Abstract
Knowledge of vector species composition and monitoring their change over time is critical to evaluate malaria transmission and assess control interventions. This is especially important in countries such as Botswana, where malaria transmission is subjected to fluctuations due to climate variability. Another important aspect that impacts vector populations is the insecticide resistance. In order to assess species composition and the presence of mutations associated with insecticide resistance, Anopheles specimens from larval samplings and indoor pyrethrum spray sheet collections were analysed. A total of 349 Anopheles were screened by morphology and PCR as belonging to the An. gambiae complex and An. funestus group. Specimens were subsequently analysed for human blood meal and Plasmodium index. Finally, knock-down resistance polymorphisms were assessed. Anopheles arabiensis accounted for the majority of specimens collected through larval (96.7%) and pyrethrum spray sheet collection (87.4%) across all sampling sites, and this species was the only one found positive for human blood and for P. falciparum. Other Anopheles species were collected in small numbers by pyrethrum spray catches, namely An. quadriannulatus, An. longipalpis type C and An. parensis. The authors speculate on changing climate patterns and their possible impact on species composition. The kdr assay revealed that Anopheles mosquitoes were homozygous wild type for both L1014F (kdr-w) and L1014S (kdr-e) mutations. These results highlight the unique vectorial role of An. arabiensis in Botswana and indicated that even with prolonged use of pyrethroids and DDT, the mosquito population has not developed kdr mutations, despite some in vivo evidence of insecticide resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malaria continues to be a significant cause of morbidity and mortality in the developing world with Africa being the most affected. In Botswana, malaria foci are localised in five northern and eastern districts (Okavango, Ngami, Chobe, Boteti and Tutume) (Chihanga et al. 2016). However, there have been recent sporadic outbreaks in areas that were not usually prone to malaria (Botswana Ministry of Health and Wellness 2017, 2018a). Over the years, Botswana has implemented numerous vector control programmes, which have made extraordinary contributions to the decline in malaria incidence in the country and allowed it to enter the elimination phase (Chihanga et al. 2016). As efforts continue towards malaria elimination, monitoring Anopheles species distribution and density over time, assessing their vectorial capacity and surveying insecticide resistance will be a basic requirement to guide malaria vector control programmes. Previous studies indicate that the main malaria vector in Botswana is An. arabiensis (Chirebvu et al. 2014, 2016; Chirebvu and Chimbari 2015, 2016; Tawe et al. 2017). However, several additional species of the An. gambiae complex and of the An. funestus group have been found, namely An. quadriannulatus, An. longipalpis type C, An. parensis and An. leesoni (Tawe et al. 2017). In addition, other secondary vector and non-vector species have been identified: An. argentolobatus, An. caliginosus, An. coustani, An. cydippis, An. demeilloni, An. distinctus, An. funestus s.s., An. listeri, An. maculipalpis, An. marshallii, An. pharoensis, An. pretoriensis, An. rhodesiensis, An. rivulorum, An. rufipes, An. seretsei, An. squamosus, An. tchekedii, An. tenebrosus, An. vaneedeni, An. walravensi, An. wellcomei ugandae, An. ziemanni (Chirebvu et al. 2016; Pachka et al. 2016; Kyalo et al. 2017; Cornel et al. 2018). There are currently 28 species of Anopheles known to inhabit Botswana. However, Plasmodium parasites have only been found in An. arabiensis, (Tawe et al. 2017) thereby confirming its malaria vectorial role in Botswana. Notably, An. arabiensis is an endo- and exophilic (with both indoors and outdoors resting behaviour), zoophilic and anthropophilic (feeding both on animals and humans) mosquito and is therefore less affected by indoor spraying campaigns than other members of the An. gambiae complex. This is an important point, since malaria elimination in Botswana is primarily focused on indoor vector control strategies. No operational research has been performed, until now, to assess the biting and resting behaviour of the local An. arabiensis populations.
