Abstract
Coccidiosis is caused by multiple species of the apicomplexan protozoa Eimeria. Among them, Eimeria tenella is frequently considered to be the most pathogenic. Zinc finger proteins (ZnFPs) are a type of protein containing zinc finger domains. In the present study, a putative Eimeria tenella AN1-like ZnFP (E. tenella AN1-like zinc finger domain-containing protein, putative partial mRNA, EtAN1-ZnFP) was cloned and characterized, and its immune protective effects were evaluated. The 798-bp ORF sequence of EtAN1-ZnFP that encoded a protein of approximately 27.0 kDa was obtained. The recombinant EtAN1-ZnFP protein (rEtAN1-ZnFP) was expressed in Escherichia coli. Western blot analysis showed that the recombinant protein was recognized by the anti-GST monoclonal antibody and anti-sporozoite protein rabbit serum. qPCR analysis revealed that EtAN1-ZnFP was highly expressed in unsporulated oocysts and sporozoites. Immunostaining with an anti-rEtAN1-ZnFP antibody indicated that EtAN1-ZnFP was uniformly distributed in the cytoplasm of sporozoites, except for the refractive body; furthermore, this protein was evenly distributed in the cytoplasm of immature schizonts but seldom distributed in mature schizonts. The results of the in vitro invasion inhibition assay indicated that the antibodies against rEtAN1-ZnFP efficiently reduced the ability of E. tenella sporozoites to invade host cells. Animal challenge experiments demonstrated that the chickens immunized with rEtAN1-ZnFP protein significantly decreased mean lesion scores and fecal oocyst output compared with challenged control group. The results suggest that EtAN1-ZnFP can induce partial immune protection against infection with E. tenella and could be an effective candidate for the development of new vaccines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The protozoan parasite Eimeria tenella is a major causative agent of avian coccidiosis, causing considerable economic losses to the poultry industry worldwide (Chapman et al. 2013). At present, the control of coccidiosis relies primarily on prevention and use of anticoccidial drugs and live vaccines. However, Eimeria spp. have developed resistance to most commercially available anticoccidial drugs (Chapman 1998). Furthermore, the residues of these drugs have led to food safety concerns, and live anticoccidial vaccines are more costly than anticoccidial drugs (Shirley et al. 2007). Subunit vaccines are much less effective than live ones but are easier to produce, convenient, and safe (Peek et al. 2008). Therefore, novel immunoprotective antigens are urgently needed. However, the coccidial antigens evaluated to date can only induce partial immune protection, probably because of the complex life cycle of coccidia. The immunoprotective antigens identified and characterized to date include apical membrane antigen-1, refractile body proteins, microneme proteins, elongation factor-1α, heat shock proteins, refractile body protein SO7, surface antigens, IMP1, profilin, and so on (Blake et al. 2017; Lin et al. 2017; Liu et al. 2018; Pastor-Fernandez et al. 2018; Plattner et al. 2008; Rafiqi et al. 2018; Yin et al. 2013).
Eukaryotic proteins containing zinc finger domains are known as zinc finger proteins (ZnFPs). ZnFPs play an essential role in the recognition and binding of DNA, RNA, and protein by a unique finger structure (Ghosh and Chatterji 2017; Liu and Heermann 2015). In plants such as rice, soybean, Arabidopsis, petunia, wheat, and cotton, ZnFPs are involved in resistance to environmental stress and plant growth and development (Jin et al. 2018). In mycobacteria, MsZnFP1 and MsZnFP2 activate transcription by interacting with RNA polymerase (Ghosh and Chatterji 2017). In eukaryotes, ZnFPs are widely expressed and participate in important biological processes, including cell differentiation, proliferation, and apoptosis (Iuchi 2001). In Toxoplasma gondii, TgZNF2 may play a key role in mRNA nuclear export (Gissot et al. 2017). Eimeria tenella is one of the most important species causing avian coccidiosis and is frequently used as a model to study species of Eimeria. It belongs to the phylum Apicomplexa with a complex life cycle and needs to invade the epithelial cells lining the intestine of chicken to grow and develop. Hence, we speculated that ZnFPs may be involved in these important biological processes in the life cycles of E. tenella.
However, to the best of our knowledge, no studies to date investigated ZnFPs in E. tenella. In the present study, the putative E. tenella AN1-like ZnFP EtAN1-ZnFP was cloned, and the recombinant protein GST-EtAN1-ZnFP (rEtAN1-ZnFP) was produced in an Escherichia coli BL21 (DE3) expression system. Anti-rEtAN1-ZnFP antibodies were produced in immunized rabbits. These antibodies were used to localize EtAN1-ZnFP in parasites by immunofluorescence assay and to assess the inhibitory effect of EtAN1-ZnFP using an in vitro assay. The levels of EtAN1-ZnFP transcripts at different development stages of the parasite were measured by quantitative real-time PCR (qPCR), and the immunogenicity of EtAN1-ZnFP was evaluated in a chicken challenge model. The results of the present study indicate that AN1-ZnFP may participate in parasite growth and development.
Materials and methods
Parasite preparation
The Shanghai strain of E. tenella was initially isolated in the 1980s from a sample collected in a farm in Shanghai, China, and was subsequently maintained in our laboratory (Resource Number CAAS21111601) (Huang et al. 1993). The parasites were propagated by passage through coccidian-free 2-week-old chickens, as described previously (Tomley 1997). Each animal was infected by the oral route with 1 × 104 sporulated oocysts (SO). Unsporulated oocysts (UO) were obtained from caeca on day 8 post-infection (p.i.). Feces were collected on days 6 to 8 p.i. and oocysts were sporulated in 2.5% potassium dichromate at 28 °C for up to 96 h (Smith et al. 1993). UO and SO were purified using a standard method, as previously described (Han et al. 2010). Sporozoites (Spz) were excysted in vitro from cleaned sporulated oocysts (Miska et al. 2004). Second-generation merozoites (Mrz) were collected from the cecal contents or mucosa of chickens at 115 h p.i. (Xie et al. 1990).
