded exosomes from the phagolysosomal membrane of internalized Leishmania (Silverman et al. 2010). However, this needs to be proven since exosomes are not usually released into the cytoplasm, and it is not possible to discard the possibility that the images correspond to areas of lysosomal fusion with the parasitophorous vacuole. Based on the data obtained by Silverman et al. (2010), it was suggested that Leishmania “uses exosomes to deliver molecular messages to infected as well as neighboring uninfected macrophages.” The same authors also showed that macrophages that were allowed to interact with exosomes secreted IL-8 in a dose-dependent manner as observed when the cells were infected with parasites; this finding indicated that the exosomes may modify specific cytokine profiles of treated macrophages. This is an important observation since it may have implications in the leishmaniasis pathogenesis. IL-8 release may lead to neutrophil recruitment to the site of infection (Peters and Sacks 2009). It is important to point out at this point that Silverman et al. (2010) performed the experiments using mainly L. donovani but also observed exosomes in Leishmania major. In addition, they mention that RNAs were detected in the Leishmania exosomes. Using macrophages infected with L. mexicana, Hassani and Olivier (2013) isolated released vesicles and identified the presence of parasitic zinc metalloprotease, GP63, in the vesicles. It was also shown that GP63-containing vesicles induced phosphorylation of signaling proteins in addition to transcription factor translocation into the host cell nucleus. More recently, Ghosh et al. (2013) reported that exosomes secreted by L. donovani caused a reduction in miR-122 activity in hepatic cells. miR-122 is a microRNA abundantly expressed in the liver that comprises > 70% of the liver microRNA pool and is largely responsible for liver homeostasis and lipid metabolism, modulating a wide range of liver functions. L. donovani infection downregulates miR-122 and genes involved in cholesterol biosynthesis in infected mice livers in order to reduce serum cholesterol. On the other hand, Leishmania mettaloprotease gp63, a component of the Leishmania exosomes, is internalized and cleaves Dicer1 preventing an active formation of miRNP that culminates in the inhibition of mR122. The authors suggested that exosomes released by infected liver Kupfer cells could be transferred to hepatocytes where they could act on Dicer1. Since the restoration of Dicer1 expression in parasite-infected livers rescues miR-122 expression and reduces liver parasite burden, these factors are estimated to be related to Leishmania infectivity (Ghosh et al. 2013).
L. donovani internalization and intracellular survival in human macrophages are dependent of a Leishmania chaperonin 10 (CPN10), which is secreted in exosomes. This chaperonin is found in host cell cytosol and negatively regulates the rate of parasitic uptake by macrophages while being required for intracellular survival. Due to this dual function, it is believed it may have immunosuppressive properties (Colineau et al. 2017). Also, involved in intracellular survival is a tyrosine phosphatase secreted by exosomes of L. major (LmPRL-1). This enzyme shows a strong structural similarity to the human phosphatases of regenerating liver (PRL1, PRL 2, and PRL 3) that regulate the proliferation, differentiation, and motility of cells (Leitherer et al. 2017). More recently, Castelli and colleagues (2019) observed that amastigotes and promastigotes of L. infantum are capable to produce and secrete EVs with different sizes (122 ± 56 nm promastigotes and 115 ± 65 nm amastigotes). However, both types are capable to increase human macrophages motility and an overproduction of interleukin IL-10 and IL-18 reduction in the same macrophages line. Motility of immune human cells is important to initiate and spread of infection, since macrophages are recruited to site of infection. With this modulation of chemostatic and immune system, it is believed that exosomes can be used to establish the infection. Atayde et al. (2015) demonstrated that Leishmania constitutively secrete exosomes in the lumen of the sand fly midgut. More interestingly, these exosomes are ingested together with the infective promastigotes during insect bite. It was also shown that exosome’s presence exacerbated the lesions, probably due to increase in production of inflammatory cytokines such as interleukin (IL)-17a. Figure 3 is a representation of exosomes released by Leishmania promastigotes and amastigotes.
Exosomes released by promastigotes (a) and amastigotes (b) from Leishmania donovanii in extracellular environment (Silverman et al. 2008) and intracellular environment (c–e). c TEM demonstrating the release of vesicles by intracellular promastigotes in the macrophages parasitoid vacuole; d and e arrows indicate the release of exosomes from promastigotes into the cytoplasm of macrophages (Silverman, 2011)
Trypanosoma cruzi
In the case of Trypanosoma cruzi, studies carried out by Schmunis et al. (1980), using transmission electron microscopy, were the first to show the presence of vesicles shed from bloodstream trypomastigotes. This phenomenon was observed in the process of capping of antigen-antibody complexes where parasites were incubated with serum-containing antibodies that recognize parasite antigens exposed on the cell surface. It is important to point out that such processes were not observed with epimastigotes. Later on, Gonçalves et al. (1991) reported the release of small (20–80 nm) vesicles, equivalent to what has been designated as exosomes, throughout the surface of tissue culture-derived trypomastigotes (those obtained in the supernatant of infected cell cultures). Labelling for Tc85 (a member of the superfamily gp85-trans sialidases that is anchored to the plasma membrane via GPI-anchors) was observed in these vesicles using an immunocytochemical approach in which gold-labeled antibodies were used. In addition, these vesicles were isolated by centrifugation and molecular sieving and were shown to contain GP85. They were subsequently injected into mice followed by a T. cruzi challenge. An increase in parasitaemia with subsequent exacerbation of heart lesions and inflammation was reported (Torrecilhas et al. 2009). Recently, de Pablos-Torró and colleagues (Pablos Torró et al. 2018) suggested that extracellular vesicles secreted by T. cruzi can be responsible for parasite–parasite and parasite–host cell transmission of cellular components (proteins, RNAs) through paracrine and/or juxtacrine signaling, since they develop in a compartmentalized environment, increasing the likelihood of EV docking and delivery within the host cell. Further analysis in epimastigotes by Garcia-Silva et al. (2010a, b) showed the presence of a homogenous population of small RNAs derived from mature tRNAs that accounted for about 25% of the small RNA population in T. cruzi. The levels of these tRNA-derived halves (tsRNAs) were more pronounced if the epimastigotes were submitted to nutritional stress, which usually triggered differentiation into infective trypomastigotes. It is important to point out that the tsRNAs were localized using an in situ hybridization approach to an organelle with morphological characteristics similar to reservosomes (Garcia-Silva et al. 2010b). The reservosomes had been previously shown to accumulate macromolecules ingested through the endocytic pathway in addition to some proteases, especially cruzipain, synthesized by the parasite (review in De Souza et al. 2009). Subsequently, co-localization studies showed that tsRNAs were localized in clathrin-associated organelles and with the T. cruzi argonate protein TcPIWI-tryp in uncharacterized cytoplasmic and in Golgi-like vesicles (Garcia-Silva et al. 2013). When epimastigotes were submitted to nutrient starvation, vesicles were released from reservosome-like structures that touch the cytoplasmic face of the plasma membrane. These vesicles presented a diameter of 20 to 200 nm. Two classes of vesicles, possibly exosomes and ectosomes, have recently been analyzed in more detail (Bayer-Santos et al. 2013). They carry small tRNAs and TcPIWI-tryp proteins. In addition, they are internalized by other parasites of the same population, thus suggesting that at least tRNA-derived small RNAs can be transferred from one parasite to another one. The vesicles also positively interfered with metacyclogenesis in vitro, a process in which epimastigotes transform into trypomastigotes. The vesicles can also be incorporated by mammalian cells susceptible to T. cruzi infection. Their incorporation via electroporation by cells that usually are not infected with T. cruzi such as K562 cells renders them susceptible to infection. In addition, incorporation of the vesicles by mammalian cells led to changes in gene expression as assessed by microarray assays. A large set of genes in HeLa cells were differentially expressed upon incorporation of T. cruzi-derived extracellular vesicles. The elicited response caused mainly a modification of host cell cytoskeleton, extracellular matrix, and immune responses pathways. Some genes were also modified by the most abundant tRNA-derived small RNAs included in extracellular vesicles. These data suggest that T. cruzi-secreted EVs could be relevant players in early events of the parasite–host cell interplay. Several studies have implicated secreted vesicles in the process of genetic information (such as miRNAs and mRNAs) and protein delivery between cells, rendering them important components of the process of cell-to-cell communication (Ogawa et al. 2008a, b). Exosomal mRNAs and microRNAs were completely functional in recipient cells, thus playing pivotal roles in cell-to-cell communication (Van Niel et al. 2006). We can then speculate that extracellular vesicles and their cargo could represent a route of intercellular communication delivering “molecular messages” to other cells. This T. cruzi-facilitated intercellular communication aimed at inducing coordinated responses in order to assure parasite survival through both the emergence of infective forms and the establishment of a cellular environment able to facilitate infection. This idea is further supported by the observation that T. cruzi trypomastigotes invade 5-fold as many susceptible cells when these are pre-incubated with purified parasite EVs (Ramirez et al. 2017). Recently, it was shown that extracellular amastigotes of T. cruzi also release 100–200 nm vesicles to the extracellular medium and they associate to each other forming “vesicle trays” (Bonfim-Melo et al. 2018) that present the carbohydrate epitope recognized by a monoclonal antibody specific to the carbohydrate component of Ssp-4, an important protein of the cell surface of amastigotes (Florentino et al. 2018). The secreted vesicles were isolated from the medium by ultracentrifugation (Bayer-Santos et al. 2013). It is important to point out that Ssp4 is also released in a free form. However, the vesicles are secreted mainly at sites of T. cruzi amastigotes-host cell interaction that appears as a phagocytic cup (Barros et al. 1996; Bonfim-Melo et al. 2018). It was also reported that amastigotes living in a parasitophorous vacuole containing Leishmania amazonensis also release EVs (Pessoa et al. 2016).