Insecticide-based vector control interventions, such as long-lasting insecticide-treated nets (LLINs), indoor residual spraying (IRS) and the recent introduction of winter bio-larviciding at a few selected sites, are used in Botswana against Anopheles mosquitoes to curb malaria transmission (Thomson et al. 2005; Chihanga et al. 2016; Mpofu et al. 2016). The use of pyrethroid-treated mosquito nets has been one of the cheapest and most effective interventions against malaria, and this technique has been regarded as comparatively harmless because of the relatively low toxicity to mammals and shorter persistence in the environment (Zaim et al. 2000). Additionally, Botswana utilises IRS with annual applications of DDT or lambda-cyhalothrin in the endemic districts (Chihanga et al. 2016). Even though the malaria situation is less worrying in southern Africa than elsewhere, the upsurge of insecticide resistance in different parts of the continent has threatened the sustainability of long-term effectiveness of vector control measures. Indeed, as far as we know, south-western Africa (Botswana and Namibia) remains the only region where pyrethroid-resistant Anopheles populations have not yet been reported in peer-reviewed journals (Riveron et al. 2018). However, according to the local Ministry of Health and Wellness in Botswana, pyrethroid resistance has been detected in all malaria endemic districts from 2016 while DDT resistance has only been reported in Bobirwa district in 2018 (Botswana Ministry of Health and Wellness 2018b). Nevertheless, the underlying resistance mechanisms were not characterised (Botswana Ministry of Health and Wellness 2018b). Additionally, there is no available published data on molecular markers linked to insecticide resistance from Botswana.
Pyrethroids and DDT act on voltage-gated sodium channels (VGSC) in the nerve cell membranes of insects by altering the gating kinetics, resulting in the prolonged opening of individual channels, paralysis and eventual death of the insect (Davies et al. 2007; Field et al. 2017). An important resistance mechanism against pyrethroids and DDT, known as knock-down resistance (kdr), has been linked to a single mutation in the VGSC gene in several insect species (Soderlund and Knipple 2003). Two main kdr mutations have been found in the African An. gambiae complex. In West Africa, kdr-w is due to a mutation resulting in a leucine to phenylalanine substitution in the S6 segment of domain II of the VGSC (L1014F) (Martinez-Torres et al. 1998). A second kdr mutation, kdr-e, which causes a change from leucine to serine at the same amino acid position (L1014S), was found in East Africa (Ranson et al. 2000). In Botswana, there is still no substantial information about insecticide resistance. Therefore, early detection and characterisation of kdr status may be critical for the development of strategies for effective vector management and the protection of the public’s health.
This study was designed to fill knowledge gaps in Anopheles species composition and explore their kdr status in Botswana.
Materials and methods
Mosquito collection
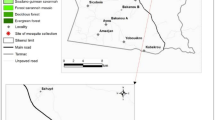
Larval collected (LC) and indoor pyrethrum spray sheet (PSS) collected adult anopheline mosquitoes preserved on silica gel were obtained from the National Malaria Programme, Ministry of Health and Wellness, Botswana. These mosquitoes were collected from four districts being Ngami (Shorobe, Mababe and Khwai villages), Okavango (Seronga village), Boteti (Motopi village) and Ghanzi (Grootlagte village) between February and March 2016 at the peak of the malaria transmission season (Fig. 1). The PSS collection was performed in the early morning for 2–3 days in each site, and 40 houses per site were sampled. The activities were performed among the established network of sentinel sites located in endemic and non-endemic districts (Chihanga et al. 2016).
Genomic DNA extraction and mosquito identification
Adult female specimens were first identified morphologically (Gillies and Coetzee 1987) before confirmatory molecular identification of specimens belonging to sibling species (An. gambiae complex and An. funestus group). DNA from body portions head/thorax and abdomen of each mosquito was extracted separately. DNA from the abdomen was extracted using DNAZol® while DNA from the head/thorax was extracted using Biomerieux NucliSENS® easyMAG® according to manufacturer’s instructions. The protocol of Scott et al. (1993) was applied for the species identification of An. gambiae s.l. specimens and the protocol of Koekemoer et al. (2002) for species identification of those belonging to the An. funestus group. Finally, to further identify the presence of An. longipalpis type C among the An. funestus samples, the protocol of Choi et al. (2010) was adopted.
Blood meal analysis and Plasmodium sporozoite positivity
A test described by Tawe et al. (2017) was applied for the detection of human β-globin DNA specific sequence in total DNA extracted from the mosquito abdomen. DNA extracts (head/thorax) were tested for the presence of P. falciparum using the molecular detection for pfmdr1 parasite gene through a nested PCR approach (Djimdé et al. 2001) with HB3 and DD2 P. falciparum as positive strains. Nested PCR was also performed using specific primers for P. vivax, as previously described by Snounou et al. (1993), since P. vivax is also circulating in some areas of Botswana (Motshoge et al. 2016). DNA of P. vivax (ATCC® 30138™) was run as positive control. PCR products were visualised on 2% agarose gels.