The chicken fibroblast cell line DF-1 derived from East Lansing Line (ELL-0) chicken embryos was used in animal infection experiments and inhibition and immunofluorescence assays (Jiang et al. 2012).
Amplification and sequence analysis of EtAN1-ZnFP
Total RNA was extracted from UO using TRIzol reagent (TaKaRa, Tokyo, Japan) according to the manufacturer’s instructions. Briefly, a total of 1.0 × 107 UO of E. tenella were oscillated and broken in 500 μL of TRIzol with a half volume of 710–1180 μm glass beads (Sigma, St. Louis, MO, USA) for 10 min. Total RNA was precipitated with isopropanol and washed with 75% ethanol and then resuspended in diethyl pyrocarbonate (DEPC)-treated water. Total RNA concentrations were determined by absorbance readings at 260 nm on a UV spectrophotometer (Eppendorf, Hamburg, Germany). The RNA quality was assessed by electrophoresis on a 1% agarose-formaldehyde ethidium bromide gel. Complementary DNA (cDNA) was synthesized from total RNA using an M-MLV Reverse Transcriptase kit (Invitrogen, Carlsbad, CA, USA) with oligo dT primers.
The ORF sequence of the EtAN1-ZnFP gene of E. tenella (GenBank accession number XM_013374066.1) was amplified using the primers 5′-ATGAGCTCAGAGCAACACG-3′ (forward) and 5′-AAAGCTTCTGGAGTTTGTCTG-3′ (reverse). Conventional PCR was carried out in a 25-μL reaction system using the cDNA of UO as a template. The amplification reactions were performed using the following conditions: pre-denaturation at 95 °C for 3 min, denaturation at 95 °C for 30 s, 62 °C for 30 s, and 72 °C for 1 min, and extension at 72 °C for 10 min. The amplified PCR products were detected by electrophoresis on a 1.0% agarose gel and purified using the QIAquick® Gel Extraction Kit (QIAGEN, Duesseldorf, Germany). The EtAN1-ZnFP fragment was subcloned into the pGEM-T-easy vector (Promega, Madison, WI, USA) using T4 DNA ligase to construct a recombinant plasmid. The size of the recombinant plasmid was confirmed by bidirectional nucleotide sequence analysis with the primers SP6: 5′-ATTTAGGTGACACTATAG-3′; and T7: 5′-TAATACGACTCACTATAGGG-3′ by Sangon (Shanghai, China).
The full-length cDNA sequence was analyzed. The molecular mass and theoretical isoelectric point were predicted using the ProtParam tool at the ExPASy server (http://web.expasy.org/protparam/). Signal peptides, transmembrane motifs, and protein motifs were predicted using the computational tools SignalP (http://www.cbs.dtu.dk/services/SignalP/), TMHMM (http://www.cbs.dtu.dk/services/TMHMM-2.0/), and Motif Scan (http://hits.isb-sib.ch/cgi-bin/motif_scan), respectively.
Expression and purification of recombinant EtAN1-ZnFP protein
The EtAN1-ZnFP open reading frame (ORF) was amplified by PCR using the primers 5′-GCGGATCCATGAGCTCAGAGCAACACGAAAACGAAAGGCCTTCTGCTCCGCCCTTGTGTGCGAAGAACTGCGGCTT-3′ (forward) and 5′-GCGTCGACTCAAAGCTTCTGGAGTTTGTCTG-3′ (reverse), incorporating the BamHI and SalI restriction sites (underlined), respectively. The amplified fragment and the pGEX-4T-1 vector were digested with BamHI/SalI. The BamHI/SalI double-digested EtAN1-ZnFP fragment and the pGEX-4T-1 vector were gel purified and ligated, and the recombinant pGEX-4T-EtAN1-ZnFP plasmid was transformed into E. coli BL21(DE3) (Tiangen, Beijing, China). The recombinant protein GST-EtAN1-ZnFP (rEtAN1-ZnFP) was expressed in E. coli BL21(DE3) as a glutathione S-transferase (GST) fusion protein. The expression of rEtAN1-ZnFP was induced in culture at an optical density of 0.6 with 1.0 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) (Sigma, St Louis, MO, USA) for 8 h at 37 °C. The cell pellets were lysed by sonication on ice, working 2 s to stop for 2 s and lasting 20 min. The rEtAN1-ZnFP protein was purified from lysate supernatants using GST-bind resin (Beyotime, Haimen, China). The protein concentration was determined using the BCA protein assay kit (Beyotime, Haimen, China). The purified protein was aliquoted and stored at − 20 °C for further analysis.
Production of anti-rEtAN1-ZnFP and anti-sporozoite polyclonal serum
The sporozoite protein was prepared using sonication according to the previous description (Jiang et al. 2012). Two-month-old New Zealand white rabbits were subcutaneously immunized with 200 μg of purified rEtAN1-ZnFP or sporozoite protein emulsified in Freund’s complete adjuvant (Sigma, St. Louis, MO, USA). Two weeks later, the rabbits were given a subcutaneous booster injection with the same amount of the protein emulsified in Freund’s incomplete adjuvant (Sigma, St Louis, MO, USA). Immunization was carried out once every 7 days, with a total of five immunizations. Seven days after the last immunization, the polyclonal antibody serum was separated from the blood of two rabbits. Negative serum was collected from the rabbits’ ear vein before immunization.
SDS-PAGE and western blot for recombinant EtAN1-ZnFP
The purified rEtAN1-ZnFP proteins were separated by SDS-PAGE and transferred to nitrocellulose membrane (Merck Millipore, Billerica, MA, USA). After being blocked with 5% (w/v) skimmed milk powder in tris-buffered saline–Tween 20 (TBST), the membranes were incubated with primary antibodies (anti-GST monoclonal antibody and anti-sporozoite protein serum, respectively) for 2 h at 37 °C (dilutions 1:1000 to anti-GST monoclonal antibody, 1:200 to anti-sporozoite protein serum). Secondary antibodies, IRDye® 800CW Goat anti-Rabbit IgG (LI-COR, Lincoln, NE, USA), were added after being washed three times with PBS. Then, the membranes were detected using Odyssey (LI-COR, Lincoln, NE, USA).