Bayer-Santos et al. (2014) isolated vesicles from T. cruzi epimastigotes and metacyclic trypomastigotes from the Dm28c strain and showed that they contained a wide variety of RNA molecules, including rRNA, mRNAs, and small RNAs. They also found differences in composition between vesicles isolated from the two developmental stages of T. cruzi. For instance, while tRNA comprised 35% of all small RNAs from epimastigotes, this percentage was only 6.5% in bloodstream trypomastigotes.
On the other hand, Nogueira et al. (2015) confirmed the release of vesicles by trypomastigotes of four different T. cruzi strains (Y, Colombiana, CL-14, and YuYu) and found that YuYu parasites released more vesicles than the other strains. These vesicles were richer in proteins, while those from the Y strain showed more alpha-galactosyl containing glycoconjugates. Differences in the ability of the vesicles from different strains to induce innate and chronic immune response have been reported.
The available information indicates that all strains of T. cruzi release EVs (exosomes). However, they seem to differ from each other. For instance, trypomastigotes from the classic CL Brener and Colombia strains produced a low amount of vesicles when compared with parasites of the G, Sylvio X10/6, and Y strains (Wyllie and Ramirez 2017). These authors also showed that differences exist in the extent of vesicular release during the interaction of metacyclic trypomastigotes of different strains with host cells. They compared strains that present differences in susceptibility to complement-mediated lysis such as the G strain (TcI), which is very sensitive, and the Y strain (TcII), which is resistant. Vesicles released during the interaction of Y strain trypomastigotes with Vero cells and THP-1 monocytes increased parasite resistance to complement-induced lysis from 50 to 80% and parasite invasion to around 50%. In the case of the G strain, the vesicles caused a doubling of both the resistance to complement-mediated lysis and cell infection. It is important to point out that vesicles from one strain did not interfere with the complement-mediated lysis or the infection capacity of the other strain (Wyllie and Ramirez 2017).
From the previous comments, it is clear that significant information were obtained concerning the vesicles released by T. cruzi. However, further studies are necessary in order to clarify the role played by the small RNAs found in the vesicles on the life cycle and behavior of different strains of T. cruzi. Recent studies have revealed that rRNA-derived small RNAs are processed by special enzymes and might play important roles in heterochromatin assembly and transcription regulation (Li et al. 2012; Cam et al. 2005). It is also important to carry out a detailed analysis of the vesicles produced by the different strains.
In view of (a) electron microscopy images showing release of EVs by trypomastigotes in addition to intracellular amastigotes, (b) the presence of some key proteins in the EVs, such as transialidase and mucin, and (c) the presence of RNA molecules, which contain genetic information and that may interfere with transcription processes, we may speculate that vesicles play some role in T. cruzi biology. EVs have important functions as (a) facilitate parasite invasion of the host cell, (b) immunomodulate the host response, and (c) help the parasite to evade this response. Retana Moreira et al. (2019) observed that EVs isolated from T. cruzi Pan4 strain are capable to raise intracellular Ca2+ levels, altering the actin filaments and arresting the cell cycle at the G0/G1 phases. They may also play a role in the pathogenesis of Chagas disease in which inflammatory processes and host cell death occur as a consequence of parasitic presence, factors released by the parasites, and molecules produced by different host cells that are part of the immunological response. In Fig. 4, examples of T. cruzi vesicles are shown.
Trypanosoma brucei
In the case of African trypanosomiasis, early studies reported the presence of surface appendages (Schepilewsky 1912; Babudieri and Tomasini 1962) that were subsequently visualized by electron microscopy (Vickerman and Luckins 1969). These structures were described as plasmanemes associated with the flagellar membrane that was released from the parasite. More recently, it has been shown that that these structures, now designated as nanotubes, which constitute highly dynamic filamentous structures that may reach a length of 20 μm, bud from the flagellar membrane and subsequently vesiculate to form extracellular vesicles with a mean diameter of 80 nm (Szempruch et al. 2016a, 2016b). Most probably, we are facing here a new mechanism of formation of EVs that do not form as a typical exosome or ectosome, but by a regular constriction of an extracellular tubule-like structure, with subsequent release of typical EVs. These structures were restricted to the posterior region of the protozoan and lacked the structures found within the flagellum of trypanosomatids, such as the axoneme and the paraflagellar rod. The formation of these vesicles could be induced by stress conditions or addition of complement-activated serum. These vesicles are able to fuse with lipid bilayers and the flagellar pocket and even with erythrocytes transferring their lipids and proteins. It is important to point out that these structures were previously seen in the blood of infected mice (Ellis et al. 1976; Wright et al. 1970). Proteomic analysis of the isolated vesicles showed the presence of a large number of proteins, including flagellar proteins in addition to some virulence factors. Following fusion with erythrocytes, these cells are rapidly cleared form the blood resulting in animal anemia. In 2010, Geiger et al. carried out a detailed proteomic analysis of the proteins secreted by T. brucei and identified 444 proteins. Bioinformatic analysis revealed that a significant number of the secreted proteins did not present the classical transit peptide, thus indicating a different sorting pathway to reach the extracellular milieu. Indeed, many of these proteins could be identified in vesicles isolated from the extracellular medium. More recently, Eliaz et al. (2017) further analyzed the vesicles produced by T. brucei and presented evidence for the existence of two independent processes: (1) the first one corresponded to vesiculation produced in connection with the flagellar membrane. These vesicles are larger, with a diameter of 300 nm and (2) the second corresponds to exosomes that form in typical cytoplasmic multivesicular structures via the endosomal sorting complex required for transport (ESCRT) machinery. Indeed, RNAi silencing of Vps36, an ESCRT component, compromised exosome secretion but not the formation of vesicles originating from the nanotubes, thus indicating that they have different origins. On the other hand, exosome secretion can be enhanced by parasite stress because it affects trans-splicing of mRNAs. The authors also purified exosomes and labeled them with the lipophilic dye, carbocyanine (DL) that was incorporated into the vesicle membrane bilayer. After using the labeled exosomes, it was shown that they were incorporated by parasites and that they interfered with the previously characterized process of “social motility,” thus enhancing nanotube repulsion between parasites engaged in such a physiological process. Figure 5 demonstrates EVs secreted by T. brucei.
Extracellular vesicle in T. brucei. a Scanning electron microscopy (SEM) of T. brucei stimulated to exosome production (Geiger et al. 2010). b TEM of spherical units similar to extracellular vesicle in T. brucei surface (black arrow) (bar = 500 nm). c Free extracellular vesicle (red arrows) at the trypanosome surface visualized by SEM (bar = 400 nm). b and c Szempruch et al., 2016
Plasmodium sp.