Conventional PCR for detection of knock-down resistance status
Two separate PCR assays for the detection of kdr-w (L1014F) and kdr-e (L1014S) were utilised on the Anopheles mosquito samples according to the protocols from Martinez-Torres et al. (1998) and Ranson et al. (2000), respectively. PCR assays were modified and optimised for template concentration (2 μl in a final volume of 20 μl reaction), annealing temperature (51 °C), number of cycles (30), and the MgCl2 concentration was adjusted to 2 mM. Amplified reaction fragments were run via ethidium bromide-stained 2.5% agarose gel electrophoresis and photographed under ultraviolet light illumination. Sensitive and resistant Anopheles strains were run together with the field samples for both tests.
TaqMan real-time PCR for detection of knock-down resistance
PCR was performed using a Bio-Rad CFX96™Real-Time system with minor modifications to the Bass et al. (2007) protocol. Primers kdr-Forward (5′-CATTTTTCTTGGCCACTGTAGTGAT-3′) and kdr-Reverse (5′-CGATCTTGGTCCATGTTAATTTGCA-3′) were used for binding the flanking region of both kdr mutation sites in the sodium channel gene. The probe WT (5′-CTTACGACTAAATTTC-3′) was labelled with VIC for the detection of the wild type allele, and the probes kdr-E (5′-ACGACTGAATTTC-3′) and kdr-W (5′-ACGACAAAATTTC-3′) were labelled with 6-FAM for detection of the kdr-e and kdr-w resistant alleles, respectively. The two standard primers (kdr-Forward and kdr-Reverse) and the WT probe were used either in one reaction with the kdr-E probe for detecting kdr-e or in another reaction with the kdr-W probe for detecting kdr-w. The 20-μL PCR reaction contained 1 μL of the genomic DNA of an individual mosquito, 10 μL of IQ™Supermix (Bio-Rad), 1.6 μM of each primer and 0.4 μM of each probe. The PCR cycling conditions were as follows: an initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 45 s. The increase in VIC and FAM fluorescence was monitored in real time by detecting fluorescence of VIC (530 nm excitation & 55 nm emission) and FAM (470 nm excitation & 510 nm emission) channels for each dye, respectively. Positive controls are comprised of a DNA template from mosquitoes with known West African (kdr-w) genotype (SENN-DDT, homozygous for the L1014F mutation) and East African (kdr-e) genotype (RSP7 homozygous for the L1014S mutation). The other positive control was DNA template from a homozygous susceptible colony (KGB). The heterozygous controls were made up by mixing equal aliquots of susceptible and resistant DNA templates.
Data analysis
Allelic discrimination scatter plots were adapted using the Bio-Rad CFX manager 3.1 software. Descriptive statistics relied on the Microsoft Excel, version 3.04.
Results
Species identification
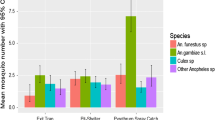
A total of 349 mosquito samples, belonging to the An. gambiae complex and An. funestus group, were analysed. Twelve (12) out of 349 mosquitoes were not identified by either morphological or molecular analysis as a consequence of poor specimen preservation (identification success rate 96.6%). Two of the sites, the villages of Motopi and Shorobe, were sampled both by LC and PSS. Overall, 150 specimens were from LC (43.0% of the total collected Anopheles). Anopheles arabiensis accounted for the majority of specimens collected through larval collection (96.7%) and PSS collection (87.4%) on overall sampling sites. Anopheles longipalpis type C (5.2%) and An. parensis (4.0%), which are both members of the An. funestus group, were also identified among the PSS specimens (Table 1).
Blood meal analysis and Plasmodium sporozoite positivity
The adult mosquitoes collected by PSS tested positive for human blood but this positivity was observed only in An. arabiensis across all study villages whereas other species from the same villages showed no human blood positivity (Table 2). Overall, a single head/thorax specimen of a human blood meal negative An. arabiensis mosquito from indoor collection in Shorobe village (Ngami district) was positive for P. falciparum. This provides a prevalence of 4.2% in this single village. An infection rate of 0% was observed for both P. falciparum and P. vivax in the other study areas.