Detection of EtAN1-ZnFP in different developmental stages of E. tenella by quantitative real-time PCR
To detect the expression of EtAN1-ZnFP at different developmental stages of E. tenella (UO, SO, Spz, and Mrz), total RNAs were isolated by TRIzol reagent (Invitrogen) from four life stages of E. tenella and treated with DNase I (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Briefly, a total of 1.0 × 107 UO or SO of E. tenella were oscillated and broken in 500 μL of TRIzol with a half volume of 710–1180 μm glass beads (Sigma, St. Louis, MO, USA) for 10 min. A total of 1.0 × 107 sporozoites or second-generation merozoites were lysed in 500 μL of TRIzol. Total RNAs were precipitated with isopropanol and washed with 75% ethanol and then resuspended in diethyl pyrocarbonate (DEPC)-treated water. First cDNA strands were synthesized using SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and random primers (Invitrogen, Carlsbad, CA, USA). qPCR was performed on an Eppendorf Mastercycler ep Realplex thermal cycler (Eppendorf, Hamburg, Germany) using the SYBR1 Green I dye and the primers 5′-CGTCGTCAGCAGTTCCCGCAGAGCA-3′ (forward) and 5′-CATCATCAGCAGGTTCTTGCGTAGG-3′ (reverse). A housekeeping gene of E. tenella (18S ribosomal RNA) was used as a normalizing control and was amplified using the primers 5′-TGTAGTGGAGTCTTGGTGATTC-3′ (forward) and 5′-CCTGCTGCCTTCCTTAGATG-3′ (reverse). Each reaction was performed in triplicate, and the experiment was repeated three times. The relative expression of EtAN1-ZnFP was calculated using the 2−ΔΔCt method (Livak and Schmittgen 2001).
Localization of EtAN1-ZnFP by indirect immunofluorescence
Indirect immunofluorescence was carried out as described (Jiang et al. 2012) using antiserum to rEtAN1-ZnFP. The DF-1 chicken fibroblast cell line was used in indirect immunofluorescence analyses (Jiang et al. 2012). Briefly, 2 × 105 DF-1 cells were cultured in six-well plates (Corning, NY, USA) with precoated glass coverslips in complete medium (CM) (Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum, 100 units/mL penicillin/streptomycin, and 2 mM l-glutamine) for 24 h at 37 °C and 5% CO2. Freshly cleaned E. tenella sporozoites (6 × 105 parasites per well) were incubated in CM for 2 h at 41 °C and were used to infect DF-1 cells. The coverslips were collected at 48, 60, and 82 h p.i. All the samples were fixed in 4% paraformaldehyde for 20 min at room temperature, permeabilized with 1% Triton X-100 in PBS for 15 min, and then blocked with 2% bovine serum albumin in PBS overnight at 4 °C. The samples were incubated with rabbit anti-rEtAN1-ZnFP polyclonal antibody at a dilution of 1:100 for 2 h at 37 °C, followed by the goat anti-rabbit IgG fluorescein isothiocyanate (FITC)-conjugated antibody (1:500 dilution) (Sigma, St. Louis, MO, USA) for 1 h at 37 °C in a moist, dark chamber. The cell nuclei were stained with 15 μg/mL 4, 6-diamidino-2-phenylindole (DAPI) (Beyotime, Haimen, China) at room temperature for 30 min in wet and dark conditions. All the samples were washed four times in PBS at the end of each step. After that, the coverslips were mounted on glass slides using 60 μL of Fluoromount Aqueous Mounting Medium (Sigma, St. Louis, MO, USA). The glass slides were examined with a fluorescence microscope (Zeiss LSM800 microscope, Carl Zeiss, Germany). Sporozoites and second-generation merozoites were prepared for immunofluorescence using the same method. Briefly, the second-generation merozoites were evenly spread on glass coverslips. Then, the coverslips were fixed with 4% paraformaldehyde, permeabilized with 1% Triton X-100 in PBS, and blocked with 2% bovine serum albumin in PBS. The samples were incubated with rabbit anti-rEtAN1-ZnFP polyclonal antibody and further incubated with FITC-conjugated antibody. Cell nuclei were labeled with DAPI.
Sporozoite invasion inhibition assay
Flat-bottomed 24-well plates (Corning, NY, USA) were seeded with DF-1 cells (3 × 105 cells per well). These cells were sequentially cultured in DMEM with 10% fetal bovine serum and 300 units/mL penicillin/streptomycin for 16 h at 37 °C and 5% CO2 and DMEM containing 5% fetal bovine serum, 300 units/mL penicillin/streptomycin for 8 h at 37 °C, and 5% CO2. Invasion assays were performed as described previously using E. tenella sporozoites infecting DF-1 cells (Jiang et al. 2012). Antibodies were purified using protein A+G agarose (Beyotime, Haimen, China), according to the manufacturer’s instructions. Freshly purified sporozoites were stained for 15 min using carboxyfluorescein diacetate succinimidyl ester (CFDA SE) (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions and incubated with 50, 100, 200, or 400 μg/mL of purified IgG against rEtAN1-ZnFP for 2 h at 37 °C. The concentration of IgG was determined by BCA (bicinchoninic acid) Protein Assay Kit (Beyotime, Haimen, China). The same amount of IgG from naive rabbit serum was used as a control. Stained and IgG-untreated sporozoites were used as a blank control. After that, DF-1 cells were infected with sporozoites (6 × 105 parasites per well) in 24-well plates (Corning, NY, USA) and cultured for 12 h at 41 °C and 5% CO2. The cells were washed, trypsinized, harvested, and analyzed on a flow cytometer (model Cytomics FC500; Beckman Coulter, CA, USA). All assays were performed in triplicate. The deduced percentages of infected cells in the presence or absence of inhibitory antibody were used to calculate inhibition rates, as detailed previously (Jahn et al. 2009).