In the case of malaria parasites, circulating vesicles were detected in the blood of patients with malaria (review in Sampaio et al. 2017). Combes et al. (2004) reported an elevated level of vesicles derived from endothelial cells in the plasma of a Malawi children with severe P. falciparum malaria complicated with coma. As previously mentioned, exosomes were first described in reticulocytes in which they allowed remodeling of the plasma membrane with the elimination of some proteins on the way to differentiating into erythrocytes. On the other hand, reticulocytes are the cells preferentially invaded by some malarial parasites as P. yoelii and more importantly, P. vivax, one of the most important agents of human malaria. High levels of vesicles were reported in the blood of patients infected with P. vivax (Campos et al. 2010; Nantakomol et al. 2011), P. malariae (Nantakomol et al. 2011), and P. falciparum (Pankoui Mfonkeu et al. 2010). Using P. berghei, it was also shown that vesicles derived from erythrocytes induced macrophage activation in vitro (Couper et al. 2010). One important contribution was made by Martin-Jaular et al. (2011), who described the presence of plasmodium proteins in exosomes isolated from reticulocytes of mice experimentally infected with P. yoelii. The exosomes were shown to present a mean diameter of 56.8 nm, to contain proteins such as Lamp 1 (CD107a), which originate from an internal compartment and not from the reticulocyte plasma membrane, and do not contain CD 401 and CD133, which are considered to be vesicular markers and membrane particles, respectively. Proteomic analysis of the isolated exosomes showed the presence of a large number of proteins such as HSP8 and HSP90, previously shown to be found in exosomes, and 31 of these proteins originated from the parasites such as merozoite surface proteins and rhoptry-associated protein 1, among others. The same group also showed that mice immunized with the isolated exosomes elicited an IgG response with antibodies that recognized P. yoelii-infected red blood cells. Immunized mice challenged with a lethal P. yoelii showed an attenuated infection with lower parasitaemia, increased survival time conferring complete and long-term protection (close to 85%) in the tested mice, and altered parasitic tropism toward reticulocytes that increased from 2 to 63%. It is important to point out that the authors were able to isolate the exosomes from the supernatant of reticulocytes maintained in vitro for 24 h in a culture medium. Taking together the results presented by Martin-Jaular et al. (2011), it was clearly shown that the exosomes they isolated were completely different from other vesicles isolated from the blood of humans or animals infected with malarial parasites.
What about the so-called EVs found in the blood of humans and animals infected with Plasmodium? Mantel et al. (2013) observed three different population of vesicles in the supernatant of a culture of human erythrocytes infected with P. falciparum with sizes varying from 100 to 400 nm. Proteomic analysis showed that the most abundant proteins found were components of the erythrocyte lipid raft components such as stomatin, band 3, and several carbonic anhydrases, thus indicating that the vesicles originated from the erythrocyte plasma membrane. Vesicular markers such as ARF-6 and V PS4 were also found. About 30 parasite proteins were also found in the EVs, including some such as SBP1, Rex 1-2, MAHRP1-2, and PfMC-2TM that are usually associated with the Maurer clefts and others that are associated with the parasitophorous vacuole membrane and erythrocyte-binding proteins used by the parasites to enter erythrocytes. Based on these information, Mantel et al. (2013) suggested that the vesicles arise by blebbing from specific sub-domains within the erythrocyte membrane and contain proteins of the erythrocyte membrane and parasite proteins present on the erythrocyte surface in addition to those found in the Maurer clefts. They also showed that vesicles isolated from the supernatant of P. falciparum cultures act as messengers for gametogenesis induction.
Regev-Rudzki et al. (2013) further analyzed P. falciparum exosomes. Based on experiments in which they provided evidence for DNA-dependent transfer of drug resistance between parasite population, they hypothesized that communication between the parasites did occur. Experiments were reported that discarded the possibility that such a process took place by direct interaction of the cells and could occur over long distances in a process in which cytoskeletal components, including microtubules and actin filaments, were involved. Since this process could be inhibited by treatment of the cells with heparin, which has been shown to suppress cell vesiculation, they hypothesized that exosomes could be involved in such a process, occurring mainly in the ring stage of the asexual P. falciparum life cycle. The exosomes were visualized by atomic force and transmission electron microscopy and appeared as vesicles with a mean diameter of 70 nm. Gene KO experiments showed that PfEMP1, a parasite protein that traffics to the Maurer clefts via budding of the parasitophorous vacuole membrane, is not required for efficient between-parasite communication. However, gene KO of PfPTP2, a protein that functions in the budding of vesicles from the Maurer clefts and the release of the extracellular vesicles, reduces cell-to-cell communication. In a seminal paper by Regev-Rudzki et al. (2013), it was also reported that communication between co-cultures of P. falciparum lines led to a disappearance of blood stages over 2 weeks with replacement by gametocytes, a developmental stage involved in the link between the mammalian and insect portions of the life cycle, which increased up to 17-fold in the culture. This observation is in close agreement with those reported by Mantel et al. (2013) and was previously discussed. Recently, Abdi et al. (2017) demonstrated that extracellular vesicles of P. falciparum were enriched in proteins found within the exomembrane compartments of infected erythrocytes such as Maurer’s clefts in addition to the secretory endomembrane compartments in the apical end of the merozoites. This finding suggests that these proteins played a role in parasite–host interactions. Using bioinformatics as a tool, Jiang et al. (2018) described a complex in P. falciparum that was composed of 10 to 11 subunits, called an exosome-like complex that appears to be directly involved in the blood infection process. Sampaio et al. (2018) showed that PfEMP1 was present only in EVs from early ring-stage parasites, many hours before it appeared on the surface of infected erythrocytes. Using the murine model of malaria, miRNAs have been found in circulating EVs. It was found that miR-16 and miR-451 decreased while miR-27a and miR-150 increased in response to infection. The level of these miRNAs changes following malaria treatment (see Cohen et al. 2018 for further analysis).
Toxoplasma gondii
Recently, for the first time, Li and colleagues isolated and identified exosomes secreted by T. gondii (Li et al. 2018a) using electron microscopy, nanoparticle tracking analysis, and Western blotting. This group demonstrated that at higher concentrations, T. gondii exosomes could reduce the host macrophage viability (Li et al. 2018a). Macrophages exposed to a lower concentration of exosomes can stimulate the production of cytokines and activate the c-jun terminal kinase (JNK) pathway, leading to a stimulus of innate immune responses and regulation of host cell–parasite communication (Li et al. 2018a; Li et al. 2018b).
Cryptosporidium
Hu et al. (2013) showed that exosomes are released in vivo from the biliary and intestinal epithelial cells from animals infected with Cryptosporidium parvum and that they contained antimicrobial peptides such as β-defensin-2 and IL-37. These exosomes bound to the surface of sporozoites and decreased both the parasite’s infectivity and viability. It was shown that release of these exosomes involved a miRNA-mediated exocytic mechanism (Hu et al. 2013; Review in Zhou et al. 2014). On the other hand, this parasite may have developed strategies to modulate host miRNA-mediated cellular functions for immune evasion (reviewed by Ming et al. 2017).
Trichomonas
In the case of anaerobic protozoa, Twu et al. (2013) carried out a detailed study on the Trichomonas vaginalis exosomes. The authors based this study on previous information showing that the surface membrane proteome of this protozoan revealed the presence of three tetraspanin proteins (Tsp), a family of proteins constitutive components of exosomes on several cell types. The authors reported vesicular isolation with a diameter of 50 to100 nm from the supernatant of protozoan cultures. Tagging of one of the tetraspanins (Tsp1) with hemagglutinin A allowed the observation that this protein is located in the vesicles. The vesicles contained a heterogenous population of small RNAs ranging in size between 25 and 200 nm. Proteomic analysis of the isolated exosomes revealed the presence of a total of 215 proteins in which 73% and 40% of them were orthologs of proteins found in mammalian and L. donovani exosomes, respectively. These include protein families such as tetraspanins, Alix, Rabs, HSP70, subunits of heterotrimeric G proteins, cytoskeletal proteins, surface proteins, and proteases that may be involved in trichomoniasis pathogenesis. When BODIPY-PC labeled exosomes were incubated in the presence of ectocervical cells in culture, labeled structures were seen inside the epithelial cells. In contrast, labeled hydrogenosomes were not incorporated by the cells. Importantly, cells which incorporated exosomes induced the production of IL-6 to the same extent as when they were incubated in the presence of the parasites. Induction of IL-8 was also observed although to a lower extent. The authors also analyzed the possible role played by exosomes on the interaction between the parasites in addition to the interaction between parasites and epithelial cells in vitro. They used two parasite strains: (1) B7RC2 and (2) G3. B7RC2 is a strain 20-fold more adherent than the G3 strain. Pre-incubation of G3 parasites with exosomes from the B7RC2 strain resulted in a 2-fold increase in their attachment to ectocervical cells. On the other hand, pre-incubation of B7RC2 exosomes with epithelial cells resulted in a 3-fold increase in G3 attachment to the cells. Similar results were obtained with other strains leading to the conclusion that exosomes from high adherent strains increase attachment of less adherent cells. Recently, Olmos-Ortiz et al. (2017) demonstrated that T. vaginalis exosomes have an immunomodulatory role on the parasite-induced cytokine profile since interactions with vaginal tissues lead to a decrease of IL-17, IL-6, and IL-13 and promote a decrease in the inflammatory process in T. vaginalis-infected mice.