Kdr status
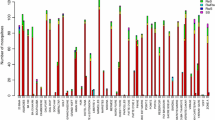
All Anopheles samples were screened for both L1014F (kdr-w) and L1014S (kdr-e) mutations with conventional and real-time PCR. Three hundred and nine (309) LC and PSS Anopheles mosquitoes yielded results. The kdr mutations, both L1014F and L1014S, were not detected (Fig. 2), and all the screened samples were homozygous wild type.
Discussion
Studies on Anopheles species identification and distribution are of importance in vector control, and such associated knowledge dictates appropriate control measures. Deployment of LLINs and IRS interventions without detailed understanding of the distribution, species composition and behaviour of local vectors complicates impact monitoring (Sinka et al. 2010). Tawe et al. (2017) emphasised the importance of vector surveillance in Botswana to understand malaria vectorial systems and transmission dynamics. The current study provides follow-up information about the distribution and diversity of Anopheles mosquitoes from six sites in four districts of Botswana. Three of these four districts, Ngami, Okavango and Boteti, are prone to malaria, whereas Ghanzi district encounters sporadic outbreaks of malaria (Botswana Ministry of Health and Wellness 2017). In all the study areas, An. arabiensis, the main known malaria vector in Botswana, accounted for the highest frequency (91.1%) among the Anopheles species found. Tawe et al. (2017) reported the frequency of An. arabiensis in 2014–2015 transmission season from six districts (Okavago, Ngami, Boteti, Tutume, Chobe and Kweneng West) being 58.9%, with presence of members of the An. funestus group at higher frequencies compared with what was observed in this study. One possible explanation for this change in species diversity could be due to the extreme drought experienced by Botswana in the 2015–2016 summer season that was driven by a very strong El Niño event (Byakatondaa et al. 2018; Siderius et al. 2018). It is well established that An. arabiensis shows considerable ecological and behavioural plasticity that allows it to survive in the harsh conditions of some arid areas, while An. funestus requires more permanent breeding grounds and it is more susceptible to changes in weather patterns (Kent et al. 2007). Furthermore, the impact of the El Niño southern oscillation (ENSO) pattern on the annual malaria incidence in southern Africa has been assessed (Mabaso et al. 2007). A possible explanation for our results is that during a very dry summer season, the Anopheles species diversity in Botswana is likely to drop, with a higher rate of persistence of An. arabiensis when compared with other species. This would decrease species competition and possibly also increase malaria transmission as reported by WHO in low transmission areas of southern Africa (WHO 2018). Longer longitudinal observations should be carried out to verify this working hypothesis.
The high frequencies of An. arabiensis in this study implicate this species as the main malaria vector in Botswana. Human DNA was detected in An. arabiensis from all the study villages indicating that the fraction of mosquitoes collected indoor readily feed on human blood, while no human DNA was detected in other collected Anopheles species, despite we should remind their very low number. Furthermore, P. falciparum DNA was detected in a single specimen of An. arabiensis from Shorobe that was negative for human DNA. This indicates that the parasite had progressed to the salivary glands of the mosquito for development into infective sporozoites. Plasmodium vivax DNA was not detected among any of the Anopheles mosquitoes.
This study was limited because it does not establish if An. arabiensis fed on humans outdoors or indoors. This is especially important because An. arabiensis populations exhibit both exophilic and endophilic behaviours and various degrees of anthropophily depending on the prevailing ecological conditions (Costantini et al. 1999; Kent et al. 2007). Understanding vector biology is very important when considering the effectiveness of vector control measures for blocking transmission (Tawe et al. 2017). Despite limited malaria vector research, Botswana has made extraordinary progress in the fight against malaria and now envisions malaria elimination by 2020, together with eight other countries under the Elimination 8 committee.
Botswana mainly depends on the use of LLINs and IRS for vector control. However, emerging resistance to pyrethroids (the only class of insecticides used in insecticide-treated nets) and DDT has the potential to render LLINs and IRS interventions ineffective. This study provides information on the status of kdr in Botswana in An. arabiensis, a target site resistance mechanism that has been associated to resistance to both pyrethroids and DDT. All specimens screened were found to be homozygous wild type, indicating that the mosquitoes do not have either the kdr-w or kdr-e.