Immunization experiment design
For the challenge experiment, forty-eight chickens (yellow-feathered broilers, Shanghai Fengxian District, China) were reared in steel cages under coccidia-free conditions from the day of hatching (day 0) until 7 days of age (Gharaibeh and Mahmoud 2013). After that, chickens were randomly divided into four groups of 12 birds. Each animal was immunized at 7 days of age by subcutaneous booster injection with 50 μg or 100 μg of purified rEtAN1-ZnFP protein emulsified in Montanide ISA 71 adjuvant (Seppic, Puteaux, France) as a 3:7 mixture (Jang et al. 2013, 2010). Two groups (one challenged and one unchallenged group) were immunized with PBS, at pH 7.2, and served as negative controls. A booster immunization was given 1 week later with the same amount of components as the first immunization. At 7-day post-secondary immunization, chickens of immunized groups and challenged-control group were orally challenged with 1.0 × 104 SO of E. tenella whereas birds of the unchallenged control group were given an equal volume of PBS orally.
Evaluation of immune protection of EtAN1-ZnFP
Protective efficacy was evaluated by the average body weight gain, mean lesion scores, fecal oocyst output, and oocyst decrease ratio. Body weight was measured on days 0 and 8 post challenge. Fecal samples were collected daily from days 6 to 8 post challenge. The number of fecal oocysts was counted using a McMaster chamber, as previously described. The oocyst decrease ratio was calculated by the formula: (number of oocysts from the challenged − unvaccinated group − number of oocysts from the challenged − vaccinated group)/number of oocysts from the challenged − unvaccinated group × 100% (Rose and Mockett 1983). The caeca of each group were collected separately. Intestinal lesions were scored according to the method of Johnson and Reid (Morehouse and Baron 1970).
Preparation of the serum
The blood samples of day 8 post challenge were collected, incubated at 37 °C for 1 h, and centrifuged at 5000 rpm for 5 min at 4 °C to isolate the serum. The sera were used for the detection of antibody, cytokines, sCD4, and sCD8.
Determination of serum antibody levels
The levels of antibody (serum IgG) against rEtAN1-ZnFP were measured by enzyme-linked immunosorbent assay (ELISA) at day 8 post challenge, as previously described (Lee et al. 2013; Lillehoj et al. 2005; Lin et al. 2017; Ding et al. 2004). Briefly, 96-well microtiter plates (Corning, NY, USA) were coated with purified rEtAN1-ZnFP (10 μg/well) at 4 °C overnight. All plates were washed with PBS (pH 7.2) containing 0.05% Tween 20 (PBS-T) more than three times and blocked with PBS containing 1% BSA for 2 h at 37 °C. Serum samples were added to the plates at a 1:25 dilution (50 μL/well) and incubated for 1 h at 37 °C. After washing five times with PBS-T, 50 μL/well HRP-donkey-anti-chicken IgG antibody (Sigma, St. Louis, MO, USA) with a 1:5000 dilution was added and incubated for 2 h at 37 °C. The plates were washed five times with PBS-T and developed with 3,3′,5,5′-tetramethylbenzidine. Optical densities at 450 nm (OD450) were measured on a microplate spectrophotometer. All assays were performed in triplicate.
Determination of cytokine, sCD4, and sCD8 levels
The immune stimulation effect of rEtAN1-ZnFP protein on chickens was evaluated by quantitative ELISA at day 8 post challenge, as previously described (Lee et al. 2013; Lillehoj et al. 2005; Lin et al. 2017; Ding et al. 2004). The cytokines, sCD4, and sCD8 used were soluble cluster of differentiation 4 (sCD4), soluble cluster of differentiation 8 (sCD8), interleukin-17 (IL-17), and transforming growth factor (TGF-β1). Chick Cytokine ELISA Quantitation Kits (catalog numbers CSB-E13114C, CSB-E14317C, CSB-E04607Ch, and CSB-E09875Ch; CUSABIO, Wuhan, China) were used to quantify sCD4, sCD8, IL-17, and TGF-β1, respectively.
Statistical analysis
SPSS version 22 (SPSS, Chicago, IL, USA) was used in body weight gain, mean lesion scores, fecal oocyst output, and oocyst decrease ratio. Microsoft Office Excel version 2016 for Windows (Redmond, WA, USA) was used in record original data of body weight, oocyst count antibody levels, and cytokine levels. GraphPad Prism version 6.0 (GraphPad, La Jolla, CA, USA) was used in these analyses. Data on real-time quantitative PCR (qPCR), invasion inhibition, body weight gain, fecal oocyst output, oocyst decrease ratio, and antibody and cytokine levels were analyzed. Differences between groups were analyzed by one-way analysis of variance (ANOVA) and Duncan’s multiple range test. The lesion scores were compared by the non-parametric Kruskal–Wallis test. p values smaller than 0.05 were considered significant, and p values smaller than 0.01 were considered highly significant. Bars with different letters were significantly different (p < 0.05) and the error bars indicate standard deviations.
Results
Characterization of the EtAN1-ZnFP sequence
PCR amplification of EtAN1-ZnFP resulted in a 798-bp product. Nucleotide sequence analysis showed that the 798-bp length of ORF sequence encodes a polypeptide of 265 amino acid residues with a predicted molecular mass of 27.0 kDa. The sequence we obtained respectively shared 100%, 92%, and 63% amino acid sequence homology with putative AN1-like ZnFPs from E. tenella, E. necatrix, and E. maxima by BLASTp analysis. The amino acid sequence had 100% homology with a putative E. tenella AN1-like ZnFP (Protein ID XP_013229520.1). SignalP program analysis revealed that the protein most likely does not have a signal peptide and a transmembrane domain. Structural module and conservative structure predictions indicated that the protein had an AN1-like zinc finger and an A20-like zinc finger domains. One zinc finger ubiquitin hydrolase domain (204-236 aa), three casein kinase II phosphorylation sites, two N-myristoylation sites, and one protein kinase C phosphorylation site were also predicted (Fig. 1).