Giardia
In relation to Giardia intestinalis, there are some interesting results although two of them are from unpublished reports. First, Deolindo et al. (2013) wrote a short review on the subject in which they mentioned that they found intense vesicular formation by trophozoites incubated at pH 3 and 8 for 5 to 10 min. The second report was found in a master’s thesis presented by Mojoli le Quesne (2019), who showed that trophozoites incubated in the presence of 1 mM Ca2+ release vesicles in a time-dependent manner reaching the highest intensity after 120 min of incubation. The vesicles were isolated and analyzed by mass spectrometry and eight proteins, most of them enzymes involved in the general metabolism and some cytoskeletal proteins, were identified. The nature of the proteins varied if the vesicles were isolated from trophozoites or from encysting cells. It was also shown that the vesicles contained a 5.8S rRNA and that when added to parasites interacting with Caco-2 cells, they increase the adhesion of trophozoites to the epithelial cells. A more detailed work was recently published by Evans-Osses et al. (2017) who presented several findings: (1) release of 60 to 150 nm vesicles when the protozoan was incubated in the presence of calcium; (2) dose-dependent inhibition of vesicular release when the protozoan was treated with methyl-β-cyclodextrin, a compound that causes cholesterol depletion from the plasma membrane; (3) EVs facilitate adhesion of the protozoan to epithelial cells in vitro; and (4) that G. intestinalis vesicles are internalized by dendritic cells via endocytosis and are subsequently upregulated for the CD25 expression, thus acquiring an activated state. The isolated EVs were submitted for a proteomic analysis. Those obtained from trophozoites revealed the presence of 11 proteins, seven of which are usually found in vesicles obtained from other cells. However, when the vesicles were isolated from parasites during the process of encystation in vitro, 80 proteins were identified, and 36 of them are still unknown. More recently, tridimensional reconstruction of Giardia trophozoites induced to release exosomes revealed that some, but not all, peripheral vacuoles constitute multivesicular bodies containing vesicles with a mean diameter of 50 nm (Midlej et al. 2019). Figure 6 shows images of these vesicles.
Electron tomography of Giardia intestinalis (non-encysted: a–c and encysted: d–f) parasites showing tomographic 3D reconstruction of peripheral vesicles (PVs) consisting of MVBs inside. Midlej et al. (2019)
EVs secreted by host cells during interaction with parasitic protozoa
There are some reports showing that cells infected with parasites able to survive within them are induced to secrete EVs. For instance, it was shown that T. cruzi induces exosome release from the host cell and that these vesicles bind to the parasites and protect them from complement-mediated lysis (Cestari et al. 2012). This protection appeared to be dependent on the T. cruzi subtype (strain). Vesicles produced during the interaction between TcII parasites and host cells increased parasite resistance to complement lysis from 50 to 80% and parasite invasion was increased to > 50%. Meanwhile, vesicles purified during the interaction between TcI parasites and host cells have stronger effects, causing a doubling of both complement resistance and parasite invasion. The complement-mediated lysis assays showed that all vesicles inhibited mainly the lectin pathway (Wyllie and Ramirez 2017).
In the case of Leishmania infection, it has been shown that bone marrow-derived dendritic cells loaded with the major Leishmania antigen produce exosomes that mediate protective immunity against cutaneous leishmaniasis (Schnitzer et al. 2010).
There are some reports concerning exosomes that are not from Apicomplexa parasites but from the infected host cells. In the case of Toxoplasma gondii and Eimeria tenella, it was shown that exosomes derived from dendritic cells previously infected with the respective parasite protect animals from a subsequent infection, thus acting as a vaccine (Beauvillain et al. 2009; Bhatnagar et al. 2007; Del Cacho et al. 2011). Dendritic cells are able to secrete exosomes expressing functional MHC classes I and II in addition to sT-cell costimulatory molecules on their surfaces (Keller et al. 2006). Proteomic analysis of exosomes released by cells infected with T. gondii (when compared with non-infected fibroblasts) demonstrated the presence of 346 proteins uniquely from the parasite and 15 proteins as being unique to the host cells. Among the proteins present in exosomes, some correspond to classical EVs markers such as extracellular matrix glycoproteins, calcium-binding proteins, filament-associated proteins, annexin family members, HSP 70, serpins, and CD63 (Wowk et al. 2017). In the case of leukocytes infected with Theileria annulata, it was shown that there are significant differences in the protein and miRNA profile between exosomes released by uninfected and T. annulata infected leukocytes (Gillan et al. 2019).
EVs in other parasites
Although out of the initial scope of this review, it is important to mention that EVs have been also found in other parasites such fungi (Cryptococus neoformans, Malassezia sympodialis, Paracoccidiodes brasilensis) in addition to helminths such as Heligmosomoides polygyrus, Schistosoma japonicum, S. mansoni, Echinostoma caproni, Dicrocoelium dendriticus, and even in the free living nematode Caenorhabditis elegans (Wang et al. 2015; Trelis et al. 2016; Nowacki et al. 2015; Samoil et al. 2018). In most cases, the vesicles have been identified by electron microscopy and their protein and miRNA content characterized by using standard biochemical and molecular techniques (reviewed in Britton et al. 2014). Furthermore, it has been shown that incubation of these vesicles with host cells interfere with various factors such as iNOS, Arg-1, TND-alpha, IL-10, and IL-12 and may act as immunomodulators (Buck et al. 2014; Coakley et al. 2015; Maizels and Yazdanbakhsh 2003; Marcilla 2012; Marcilla et al. 2014; Trelis et al. 2016; Wang et al. 2015). In all the studies, the authors suggest that exosomes could play important roles in host–parasite interactions and could be a useful tool in vaccine and therapeutic development against parasitic diseases.
Perspectives
The huge number of papers that have been published on EVs indicate the interest and importance of this topic that even originated an international organization and a highly specialized scientific journal (Journal of Extracellular Vesicles). There are reasons to convince several research groups that these structures may constitute potential biotechnology tools and are important players of what has been designated as nanobiotechnology. They may constitute an important delivery system for gene therapy and molecular-displaying cell regulation capabilities when incorporated into other cells and even by interfering with the exosomal membrane during its biogenesis, targeting the vesicles via specific ligands to different cell types. These vesicles may reach the bloodstream, extravasate through intercellular junctions and even passing the central nervous system blood barrier. There is evidence that it is possible to interfere with the composition of the exosomes by interfering with multivesicular body biogenesis. Finally, it is important to recognize that the same approach in drug development for targeting cancer cells may also work for extracellular and intracellular parasites, especially those that are able to deliver their proteins to the plasma membrane of the host cell. In addition, exosome’s great practical potential provides an incentive to some groups to produce fully artificial exosomes as reviewed by García-Manrique et al. (2018a, b).