Though, our study was limited by the lack of phenotypic data showing absence/presence of insecticide resistance. In addition, the sampling strategy of PSS may impact on our results. Moreover, kdr is not the only mechanism conferring resistance. In fact, there are several other insecticide resistance mechanisms such as metabolic resistance, behavioural and cuticular resistance that can be also explored. Our results highlight the absence of kdr-resistant alleles in the An. arabiensis population of Botswana but they do not conflict with the possibility of in vivo resistance to insecticides. During 2016–2018, the National Malaria Programme in Botswana has carried out the phenotypic resistance assay for the local population of An. arabiensis using the WHO guidelines, but did not characterise the underlying resistance mechanisms (Botswana Ministry of Health and Wellness 2018b). In summary, resistance to pyrethroid (lambda-cyhalothrin) was identified for An. arabiensis in 5 monitored sentinel sites. There are also indications of possible resistance to DDT in 4 sentinel sites. On the other hand, susceptibility to bendiocarb was confirmed in one site. In line with our results, in the two other southern African countries where pyrethroid resistance has been detected in An. arabiensis (Zimbabwe and South Africa), no kdr mutations were seen (Munhenga et al. 2008; Nardini et al. 2013). It is likely that southern African An. arabiensis are not prone to having mutations arise in the sodium channel gene and indicate marginal incongruence when compared with other members of the An. gambiae complex and to populations occurring elsewhere in Africa.
Malaria control interventions in Botswana target indoor feeding and resting malaria vectors, but it is also important to evaluate other possible measures targeting outdoor resting vectors, when accounting for the presence of An. arabiensis (Tawe et al. 2017). Even though LLINs and IRS are highly effective, they are generally insufficient to stop malaria transmission in many settings because of operational constraints, possible resistance to available insecticides and mosquitoes that behaviourally avoid contact with these interventions (Killeen et al. 2017). Bio-larviciding using Bacillus thuringiensis var. israelensis as an additional vector control measure targeting larvae, has been evaluated in Bobirwa district, Botswana (Mpofu et al. 2016). This resulted in reduced larval density and ultimately reduced the density of adult mosquitoes. Therefore, further strengthening of vector control interventions through integrated vector management (IVM) is relevant in Botswana. Further efforts are required to go from low transmission situations to sustained local and country-wide malaria elimination (Beier et al. 2008).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bass C, Nikou D, Donnelly MJ, Williamson MS, Ranson H, Ball A, Vontas J, Field LM (2007) Detection of knockdown resistance kdr mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar J 6:111. https://doi.org/10.1186/1475-2875-6-111
Beier JC, Keating J, Githure JI, Macdonald MB, Impoinvil DE, Novak RJ (2008) Integrated vector management for malaria control. Malar J 7:S4. https://doi.org/10.1186/1475-2875-7-S1-S4
Botswana Ministry of Health and Wellness (2017). Press release: Malaria Outbreak Awareness. https://www.moh.gov.bw/press%20release/MALARIA%20PRESS%20RELEASE.pdf. Accessed 30 September 2018
Botswana Ministry of Health and Wellness (2018a). Press release: public awareness on malaria. http://www.moh.gov.bw/press%20release/awareness_malaria.pdf. Accessed 30 September 2018
Botswana Ministry of Health and Wellness (2018b) National plan for insecticide resistance prevention and management in malaria vectors 2018–2021. National Malaria Programme, Gaborone Accessed 2 December 2019
Byakatondaa J, Parida BP, Moalafhi DB, Kenabatho PK (2018) Analysis of long term drought severity characteristics and trends across semiarid Botswana using two drought indices. Atmos Res 213:492–508. https://doi.org/10.1016/j.atmosres.2018.07.002
Chihanga S, Haque U, Chanda E, Mosweunyane T, Moakofhi K, Jibril HB, Motlaleng M, Zhang W, Glass GE (2016) Malaria elimination in Botswana, 2012-2014: achievements and challenges. Parasit Vectors 9:99. https://doi.org/10.1186/s13071-016-1382-z