Nucleotide and predicted amino acid sequences of EtAN1-ZnFP. The stop codon is indicated with an asterisk. Casein kinase II phosphorylation sites are double underlined. Zn-finger ubiquitin hydrolases are underlined by a wavy line. N-Myristoylation sites are shaded black with white lettering. Protein kinase C phosphorylation sites are shaded gray. The AN1-like zinc finger is indicated by a black box. The A20-like zinc finger is indicated by a black box with a dotted line. The alanine-rich region is indicated with red lettering
Expression and purification of recombinant EtAN1-ZnFP
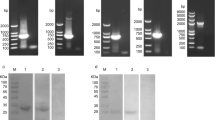
rEtAN1-ZnFP was successfully expressed in E. coli BL21(DE3) using the expression vector pGEX-4T-1 after induction with 0.8 mM IPTG for 8 h at 37 °C. The purified protein was purified from the supernatant using a GST-Bind Purification kit. The molecular mass of rEtAN1-ZnFP fused to the GST-tag was approximately 72 kDa (Fig. 2) but was larger than expected (55 kDa). Western blot analysis showed that the recombinant protein was recognized by the anti-GST monoclonal antibody (Fig. 2) and rabbit sera against sporozoites of E. tenella (Fig. 2, lane 2), but was not detected by naïve rabbit serum (Fig. 2C, lane 4).
Expression and purification of rEtAN1-ZnFP. (A) SDS-PAGE of the rEtAN1-ZnFP negative control (not induced with IPTG) (lane 2), induced with IPTG for 3 to 9 h (lanes 3 to 5). (B) Western blot analysis of purified rEtAN1-ZnFP protein recognized by an anti-GST monoclonal antibody (lane 2). (C) Western blot analysis of purified rEtAN1-ZnFP protein recognized by rabbit sera against sporozoite of E. tenella (lane 2) and incubated with naïve rabbit serum (lane 4). Lane 1 and lane 3 contain protein markers. The sizes of protein markers (kDa) are shown on the left
EtAN1-ZnFP transcription at different developmental stages of E. tenella
The transcription of EtAN1-ZnFP at different developmental stages (UO, SO, Spz, and Mrz) was assessed by qPCR. The relative expression of EtAN1-ZnFP was calculated using the 2−ΔΔCt method. Among the four development stages, EtAN1-ZnFP transcripts were most abundant in UO, moderately expressed in Spz stages, and much lower in SO and Mrz (Fig. 3). Compared with Spz, the level of EtAN1-ZnFP transcripts was significantly higher in UO (p < 0.05) and significantly lower in SO and Mrz (p < 0.05).
Quantitative real-time PCR amplification of EtAN1-ZnFP mRNA in different life stages of E. tenella UO, unsporulated oocysts; SO, sporulated oocysts; Spz, sporozoites; Mrz, merozoites. Bars with different letters indicate significant differences (p < 0.05) and the error bars indicate standard deviations
EtAN1-ZnFP localization by indirect immunofluorescence during in vitro infection
EtAN1-ZnFP was detected in sporozoites and second-generation merozoites, and during first-generation schizogony in vitro using anti-rEtAN1-ZnFP polyclonal antibody. In sporozoites incubated with PBS or CM, EtAN1-ZnFP was uniformly distributed in the cytoplasm except for the refractive body (Fig. 4A and B). After incubating for 2 h in CM, the expression level of EtAN1-ZnFP was the same as that expressed in PBS for 2 h. After sporozoite-infected DF-1 cells for 48–60 h, EtAN1-ZnFP was evenly distributed in the cytoplasm of immature schizonts (Fig. 4 and ). However, after immature schizonts differentiated into first-generation mature schizonts at 82 h p.i., the expression levels of EtAN1-ZnFP decreased, and the green fluorescence intensity was lower (Fig. 4). Furthermore, EtAN1-ZnFP was primarily located in the membrane and cytoplasm of second-generation merozoites (Fig. 4F).
EtAN1-ZnFP localization in infected DF-1 cells by indirect immunofluorescence at different developmental stages of E. tenella. Parasites were injected with anti-rEtAN1-ZnFP, stained with FITC (green), and counterstained with DAPI (blue). Infected DF-1 cells were harvested at the indicated times. (A) Sporozoites (Spz) in PBS, pRB, posterior refractile body; (B) Spz in complete medium. Infected DF-1 cells were collected at the indicated time points post-infection (pi); (C) immature schizonts (iSC) 48 h pi (hpi); (D) immature schizonts (iSC) 60 hpi; (E) intracellular merozoites (iMrz) 82 hpi; (F) merozoites (Mrz) in PBS
In vitro invasion inhibition assay
To assess the ability of EtAN1-ZnFP to inhibit the infection of E. tenella, sporozoites were purified, incubated with different concentrations of purified anti-rEtAN1-ZnFP antibody (50, 100, 200, and 300 μg/mL), and used to infect DF-1 cells. Protein function was blocked by pre-incubation of sporozoites with purified antibody against rEtAN1-ZnFP before DF-1 cell infection. The infection inhibition rate was up to 30% at 100 μg/mL (Fig. 5). Compared with the same dose of naive rabbit sera IgG (negative control), pretreatment with 50 μg/mL anti-EtAN1-ZnFP IgG had no significant effect on the invasion capacity of sporozoites (p > 0.05), while pretreatment with 100, 200, and 300 μg/mL significantly decreased invasion (p < 0.05). In contrast, naïve rabbit sera IgG did not significantly affect the rate of infection.
Inhibition of sporozoite invasion in vitro. Anti-rEtAN1-ZnFP, rabbit antiserum against recombinant EtAN1-ZnFP protein; NA, naïve rabbit serum. All assays were performed in triplicate. *p < 0.01 for differences between treatment with antibody against rEtAN1-ZnFP and naïve rabbit serum at the same IgG concentration
Protective efficacy of vaccination on E. tenella challenge
Chickens were immunized twice subcutaneously with 50 or 100 μg of purified rEtAN1-ZnFP. The body weight gain in immunized-challenged chickens was higher than that in unimmunized-challenged animals, but the difference was not significant (p > 0.05) (Table 1). Body weight gain was lower in the 50-μg– and 100-μg–immunized groups than the unimmunized-unchallenged group, but the difference was only significant (p < 0.05) in the latter group. The mean lesion scores and fecal oocyst output in rEtAN1-ZnFP-immunized chickens were significantly lower than those in unimmunized-challenged birds (p < 0.05). Vaccination with 50 μg of recombinant protein resulted in better protection than vaccination with 100 μg in terms of lesion score and body weight gain. However, oocyst decrease ratio of 100-μg–immunized group is more obvious than 50-μg–immunized group (Table 1).