References
Abdi A, Yu L, Goulding D, Rono MK, Bejon P, Choudhary J, Rayner J (2017) Proteomic analysis of extracellular vesicles from a Plasmodium falciparum Kenyan clinical isolate defines a core parasite secretome. Wellcome Open Res 2:50. https://doi.org/10.12688/wellcomeopenres.11910.2
Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P (2015) Molecular biology of the cell, 6th edn. Garland Science, New York. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21054. Accessed Nov 2019
Atayde VD, Aslan H, Townsend S, Hassani K, Kamhawi S, Olivier M (2015) Exosome secretion by the parasitic protozoan Leishmania within the sand fly midgut. Cell Rep 13(5):957–967
Babudieri B, Tomasini N (1962) Fine struttura dei trypanosomi. Parassitologia 4:89–95
Barros HC, da Silva S, Verbisck NV, Araguth MF, Tedesco RC, Procopio DO (1996) Release of membrane-bound trails by Trypanosoma cruzi amastigotes onto modified surfaces of mammalian cells. J Eukaryot Microbiol 43:275–285
Bayer-Santos E, Aguliar-Bonavides C, Rodrigues SP, Cordero EM, Marques AF, Valera-Ramirez A, Choi H, Yoshida N, da Silveira JF, Almeida IC (2013) Proteomic analysis of Trypanosoma cruzi secretome: characterization of two populations of extracellular vesicles and soluble proteins. J Proteome Res 12:883–897
Bayer-Santos E, Lima FB, Ruiz JC, Almeida IC, da Silveira JF (2014) Characterization of the small RNA content of Trypanosma cruzi extracellular vesicles. Mol Biochem Parasitol 193(2):71–74
Beauvillain C, Juste MO, Dion S, Pierre J, Dimier-Poisson I (2009) Exosomes are an effective vaccine against congenital toxoplasmosis in mice. Vaccine. 27(11):1750–1757
Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS (2007) Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 110(9):3234–3244
Bianco NR, Kim SH, Ruffner MA, Robbins PD (2009) Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase-positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis Rheum 60(2):380–389
Bissig C, Lenoir M, Velluz MC, Kufareva I, Abagyan R, Overduin M, Gruenberg J (2013) Viral infection controlled by a calcium-dependent lipid-binding module in ALIX. Dev Cell 25(4):364–373
Bonfim-Melo A, Ferreira ER, Florentino PTV, Mortara RA (2018) Amastigote synapse: the tricks of Trypanosoma cruzi extracellular amastigotes. Front Microbiol 27(9):1341. https://doi.org/10.3389/fmicb.2018.01341
Britton C, Winter AD, Gillan V, Devaney E (2014) microRNAs of parasitic helminths - identification, characterization and potential as drug targets. Int J Parasitol Drugs Drug Resist 4(2):85–94
Brown M, Johnson LA, Leone DA, Majek P, Vaahtomeri K, Senfter D, Bukosza N, Schachner H, Asfour G, Langer B, Hauschild R, Parapatics K, Hong Y-K, Bennett KL, Kain R, Detmar M, Sixt M, Jackson DG, Kerjaschki D (2018) Lymphatic exosomes promote dendritic cell migration along guidance cues. J Cell Biol 217(6):2205–2221
Bruno S, Porta S, Bussolati B (2016) Extracellular vesicles in renal tissue damage and regeneration. Eur J Pharmacol 790:83–89
Buck AH, Coakley G, Simbari F, McSorley HJ, Quintana JF, Le Bihan T (2014) Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat Commun Nature Publishing Group 5:5488
Bukong TN, Momen-Heravi F, Kodys K, Bala S, Szabo G (2014) Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS Pathog 10:e10004424
Cam H, Sugiyama T, Chen ES, Chen X, FisGeral PC, Grewal SI (2005) Comprehensive analysis of heterochromatin- and RNA-mediated epigenetic control of the fission yeast genome. Nat Genet 37(8):809–819
Campos FM, Franklin BS, Teixeira-Carvalho A, Filho AL, de Paula SC, Fontes CJ, Brito CF, Carvalho LH (2010) Augmented plasma microparticles during acute Plasmodium vivax infection. Malar J 16(9):327. https://doi.org/10.1186/1475-2875-9-327
Castelli G, Bruno F, Saieva L, Alessandro R, Galluzzi L, Diotallevi A, Vitale F (2019) Exosome secretion by Leishmania infantum modulate the chemotactic behavior and cytokinic expression creating an environment permissive for early infection. Exp Parasitol 198:39–45
Cestari I, Ansa-Addo E, Deolindo P, Inai JM, Ramirez MI (2012) Trypanosoma cruzi immune evasion mediated by host cell derived microvesicles. J Immunol 188(4):1942–1952. https://doi.org/10.4049/jimmunol.1102053
Chaput N, Flament C, Viaud S, Taieb J, Roux S, Spatz A, André F, LePecq JB, Boussac M, Garin J, Amigorena S, Théry C, Zitvogel L (2006) Dendritic cell derived-exosomes: biology and clinical implementations. J Leukoc Biol 80(3):471–478
Chen C, Zong S, Wang Z, Lu J, Zhu D, Zhang Y, Cui Y (2016) Imaging and intracellular tracking of cancer-derived exosomes using single-molecule localization-based super-resolution microscope. ACS Appl Mater Interfaces 8(39):25825–25833
Coakley G, Maizels RM, Buck AH (2015) Exosomes and other extracellular vesicles: the new communicators in parasite infections. Trends Parasitol 31(10):477–489
Cohen A, Zinger A, Tiberti N, Grau GER, Combes V (2018) Differential plasma microvesicle and brain profiles of microRNA in experimental cerebral malaria. Malar J 17(1):192. https://doi.org/10.1186/s12936-018-2330-5
Colineau L, Clos J, Moon KM, Foster LJ, Reiner NE (2017) Leishmania donovani chaperonin 10 regulates parasite internalization and intracellular survival in human macrophages. Med Microbiol Immunol 206(3):235–257
Combes V, Taylor TE, Johan-Vacue I, Mege JL, Mwenechanya J, Grau GE, Molyneux ME (2004) Circulating endothelial microparticles in Malawian children with severe falciparum malaria complicated with coma. JAMA 291(21):2542–2544
Corrigan L, Redhai S, Leiblich A, Fan SJ, Perera SM, Patel R, Gandy C, Wainwright S, Morris JF, Hamdy F, Goberdhan DC, Wilson C (2014) BMP-regulated exosomes from Drosophila male reproductive glands reprogram female behavior. J Cell Biol 206(5):671–688
Costa-Verdera H, Gitz-Francois JJ, Schiffelers RM, Vader P (2017) Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J Control Release 266:100–108
Couper KN, Barnes T, Hafalla JC, Combes V, Ryffel B, Secher T, Grau GE, Riley EM, de Souza JB (2010) Parasite-derived plasma microparticles contribute significantly to malaria infection-induced inflammation through potent macrophage stimulation. PLoS Pathog 6(1):e1000744. https://doi.org/10.1371/journal.ppat.1000744
De Souza W, Sant’anna C, Cunha e Silva NL (2009) Electron microscopy and cytochemistry analysis of the endocytic pathway in pathogenic protozoa. Prog Histochem Cytochem 44(2):67–124. https://doi.org/10.1016/j.proghi.2009.01.001
Del Cacho E, Gallego M, Lee SH, Lillehoj HS, Quilez J, Lillehoj EP, Sánchez-Acedo C (2011) Induction of protective immunity against Eimeria tenella infection using antigen-loaded dendritic cells (DC) and DC-derived exosomes. Vaccine. 29(21):3818–3825
Denzer K, Keijmeer MJ, Heijnen HF, Stoorvogel W, Geuze H (2000) Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 113(19):3365–3374
Deolindo P, Evans-Osses I, Ramirez MI (2013) Microvesicles and exosomes as vehicles between protozoan and host cell communication. Biochem Soc Trans 41(1):252–257. https://doi.org/10.1042/BST20120217
Eliaz D, Kannan S, Shaked H, Arvatz G, Tkacz ID, Binder L, Ben-Asher HW, Okalang V, Chikne V, Cohen-Chalamish S, Michaeli S (2017) Exosome secretion affects social motility in Trypanosoma brucei. PLOS Pathol 13(3):e1006245. https://doi.org/10.1371/journal.ppat.1006245