Chirebvu E, Chimbari MJ (2015) Characteristics of Anopheles arabiensis larval habitats in Tubu village, Botswana. J Vector Ecol 40:129–138. https://doi.org/10.1111/jvec.12141
Chirebvu E, Chimbari MJ (2016) Characterization of an indoor-resting population of Anopheles arabiensis (Diptera: Culicidae) and the implications on malaria transmission in Tubu Village in Okavango subdistrict, Botswana. J Med Entomol 53:569–576. https://doi.org/10.1093/jme/tjw024
Chirebvu E, Chimbari MJ, Ngwenya BN (2014) Assessment of risk factors associated with malaria transmission in Tubu village, northern Botswana. Malar Res Treat 2014:403069. https://doi.org/10.1155/2014/403069
Chirebvu E, Chimbari MJ, Ngwenya BN, Sartorius B (2016) Clinical malaria transmission trends and its association with climatic variables in Tubu village, Botswana: a retrospective analysis. PLoS One 11:e0139843. https://doi.org/10.1371/journal.pone.0139843
Choi KS, Coetzee M, Koekemoer LL (2010) Simultaneous identification of the Anopheles funestus group and Anopheles longipalpis type C by PCR-RFLP. Malar J 9:316. https://doi.org/10.1186/1475-2875-9-316
Cornel AJ, Lee Y, Almeida APG, Johnson T, Mouatcho J, Venter M, de Jager C, Braack L (2018) Mosquito community composition in South Africa and some neighboring countries. Parasit Vectors 11:331. https://doi.org/10.1186/s13071-018-2824-6
Costantini C, Sagnon N, della Torre A, Coluzzi M (1999) Mosquito behavioural aspects of vector-human interactions in the Anopheles gambiae complex. Parassitologia 41:209–217
Davies TG, Field LM, Usherwood PN, Williamson MS (2007) DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life 59:151–162. https://doi.org/10.1080/15216540701352042
Djimdé A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourté Y, Coulibaly D, Dicko A, Su XZ, Nomura T, Fidock DA, Wellems TE, Plowe CV (2001) A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med 344:257–263. https://doi.org/10.1056/NEJM200101253440403
Field LM, Emyr Davies TG, O’Reilly AO, Williamson MS, Wallace BA (2017) Voltage-gated sodium channels as targets for pyrethroid insecticides. Eur Biophys J 46:675–679. https://doi.org/10.1007/s00249-016-1195-1
Gillies MT, Coetzee M (1987) A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical region). Publications of the South African Institute for Medical Research, Johannesburg
Kent RJ, Thuma PE, Mharakurwa S, Norris DE (2007) Seasonality, blood feeding behavior, and transmission of Plasmodium falciparum by Anopheles arabiensis after an extended drought in southern Zambia. Am J Trop Med Hyg 76:267–274. https://doi.org/10.4269/ajtmh.2007.76.267
Killeen GF, Tatarsky A, Diabate A, Chaccour CJ, Marshall JM, Okumu FO, Brunner S, Newby G, Williams YA, Malone D, Tusting LS, Gosling RD (2017) Developing an expanded vector control toolbox for malaria elimination. BMJ Glob Health 2:e000211. https://doi.org/10.1136/bmjgh-2016-000211
Koekemoer LL, Kamau L, Hunt RH, Coetzee M (2002) A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg 66:804–811
Kyalo D, Amratia P, Mundia CW, Mbogo CM, Coetzee M, Snow RW (2017) A geo-coded inventory of anophelines in the Afrotropical region south of the Sahara: 1898-2016. Wellcome Open Res 2:57. https://doi.org/10.12688/wellcomeopenres.12187.1
Mabaso ML, Kleinschmidt I, Sharp B, Smith T (2007) El Niño southern oscillation (ENSO) and annual malaria incidence in southern Africa. Trans R Soc Trop Med Hyg 101:326–330. https://doi.org/10.1016/j.trstmh.2006.07.009
Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Bergé JB, Devonshire AL, Guillet P, Pasteur N, Pauron D (1998) Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol 7:179–218. https://doi.org/10.1046/j.1365-2583.1998.72062.x
Motshoge T, Ababio GK, Aleksenko L, Read J, Peloewetse E, Loeto M, Mosweunyane T, Moakofhi K, Ntebele DS, Chihanga S, Motlaleng M, Chinorumba A, Vurayai M, Pernica JM, Paganotti GM, Quaye IK (2016) Molecular evidence of high rates of asymptomatic P. vivax infection and very low P. falciparum malaria in Botswana. BMC Infect Dis 16:520. https://doi.org/10.1186/s12879-016-1857-8
Mpofu M, Becker P, Mudambo K, de Jager C (2016) Field effectiveness of microbial larvicides on mosquito larvae in malaria areas of Botswana and Zimbabwe. Malar J 15:586. https://doi.org/10.1186/s12936-016-1642-6