IgG titers and cytokine, sCD4 and sCD8 concentrations in sera of immunized chickens
The serum IgG titers and cytokine concentrations, sCD4, and sCD8 in sera of chickens following immunization with rEtAN1-ZnFP protein are shown in Fig. 6. The IgG titers of both 50 μg rEtAN1-ZnFP-immunized group and 100 μg rEtAN1-ZnFP-immunized group were significantly higher compared with those of the unimmunized-challenged group (p < 0.05) (Fig. 6). As depicted in Fig. 6C, serum from chickens immunized with 50 μg rEtAN1-ZnFP and 100 μg rEtAN1-ZnFP protein showed significantly high levels of sCD8 (p < 0.05) compared with that from unimmunized-challenged group. But no significant differences (p > 0.05) of sCD4, IL-17, and TGF-β1 were observed between the rEtAN1-ZnFP-immunized and unimmunized-challenged groups (Fig. 6B, D, and E).
Levels of IgG (A), sCD4 (B), sCD8 (C), cytokines IL-17 (D), and TGF-β1 (E) in chicken sera were measured using ELISA. Chickens were infected with E. tenella except the unchallenged control group. Chickens of group rEtAN1-ZnFP-50 μg and group rEtAN1-ZnFP-100 μg were immunized with 50 μg or 100 μg of rEtAN1-ZnFP protein, respectively. Challenged and unchallenged groups were immunized with PBS and served as controls. The IgG titers and the concentrations of sCD4, sCD8, and cytokines are expressed as mean ± SD. Bars with different letters were considered significantly different (p < 0.05)
Discussion
ZnFPs are formed by a protein structural motif containing conserved amino acid residues tetrahedrally coordinated to one or more zinc ions containing a finger structure, which is a finger-like tetrahedral structure formed by the combination of zinc ions and several conserved amino acid residues. The zinc finger structure of ZnFPs was first discovered by Miller in 1985 in the Xenopus laevis transcription factor TFIIIA (Miller et al. 1985). Zinc ions help maintain the function of the protein (Miller et al. 1985). A previous study indicated that plant A20/AN1 ZnFPs serve as an important hub to mediate antiviral immunity (Chang et al. 2018). Zinc finger protein is a kind of transcription factor with a finger-like domain, which plays an important role in life regulation such as gene expression regulation, cell differentiation, and embryo development. Therefore, we speculated that EtAN1-ZNFP also may be involved in invasion and survival of the parasite. Whether this protein is related to the virulence or growth of the parasite in the host cells needs to be further researched. Nonetheless, few studies to date investigated ZnFPs in protozoa.
The AN1-like zinc finger and A20-like ZnFPs have been described in the literature (Mukhopadhyay et al. 2004; Vij and Tyagi 2008). In this study, we cloned and characterized a ZnFP of E. tenella. BLASTp analysis indicated that the cloned sequence shared 100%, 92%, and 63% amino acid sequence homology with putative AN1-like ZnFPs from E. tenella, E. necatrix, and E. maxima, respectively. These results indicate that AN1-like ZnFPs of E. tenella and AN1-like ZnFPs of E. necatrix have high homology. E. tenella and E. necatrix are the most pathogenic species among Eimeria spp. and they can all reside in cecal mucosa (Sharma et al. 2015).
We successfully expressed rEtAN1-ZnFP using a recombinant prokaryotic expression system. The molecular mass of rEtAN1-ZnFP was approximately 72 kDa, which is higher than that of the deduced amino acid sequence. This difference may be due to several reasons. Muh et al. compared the migration of proteins reduced or not with DTT on SDS-PAGE and found that DTT could affect migration (Muh et al. 2018). Samsó et al. (1995) reported that the large micelles of the complex could cause an abnormal electrophoretic migration of the sodium dodecyl sulfate/histone H5 complex (Samso et al. 1995). Moreover, it is well known that strongly basic or acidic proteins may migrate anomalously on SDS-PAGE gels (Papageorgiou and Soteriadou 2002).
E. tenella undergoes meiosis in the unsporulated oocyst stage when exposed to oxygen and moisture in the environment (Belli et al. 2006). In this study, the results of qPCR showed that the levels of the mRNA transcripts of EtAN1-ZnFP were high in unsporulated oocyst and sporozoite. Gissot et al. (2017) have found that the G1 phase is the period of intense production of transcripts in T. gondii, and parasite development is blocked in G1 phase when TgZnFP2 is depleted (Gissot et al. 2017). Additionally, it has been found that ZnFPs can bind to RNA in rice (Jin et al. 2018). TgZnFP2 and EtAN1-ZnFP belong to the zinc finger protein family. We supposed that the high expression of EtAN1-ZnFP in unsporulated oocyst may be related to the binding of EtAN1-ZnFP to RNA. Immunofluorescence showed that staining with EtAN1-ZnFP was stronger in the sporozoite and schizogony stages, and the result of qPCR also indicated that EtAN1-ZnFP has a high level of transcription in sporozoite stages; these suggest that this protein may be related to parasite invasion and development. Our hypothesis was confirmed by the results of the invasion inhibition assay. The inhibitory effect of polyclonal anti-rEtAN1-ZnFP IgG on sporozoites was gradually increased by up to 30%. Gissot et al. (2017) have demonstrated that TgZnFP2 is essential for parasite survival in vivo and in vitro (Gissot et al. 2017). Therefore, we postulated that EtAN1-ZnFP is involved in E. tenella invasion and development. Furthermore, no studies to date investigated ZnFPs in E. tenella; then our study provides information on this research. This new antigen might be used to identify novel vaccine targets and improve knowledge of immunogenic proteins in E. tenella.