Ellis DS, Ormerod WE, Lumsden WH (1976) Filaments of Trypanosoma brucei: some notes on differences in origin and structure in two strains of Trypanosoma (Trypanozoon) brucei rhodesiense. Acta Trop 33(2):151–168
Evans-Osses I, Mojoli A, Monguió-Tortajada M, Marcilla A, Aran V, Amorim M, Inal J, Borràs FE, Ramirez MI (2017) Microvesicles released from Giardia intestinalis disturb host-pathogen response in vitro. Eur J Cell Biol 96(2):131–142. https://doi.org/10.1016/j.ejcb.2017.01.005
Février B, Raposo G (2004) Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Op Cell Biol 16(4):415–421
Florentino PTV, Real F, Orikaza CM, da Cunha JPC, Vitorino FNL, Cordero EM, Sobreira TJP, Mortara RA (2018) A carbohydrate moiety of secreted stage-specific glycoprotein 4 participates in host cell invasion by Trypanosoma cruzi extracelular amastigotes. Front Microbiol 10(9):693. https://doi.org/10.3389/fmicb.2018.00693
Frey B, Gaipl US (2011) The immune functions of phosphatidylserine in membranes of dying cells and microvesicles. Semin Immunopathol 33(5):497–516
Fu Y, Zhang L, Zhang F, Tang T, Zhou Q, Feng C, Jin Y, Wu Z (2017) Exosome-mediated miR-146a transfer suppresses type I interferon response and facilitates EV71 infection. PLoS Pathog 13(9):e1006611
García-Manrique P, Gutiérrez G, Blanco-López MC (2018a) Fully artificial exosomes: towards new theranostic biomaterials. Trends Biotechnol 36(1):10–14
García-Manrique P, Matos M, Gutiérrez G, Pazos C, Blanco-López MC (2018b) Therapeutic biomaterials based on extracellular vesicles: classification of bio-engineering and mimetic preparation routes. J Extracell Vesicles 7(1):1422676
Garcia-Silva MR, Cura das Neves RF, Cabrera-Cabrera F, Sanguinetti J, Medeiros LC, Robello C, Naya H, Fernadez-Galero T, Souto-Padron T, de Souza W (2013) Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation and susceptibility to infection of mammalian cells. Parasitol Res 113(1):285–304
Garcia-Silva MR, Frugier M, Tosar JP, Correa-Domingues A, Ronalte-Alves I, Parodi-Talice A, Rovira C, Robello C, Goldenberg S, Cayota A (2010a) A population of t-RNA-derived small RNAs is actively produced in Trypanosoma cruzi and recruited to a specific cytoplasmic organelle. Mol Biochem Parasitol 171(2):64–73
Garcia-Silva MR, Tosar JP, Frugier M, Pantano S, Bonilla B, Esteban L, Serra E, Rovira C, Robeloo C, Cayota A (2010b) Cloning, characterization and subcellular localization of a Trypanosoma cruzi argonaute protein defining a new subfamily distinctive of trypanosomatids. Gene 466(91–2):26–35
Geiger A, Hirtz C, Bécue T, Bellard E, Centeno D, Gargani D, Rossignol M, Cuny G, Peltier JB (2010) Exocytosis and protein secretion in Trypanosoma. BMC Microbiol 26(10):20
Ghosh J, Bose M, Roy S, Bhattacharyya SN (2013) Leishmania donovani targets Dicer1 to downregulate miR-122, lower serum cholesterol, and facilitate murine liver infection. Cell Host Microbe 13(3):277–288
Gillan V, Simpson DM, Kinnaird J, Maitland K, Shiels B, Devaney E (2019) Characterization of infection associated microRNA and protein cargo in extacellular vesicles of Theilleria annulata infected leukocytes. Cell Microbiol 21(1):e12969
Gonçalves MFL, Umezawa ES, Katzin AM, de Souza W, Alves MJM, Zingales B, Colli W (1991) Trypanosoma cruzi: shedding of surface antigens as membrane vesicles. Exp.Parasitol. 72(1):43–53
Greening DW, Xu R, Ji H, Tauro BJ, Simpson RJ (2015) A protocol for exosome isolation and characterization: evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol Biol 129(5):179–209
Hassani K, Olivier M (2013) Immunomodulatory impact of leishmania-induced macrophage exosomes: a comparative proteomic and functional analysis. PLoS Negl Trop Dis 7(5):e2185
Hassani K, Antoniak E, Jardim A, Olivier M, Langsley G (2011) Temperature-induced protein secretion by Leishmania mexicana modulates macrophage signalling and function. PLoS One 6(5):e18724
Heinemann ML, Vykoukal J (2017) Sequential filtration: a gentle method for the isolation of functional extracellular vesicles. Methods Mol Biol 1660:33–41
Heusermann W, Hean J, Trojer D, Steib E, von Bueren S, Graff-Meyer A, Genoud C, Martin K, Pizzato N, Voshol J, Morrissey DV, Andaloussi SE, Wood MJ, Meisner-Kober NC (2016) Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J Cell Biol 213(2):173–184
Hou J, Jiang W, Zhu L, Zhong S, Zhang H, Li J, Zhou S, Yang S, He Y, Wang D, Chen X, Deng F, Zhang Q, Wang J, Hu J, Zhang W, Ding L, Zhao J, Tang J (2018) Circular RNAs and exosomes in cancer: a mysterious connection. Clin Transl Oncol 20(9):1109–1116
Hu G, Gong AY, Roth AL, Huang BQ, Ward HD, Zhu G, Larusso NF, Hanson ND, Chen XM (2013) Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog 9(4):e1003261. https://doi.org/10.1371/journal.ppat.1003261
Huotari J, Helenius A (2011) Endosome maturation. EMBO J 30(17):3481–3500
Hurley JH, Hanson PI (2010) Membrane budding and scission by the ESCRT machinery: it's all in the neck. Nat Rev Mol Cell Biol 11(8):556–566
Hurley JH (2008) ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol 20(1):4–11
Izquierdo-Useros N, Puertas MC, Borràs FE, Blanco J, Martinez-Picado J (2011) Exosomes and retroviruses: the chicken or the egg? Cell Microbiol 13(1):10–17
Jiang N, Yu S, Yang N, Feng Y, Sang X, Wang Y, Wahlgren M, Chen Q (2018) Characterization of the catalytic subunits of the RNA exosome-like complex in Plasmodium falciparum. J Eukaryot Microbiol 65(6):843–853
Kastelowitz N, Yin H (2014) Exosomes and microvesicles: identification and targeting by particle size and lipid chemical probes. ChemBioChem 15(7):923–928
Katzmann DJ, Sarkar S, Chu T, Audhya A, Emr SD (2004) Multivesicular body sorting: ubiquitin ligase Rsp5 is required for the modification and sorting of carboxypeptidase S. Mol Biol Cell 15(2):468–480
Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, Gangoda L, Mathivanan S (2016) ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol 428(4):688–692
Keller S, Sanderson MP, Stoeck A, Altevogt P (2006) Exosomes: from biogenesis and secretion to biological function. Immunol Lett 107(2):102–108
Keshtkar S, Azarpira N, Ghahremani MH (2018) Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther 9(1):63
Kibria G, Ramos EK, Wan Y, Gius DR, Liu H (2018) Exosomes as a drug delivery (2015). System in cancer therapy: potential and challenges. Mol Pharm 15(9):3625–3633
Lakkaraju A, Rodriguez-Boulan E (2008) Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol 18(5):199–209
Laulagnier K, Grand D, Dujardin A, Hamdi S, Vincent-Schneider H, Lankar D, Salles JP, Bonnerot C, Perret B, Record M (2004) PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett 572(1–3):11–14
Leitherer S, Clos J, Liebler-Tenorio EM, Schleicher U, Bogdan C, Soulat D (2017) Characterization of the protein tyrosine phosphatase LmPRL-1 secreted by Leishmania major via the exosome pathway. Infect Immun 85(8):e00084–e00017
Li Y, Liu Y, Xiu F, Wang J, Cong H, He S, Shi Y, Wang X, Li X, Zhou H (2018a) Characterization of exosomes derived from Toxoplasma gondii and their functions in modulating immune responses. Int J Nanomedicine 19(13):467–477
Li Y, Xiu F, Mou Z, Xue Z, Du H, Zhou C, Li Y, Shi Y, He S, Zhou H (2018b) Exosomes derived from Toxoplasma gondii stimulate an inflammatory response through JNK signaling pathway. Nanomedicine (London) 13(10):1157–1168