Munhenga G, Masendu HT, Brooke BD, Hunt RH, Koekemoer LK (2008) Pyrethroid resistance in the major malaria vector Anopheles arabiensis from Gwave, a malaria-endemic area in Zimbabwe. Malar J 7:247. https://doi.org/10.1186/1475-2875-7-247
Nardini L, Christian RN, Coetzer N, Koekemoer LL (2013) DDT and pyrethroid resistance in Anopheles arabiensis from South Africa. Parasit Vectors 6:229. https://doi.org/10.1186/1756-3305-6-229
Pachka H, Annelise T, Alan K, Power T, Patrick K, Véronique C, Janusz P, Ferran J (2016) Rift Valley fever vector diversity and impact of meteorological and environmental factors on Culex pipiens dynamics in the Okavango Delta, Botswana. Parasit Vectors 9:434. https://doi.org/10.1186/s13071-016-1712-1
Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH (2000) Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol 9:491–497. https://doi.org/10.1046/j.1365-2583.2000.00209.x
Riveron JM, Tchouakui M, Mugenzi L, Menze BD, Chiang M, Wondji CS (2018) Insecticide resistance in malaria vectors: an update at a global scale. In: Manguin S, Dev V (eds) Towards malaria elimination - a leap forward. https://doi.org/10.5772/intechopen.78375
Scott JA, Brogdon WG, Collins FH (1993) Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg 49:520–529. https://doi.org/10.4269/ajtmh.1993.49.520
Siderius C, Gannon KE, Ndiyoi M, Opere A, Batisani N, Olago D, Pardoe J, Conway D (2018) Hydrological response and complex impact pathways of the 2015/2016 El Niño in eastern and southern Africa. Earth’s Future 6:2–22. https://doi.org/10.1002/2017EF000680
Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, Patil AP, Temperley WH, Gething PW, Kabaria CW, Okara RM, Van Boeckel T, Godfray HC, Harbach RE, Hay SI (2010) The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit Vectors 3:117. https://doi.org/10.1186/1756-3305-3-117
Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN (1993) Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol 58:283–292. https://doi.org/10.1016/0166-6851(93)90050-8
Soderlund DM, Knipple DC (2003) The molecular biology of knockdown resistance to pyrethroid insecticides. Insect Biochem Mol Biol 33:563–577. https://doi.org/10.1016/S0965-1748(03)00023-7
Tawe L, Ramatlho P, Waniwa K, Muthoga CW, Makate N, Ntebela DS, Quaye IK, Pombi M, Paganotti GM (2017) Preliminary survey on Anopheles species distribution in Botswana shows the presence of Anopheles gambiae and Anopheles funestus complexes. Malar J 16:106. https://doi.org/10.1186/s12936-017-1756-5
Thomson MC, Mason SJ, Phindela T, Connor SJ (2005) Use of rainfall and sea surface temperature monitoring for malaria early warning in Botswana. Am J Trop Med Hyg 73:214–221. https://doi.org/10.4269/ajtmh.2005.73.214
World Health Organization (2018) World malaria report. https://apps.who.int/iris/bitstream/handle/10665/275867/9789241565653-eng.pdf?ua=1. Accessed 16 February 2019
Zaim M, Aitio A, Nakashima N (2000) Safety of pyrethroid-treated mosquito nets. Med Vet Entomol 14:1–5. https://doi.org/10.1046/j.1365-2915.2000.00211.x
Acknowledgements
We thank Dr. Lizette Koekemoer and the Malaria Entomology Research Group at the University of the Witwatersrand, Johannesburg, South Africa, for assisting with kdr genotyping; Dr. Verena Pichler at Sapienza University of Rome, Italy, for providing the control DNA of An. arabiensis specimen of the three different kdr genotypes; finally, Mr. Charles Waithaka Muthoga, Mr. Leabaneng Tawe and Mr. Zackary Bango from Botswana-University of Pennsylvania Partnership, for their assistance and endless efforts in helping the team.
Funding
This study was funded by the National Malaria Programme in the Ministry of Health and Wellness of Botswana, Department of Biological Sciences of the University of Botswana, University of Botswana (grant # P1130) and with support from the Botswana-University of Pennsylvania Partnership, Penn Center for AIDS Research (grant # P30 AI045008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Helge Kampen
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kgoroebutswe, T.K., Ramatlho, P., Reeder, S. et al. Distribution of Anopheles mosquito species, their vectorial role and profiling of knock-down resistance mutations in Botswana. Parasitol Res 119, 1201–1208 (2020). https://doi.org/10.1007/s00436-020-06614-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06614-6