In previous research, there was a significant decrease in lesion scores and fecal oocyst output after immunization of chickens with recombinant protein or recombinant plasmids (Huang et al. 2015; Zhai et al. 2016). In our study, following challenge infection, chickens vaccinated with rEtAN1-ZnFP had significantly lower lesion scores and fecal oocyst output compared with unimmunized birds. Our data showed that immunization with rEtAN1-ZnFP could induce partial protection against live E. tenella infection. Due to the “crowding effect,” an infective dose lower than the crowding threshold should be used for experiments on effects of immunization on oocyst production (Williams 2001). Williams (1973) have pointed that increasing doses of oocysts give rise to progressively higher oocyst yields when the infection dose is less than 2.5 × 104 oocysts per bird (Williams 1973). Our experiment which infected 1 × 104 oocysts per chicken was consistent with this report. This result highlights the importance of AN1-ZnFP in E. tenella and demonstrates its vital role in coccidiosis.
In recent years, many researchers have performed immunizations with recombinant proteins and determined the effects of these proteins by measuring cytokine levels in spleen or serum by RT-qPCR or ELISA (Ding et al. 2004; Lin et al. 2017). Kundu et al. (2017) found that chickens immunized with rEtIMP-1 produced a significantly strong IgG response (Kundu et al. 2017). Smith et al.(1993) observed that there was a slight increase in IgG levels after primary infection but a strong increase occurred after challenge (Smith et al. 1993). In the present study, the IgG concentrations in the serum of immunized chickens were significantly higher than those in the control groups by using ELISA after 8 days of challenge. A previous report also showed that chickens infected with coccidiosis can stimulate humoral immune response (Wallach 2010).
After chicken infection with coccidia, intestinal intraepithelial lymphocytes (IELs) express high levels of Th1-related cytokines and the Th2 cytokines (Cornelissen et al. 2009). The soluble sCD4 and sCD8 antigens are released by CD4+ and CD8+ lymphocytes, respectively (Zajkowska et al. 2001). The concentrations of sCD4 and sCD8 in serum are consistent with the number of CD4+ and CD8+ lymphocytes (Willsie et al. 1996). And sCD8 is a sensitive and specific parameter of cytotoxic and suppressor T cell activation (Orditura et al. 1998). In a previous study on malaria, patients infected with Plasmodium had a gradual increase in sCD8 levels, which was associated with malaria-associated immunosuppression (Harpaz et al. 1992). Liu et al. reported that the levels of sCD4 and sCD8 were higher in the groups immunized with rEmSAG compared with the control groups (Liu et al. 2018). The results in our study showed that sCD8 concentrations of the vaccinated groups were significantly higher than the control groups. These results indicate that sCD4 and sCD8 may play a role in immunization against coccidiosis and suggest that EtAN1-ZnFP can stimulate cellular immunity.
The Th2-type cytokines IL-17 which produced by Th17 cells are also involved in the immune response to coccidial challenge. Previous studies reported that the induction of Th1 cytokine-producing cells is correlated with a marked reduction in the numbers of Th2 cytokine-synthesizing cells (Bozza et al. 2004; Lu and Zhong 1999). Thus, del Cacho et al. (2012) considered that the immune response is polarized toward a Th1 response following immunization with Ag-loaded exosomes (del Cacho et al. 2012). Further, IL-17 facilitates the immunopathology during E. tenella infection and the elevated IL-17 might be harmful to the host (Huang et al. 2015). Two earlier reports showed that serum IL-17 levels were increased in all immunized birds (Huang et al. 2015; Pastor-Fernandez et al. 2018). However, our results showed that serum IL-17 was not significantly different in all immunized chicken compared with that in non-immunized chicken.
TGF-β is another cytokine that related with the immune suppression mechanism (Kehrl et al. 1986). Zhu et al. (2012) and Song et al. (2010) found that TGF-β was significantly higher in chickens immunized with recombinant EbAMA1 (Song et al. 2010; Zhu et al. 2012), which produces Treg cells involved in regulating immune responses (Chen et al. 1995; Fukaura et al. 1996). In the current study, the level of the Treg-type cytokine TGF-β1 in the rEtAN1-ZnFP-immunized groups was similar to that in the unimmunized group (p > 0.05). We speculate that the discrepancy in the results may be associated with the different parasite strains and proteins used in animal challenge experiments.
In conclusion, Th2-type cytokines regulate the immune response through different pathways, which downregulates the expression level of Th1-type cytokines (Hong et al. 2006). Th1-type cytokines predominate in coccidial infections in chickens (Choi et al. 1999; Laurent et al. 2001). In our study, serum IgG level highly increased following immunization, while high levels of sCD4 and sCD8 were increased. In contrast, IL-17 and TGF-β1 did not increase significantly. Our results corroborated this finding and indicated that Th1-type cytokines were higher after immunization with rEtAN1-ZnFP, suggesting that rEtAN1-ZnFP elicits strong immune responses to E. tenella infection.
Conclusions
This study focused on the amplification of the gene encoding a ZnFP of E. tenella, and its nucleic acid and amino acid sequences were analyzed. Moreover, the distribution of rEtAN1-ZnFP in the sporozoite and merozoite stages of the parasite was determined, and its polyclonal antibody was used to detect the inhibitory effect of this protein on sporozoite invasion. The antibody against rEtAN1-ZnFP could reduce the rate of sporozoite invasion. Animal immune protection assays with recombinant proteins were performed, and the results were satisfactory. Vaccination with rEtAN1-ZnFP could elicit partial protective immunity against E. tenella. However, the mechanism of action of rEtAN1-ZnFP in coccidial infections needs to be further studied.