Li ZH, De Gaudenzi JG, Alvarez VE, Mendiondo N, Wang H, Kissinger JC, Frasch AC, Docampo R (2012) A 43-nucleotide U-rich element in 3′-untranslated region of large number of Trypanosoma cruzi transcripts is important for mRNA abundance in intracellular amastigotes. J BiolChem 287(23):19058–19069
Liégeois S, Benedetto A, Garnier JM, Schwab Y, Labouesse M (2006) The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. J Cell Biol 173(6):949–961
Lorentzen E, Conti E (2006) The exosome and the proteasome: nano-compartments for degradation. Cell. 125(4):651–654
Maizels RM, Yazdanbakhsh M (2003) Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol 3(9):733–744
Mantel PY, Hoang AN, Goldowitz I, Potashnikova D, Hamza B, Vorobjev I, Ghiran I, Toner M, Irimia D, Ivanov AR, Barteneva N, Marti M (2013) Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe 13(5):521–534
Marcilla A (2012) Extracellular vesicles from parasitic helminths contain specific excretory/secretory proteins and are internalized in intestinal host cells. PLoS One 7(9):e45974. https://doi.org/10.1371/journal.pone.0045974
Marcilla A, Martin-Jaular L, Trellis M, Menezes-Neto A, Osuna A, Bernal D, Fernandez-Becerra C, Almeida IG, del Portillo HA (2014) Extracellular vesicles in parasitic diseases. J Extrac Ves 3:2504 [37]
Martin-Jaular L, Nakayasu ES, Ferrer M, Almeida IC, Del Portillo HA (2011) Exosomes from Plasmodium yoelii-infected reticulocytes protect mice from lethal infections. PLoS One 6(10):e26588. https://doi.org/10.1371/journal.pone.0026588
Mathivanan S, Ji H, Simpson RJ (2010) Exosomes: extracellular organelles important in intercellular communication. J Proteome 73(10):1907–1920
McNamara RP, Chugh PE, Bailey A, Constantini LM, Ma Z, Bigi R, Cheves A, Eason AB, Landis JT, Host KM, Xiong J, Griffith JD, Damania B, Dittmer DP (2019) Exracellular vesicles from Kaposi Sarcoma-associated herpesvirus lymphoma induce long-term endothelial cell reprogramming. PLOS Path 15(2):e1007536
Meckes DG Jr, Shair KH, Marquitz AR, Kung CP, Edwards RH, Raab-Traub N (2010) Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci U S A 107(47):20370–203075
Midlej V, de Souza W, Benchimol M (2019) The peripheral vesicles gather multivesicular bodies with different behavior during the Giardia intestinalis life cycle. J Struct Biol 207(3):301–311. https://doi.org/10.1016/j.jsb.2019.07.002
Ming Z, Zhou R, Chen XM (2017) Regulation of host epithelial responses to Cryptosporidium infection by microRNAs. Parasite Immunol 39(2). https://doi.org/10.1111/pim.12408
Mojoli Le Quesne AH (2019) Characterization of microvesiculation mechanisms of Giardia instestinalis trophozoites and determination of the microvesicles role in the interaction with host cells. Rio de Janeiro; s.n; 2014. xiii,96 p. ilus, graf, tab, mapas. Tese em Português | LILACS | ID: lil-746878. Biblioteca responsável: BR15.1
Momen-Heravi F, Balaj L, Alian S, Mantel PY, Halleck AE, Trachtenberg AJ, Soria CE, Oquin S, Bonebreak CM, Saracoglu E, Skog J, Kuo WP (2013) Current methods for the isolation of extracellular vesicles. Biol Chem 394(10):1253–1262
Morelli AE, Larregina A, Shufesky WJ, Sullivan MG, Stolz DB, Papwoth GD, Zahochak AF, Logar AJ, Wang Z, Watkins SC, Falo LD, Thompson AW (2004) Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 104(10):3257–3266
Nantakomol D, Dondorp AM, Krudsood S, Udomsangpetch R, Pattanapanyasat K, Combes V, Grau GE, White NJ, Viriyavejakul P, Day NP, Chotivanich K (2011) Circulating red cell-derived microparticles in human malaria. J Infect Dis 203(5):700–706
Nieuwland R, Berckmans RJ, Rotteveel-Eijkman RC, Maquelin KN, Roozendaal KJ, Jansen PGM, Have Kt, Eijsman L, Hack CE, Sturk A (1997) Cell-derived microparticles generated in patients during cardiopulmonary bypass are highly procoagulant. Circulation 96(10):3534–3541
Nogueira P, Ribeiro K, Silveira CO, Campos JH, Martins Filho CA, Bela SR, Campos MA, Pessoa ML, Colli W, Alves MJM, Soares RP, Torrecilhas AC (2015) Vesicles from different Trypanosoma cruzi strains trigger differential innate and chronic immune response. J Extrac Ves 26(4):28734
Nowacki FC, Swain MT, Klychnikov OI, Niazi U, Ivens A, Quintana JF et al (2015) Protein and small non-coding RNA-enriched extracellular vesicles are released by the pathogenic blood fluke Schistosoma mansoni. J Extracell Vesicles Available:http://www.journalofextracellularvesicles.net/index.php/jev/article/view/28665/xml_30
Ogawa Y, Kanai-Azuma M, Akimoto Y, Kawakami H, Yanoshita R (2008a) Exosome-like vesicles in Gloydius blomhoffii blomhoffii venom. Toxicon. 51(6):984–993
Ogawa Y, Kanai-Azuma M, Akimoto Y, Kawakami H, Yanoshita R (2008b) Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol Pharm Bull 31(6):1059–1062
Olmos-Ortiz LM, Barajas-Mendiola MA, Barrios-Rodiles M, Castellano LE, Arias-Negrete S, Avila EE, Cuéllar-Mata P (2017) Trichomonas vaginalis exosome-like vesicles modify the cytokine profile and reduce inflammation in parasite-infected mice. Parasite Immunol 39(6). https://doi.org/10.1111/pim.12426
Pablos Torró LM, Retana Moreira L, Osuna A (2018) Extracellular vesicles in Chagas disease: a new passenger for an old disease. Front Microbiol 1(9):1190
Pan BT, Johnstone RM (1983) Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 33(3):967–978
Pan BT, Teng K, Wu C, Adam M, Johnstone RM (1985) Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 101(3):942–948
Pankoui Mfonkeu JB, Gouado I, Fotso Kuaté H, Zambou O, Amvam Zollo PH, Grau GE, Combes V (2010) Elevated cell-specific microparticles are a biological marker for cerebral dysfunctions in human severe malaria. PLoS One 5(10):e13415. https://doi.org/10.1371/journal.pone.0013415
Pelchen-Matthews A, Raposo G, Marsh M (2004) Endosomes, exosomes and Trujan viruses. Trends Microbiol 12(7):310–316
Pessoa CC, Ferreira ER, Bayer-Santos E, Rabinovitch M, Mortara RA, Real F (2016) Trypanosoma cruzi differentiates and multiplies within chimeric parasitophorous vacuoles in macrophages coinfected with Leishmania amazonensis. Infct Immu 84(5):1603–1614
Peters NC, Sacks DL (2009) The impact of vector-mediated neutrophil recruitment on cutaneous leishmaniasis. Cell Microbiol 11(9):1290–1296
Pfeffer S (2010) Two Rabs for exosome release. Nat Cell Biol 12(1):3–4
Piper RC, Katzmann DJ (2007) Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol 23:519–547
Ramirez MI, Deolindo P, de Messias-Reason IJ, Arigi EA, Choi H, Almeida IC, Evans-Osses I (2017) Dynamic flux of microvesicles modulate parasite-host cell interaction of Trypanosoma cruzi in eukaryotic cells. Cell Microbiol 19(4). https://doi.org/10.1111/cmi.12672
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183(3):1161–1172
Record M, Subra C, Silvente-Poirot S, Poirot M (2011) Exosomes as intracellular signalosomes and pharmacological effectors. Biochem Pharmacol 81(10):1171–1182
Regev-Rudzki N, Wilson DW, Carvalho TG, Sisquella X, Coleman BM, Rug M, Bursac D, Angrisano F, Gee M, Hill AF, Baum J, Cowman AF (2013) Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell. 153(5):1120–1133
Retana Moreira L, Rodríguez Serrano F, Osuna A, Almeida IC (2019) Extracellular vesicles of Trypanosoma cruzi tissue-culture cell-derived trypomastigotes: induction of physiological changes in non-parasitized culture cells. PLoS Negl Trop Dis 13(2):e0007163
Rodrigues ML, Nimrichter L, Oliveira DL, Nosanchuk JD, Casadevall A (2008) Vesicular trans-cell wall transport in fungi: a mechanism for the delivery of virulence-associated macromolecules? Lipid Insights 2(1):27–40
Sadallah S, Eken C, Martin PJ, Schifferli JA (2011) Microparticles (ectosomes) shed by stored human platelets downregulate macrophages and modify the development of dendritic cells. J Immunol 186(11):6543–6552
Samoil V, Dagenais M, Ganapathy V, Aldridge J, Glebov A, Jardim A, Ribeiro P (2018) Vesicle-based secretion in schistosomes: analysis of protein and microRNA (miRNA) content of exosome-like vesicles derived from Schistosoma mansoni. Sci Rep 8(1):3286. https://doi.org/10.1038/s41598-018-21587-4
Sampaio NG, Emery SJ, Garnham AL, Tan QY, Sisquella X, Pimentel MA, Rex AR, Regev-Rudzki N, Schofield L, Eriksson EM (2018) Extracellular vesicles from early stage Plasmodium falciparum-infected red blood cells contain PfEMP1 and induce transcriptional changes in human monocytes. Cell Microbiol 20(5):e12822. https://doi.org/10.1111/cmi.12822
Sampaio NG, Cheng L, Eriksson EM (2017) The role of extracellular vesicles in malaria biology and pathogenesis. Malar J 16(1):245. https://doi.org/10.1186/s12936-017-1891-z
Samuelson I, Vidal-Puig AJ (2018) Fed-EXosome: extracellular vesicles and cell-cell communication in metabolic regulation. Essays Biochem 62(2):165–175
Schepilewsky E (1912) Fadenförmige Anhängsel bei den Trypanosomen. Zbl Bakt I Abt Orig 65:79–83
Schmunis GA, Szarfman A, de Souza W, Langembach T (1980) Trypanosoma cruzi: antibody-induced mobility of surface antigens. Exp Parasitol 50(1):90–102
Schnitzer JK, Berzel S, Fajardo-Moser M, Remer KA, Moll H (2010) Fragments of antigen-loaded dendritic cells (DC) and DC-derived exosomes induce protective immunity against Leishmania major. Vaccine. 28(36):5785–5793
Schorey JS, Bhatnagar S (2008) Exosome function: from tumor immunology to pathogen biology. Traffic. 9(6):871–881. https://doi.org/10.1111/j.1600-0854.2008.00734
Silverman JM, Chan SK, Robinson DP, Dwyer DM, Nandan D, Foster LJ, Reiner NE (2008) Proteomic analysis of the secretome of Leishmania donovani. Genome Biol 9(2):R35
Silverman JM, Clos J, de Oliveira CC, Shirvani O, Fang Y, Wang C, Foster LJ, Reiner NE (2010) An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J Cell Sci 123(6):842–852
Silverman JM, Reiner NE (2011) Exosomes and other microvesicles in infection biology: organelles with unanticipated phenotypes. Cell Microbiol 13(1):1–9
Stein JM, Luzio JP (1991) Ectocytosis caused by sublytic autologous complement attack on human neutrophils. The sorting of endogenous plasma-membrane proteins and lipids into shed vesicles. Biochem J 274(2):381–386
Sullivan R, Saez F (2013) Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction. 146(1):R21–R35
Szempruch AJ, Dennison L, Kieft R, Harrington JM, Hadjuk SL (2016a) Sending a message: extracellular vesicles of pathogenic protozoan parasites. Nat Rev Microbiol 14(11):669–675
Szempruch AJ, Sykes SE, Kieft R, Dennison L, Becker AC, Gartrell A, Martin WJ, Nakayasu ES, Almeida IC, Hajduk SL, Harrington JM (2016b) Extracellular vesicles from Trypanosoma brucei mediate virulence factor transfer and cause host anemia. Cell. 164(1–2):246–257
Théry C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2(8):569–579
Torrecilhas AC, Tonelli RR, Pavanelli WR, da Silva JS, Schumacher RI, de Souza W, Cunha e Silva N, Abrahamsohn IA, Colli W, Alves MJM (2009) Trypanosoma cruzi: parasite shed vesicles increase heart parasitism and generate an acute inflammatory response. Microbes Infect 11(1):29–39
Trelis M, Galiano A, Bolado A, Toledo R, Marcilla A, Bernal D (2016) Subcutaneous injection of exosomes reduces symptom severity and mortality induced by Echinostoma caproni infection in BALB/c mice. Int J Parasitol 46(12):799–808
Trissl D, Martínez-Palomo A, Argüello C, de la Torre M, de la Hoz R (1977) Surface properties related to concanavalin A-induced agglutination. A comparative study of several Entamoeba strains. J Exp Med 145(3):652–665
Twu O, de Miguel N, Lustig G, Stevens GC, Vashisht AA, Wohlschlegel JA, Johnson PJ (2013) Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host∶parasite interactions. PLoS Pathog 9(7):e1003482
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JL, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9(6):654–659
Van Niel G, Porto-Carreiro I, Simões S, Raposo G (2006) Exosomes: a common pathway for a specialized function. J Biochem (Tokyo) 140(1):13–21
Vickerman K, Luckins AG (1969) Localization of variable antigens in the surface coat of Trypanosoma brucei using ferritin conjugated antibody. Nature 224(5224):1125–1126
Vidakovics ML, Jendholm J, Mörgelin M, Månsson A, Larsson C, Cardell LO, Riesbeck K (2010) B cell activation by outer membrane vesicles-a novel virulence mechanism. PLoS Pathog 6(1):e1000724
Vidal M, Mangeat P, Hoekstra D (1997) Aggregation reroutes molecules from a recycling to a vesicle-mediated secretion pathway during reticulocyte maturation. J Cell Sci 110(16):1867–1877
Wang L, Li Z, Shen J, Liu Z, Liang J, Wu X, Sun X, Wu Z (2015) Exosome-like vesicles derived by Schistosoma japonicum adult worms mediates M1 type immune- activity of macrophage. Parasitol Res 114(5):1865–1873
Williams C, Royo F, Aizpurua-Olaizola O, Pazos R, Boons GJ, Reichardt NC, Falcon-Perez JM (2018) Glycosylation of extracellular vesicles: current knowledge, tools and clinical perspectives. J Extracell Vesicles 7(1):1442985
Witwer KW, Théry C (2019) Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J Extracell Vesicles 8(1):1648167
Wowk PF, Zardo ML, Miot HT, Goldenberg S, Carvalho PC, Mörking PA (2017) Proteomic profiling of extracellular vesicles secreted from Toxoplasma gondii. Proteomics. 17(15–16). https://doi.org/10.1002/pmic.201600477
Wright KA, Lumsden WHR, Hales H (1970) The formation of filopodium-like processes by Trypanosoma (Trypanozoon) brucei. S Cell Sci 6(1):285–297
Wyllie MP, Ramirez MI (2017) Microvesicles released during the interaction between Trypanosoma cruzi TcI and TcII strains and host blood cells inhibit complement system and increase the infectivity of metacyclic forms of host cells in a strain-independent process. Pathog Dis 75(7). https://doi.org/10.1093/femspd/ftx077
Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pállinger É, Del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4:27066
Yang F, Liao X, Tian Y, Li G (2017) Exosome separation using microfluidic systems: size-based, immunoaffinity-based and dynamic methodologies. Biotechnol J 12(4). https://doi.org/10.1002/biot.201600699
Zhou J, Yang Y, Wang W, Zhang Y, Chen Z, Hao C, Zhang J (2018) Melanoma-released exosomes directly activate the mitochondrial apoptotic pathway of CD4+ T cells through their microRNA cargo. Exp Cell Res 4827(18):30788–30792
Zhou R, Feng Y, Chen XM (2014) Non-coding RNAs in epithelial immunity to Cryptosporidium infection. Parasitology. 141(10):1233–1243
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Julia Walochnik
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Souza, W., Barrias, E.S. Membrane-bound extracellular vesicles secreted by parasitic protozoa: cellular structures involved in the communication between cells. Parasitol Res 119, 2005–2023 (2020). https://doi.org/10.1007/s00436-020-06691-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06691-7