References
Belli SI, Smith NC, Ferguson DJ (2006) The coccidian oocyst: a tough nut to crack! Trends Parasitol 22(9):416–423. https://doi.org/10.1016/j.pt.2006.07.004
Blake DP, Pastor-Fernandez I, Nolan MJ, Tomley FM (2017) Recombinant anticoccidial vaccines - a cup half full? Infect Genet Evol 55:358–365. https://doi.org/10.1016/j.meegid.2017.10.009
Bozza S, Montagnoli C, Gaziano R, Rossi G, Nkwanyuo G, Bellocchio S, Romani L (2004) Dendritic cell-based vaccination against opportunistic fungi. Vaccine 22(7):857–864. https://doi.org/10.1016/j.vaccine.2003.11.031
Chang L, Chang HH, Chang JC, Lu HC, Wang TT, Hsu DW, Tzean Y, Cheng AP, Chiu YS, Yeh HH (2018) Plant A20/AN1 protein serves as the important hub to mediate antiviral immunity. PLoS Pathog 14(9):e1007288. https://doi.org/10.1371/journal.ppat.1007288
Chapman HD (1998) Evaluation of the efficacy of anticoccidial drugs against Eimeria species in the fowl. International journal for parasitology 28(7):3
Chapman HD, Barta JR, Blake D, Gruber A, Jenkins M, Smith NC, Suo X, Tomley FM (2013) A selective review of advances in coccidiosis research. Adv Parasitol 83:93–171. https://doi.org/10.1016/B978-0-12-407705-8.00002-1
Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL (1995) Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature 376(6536)
Choi KD, Lillehoj HS, Zalenga DS (1999) Changes in local IFN-gamma and TGF-beta4 mRNA expression and intraepithelial lymphocytes following Eimeria acervulina infection. Vet Immunol Immunopathol 71(3-4):263–275
Cornelissen JB, Swinkels WJ, Boersma WA, Rebel JM (2009) Host response to simultaneous infections with Eimeria acervulina, maxima and tenella: a cumulation of single responses. Vet Parasitol 162(1-2):58–66. https://doi.org/10.1016/j.vetpar.2009.02.001
del Cacho E, Gallego M, Lee SH, Lillehoj HS, Quilez J, Lillehoj EP, Sánchez-Acedo C (2012) Induction of protective immunity against Eimeria tenella, Eimeria maxima, and Eimeria acervulina infections using dendritic cell-derived exosomes. Infect Immun 80(5):1909–1916. https://doi.org/10.1128/IAI.06413-11
Ding X, Lillehoj HS, Quiroz MA, Bevensee E, Lillehoj EP (2004) Protective immunity against Eimeria acervulina following in ovo immunization with a recombinant subunit vaccine and cytokine genes. Infect Immun 72(12):6939–6944. https://doi.org/10.1128/iai.72.12.6939-6944.2004
Fukaura H, Kent SC, Pietrusewicz MJ, Khoury SJ, Weiner HL, Hafler DA (1996) Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-b1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest 98(1)
Gharaibeh S, Mahmoud K (2013) Decay of maternal antibodies in broiler chickens. Poult Sci 92(9):2333–2336. https://doi.org/10.3382/ps.2013-03249
Ghosh S, Chatterji D (2017) Two zinc finger proteins from Mycobacterium smegmatis: DNA binding and activation of transcription. Genes Cells 22(8):699–714. https://doi.org/10.1111/gtc.12507
Gissot M, Hovasse A, Chaloin L, Schaeffer-Reiss C, Van Dorsselaer A, Tomavo S (2017) An evolutionary conserved zinc finger protein is involved in Toxoplasma gondii mRNA nuclear export. Cell Microbiol 19(2). https://doi.org/10.1111/cmi.12644
Han HY, Lin JJ, Zhao QP, Dong H, Jiang LL, Xu MQ, Zhu SH, Huang B (2010) Identification of differentially expressed genes in early stages of Eimeria tenella by suppression subtractive hybridization and cDNA microarray. J Parasitol 96(1):95–102. https://doi.org/10.1645/GE-2221.1
Harpaz R, Edelman R, Wasserman SS, Levine MM, Davis JR, Sztein MB (1992) Serum cytokine profiles in experimental human malaria. Relationship to protection and disease course after challenge. J Clin Invest 90(2):515–523. https://doi.org/10.1172/jci115889
Hong YH, Lillehoj HS, Lillehoj EP, Lee SH (2006) Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection of chickens. Vet Immunol Immunopathol 114(3-4):259–272. https://doi.org/10.1016/j.vetimm.2006.08.006
Huang BZQ, Wu XZ, Shi TW, Chen ZG (1993) Study on the identification and pathogenicity of the pure species of Eimeria tenella. Chinese Journal of Veterinary Parasitology 1(4):18–20
Huang J, Zhang Z, Li M, Song X, Yan R, Xu L, Li X (2015) Immune protection of microneme 7 (EmMIC7) against Eimeria maxima challenge in chickens. Avian Pathol 44(5):392–400. https://doi.org/10.1080/03079457.2015.1071780
Iuchi S (2001) Three classes of C2H2 zinc finger proteins. Cell Mol Life Sci 58(4):625–635. https://doi.org/10.1007/pl00000885
Jahn D, Matros A, Bakulina AY, Tiedemann J, Schubert U, Giersberg M, Haehnel S, Zoufal K, Mock HP, Kipriyanov SM (2009) Model structure of the immunodominant surface antigen of Eimeria tenella identified as a target for sporozoite-neutralizing monoclonal antibody. Parasitol Res 105(3):655–668. https://doi.org/10.1007/s00436-009-1437-6
Jang SI, Lillehoj HS, Lee SH, Lee KW, Park MS, Bauchan GR, Lillehoj EP, Bertrand F, Dupuis L, Deville S (2010) Immunoenhancing effects of Montanide ISA oil-based adjuvants on recombinant coccidia antigen vaccination against Eimeria acervulina infection. Vet Parasitol 172(3-4):221–228. https://doi.org/10.1016/j.vetpar.2010.04.042
Jang SIKD, Lillehoj HS, Lee SH, Lee KW, Bertrand F, Dupuis L, Deville S, Ben Arous J, Lillehoj EP (2013) Evaluation of Montanide™ ISA 71 VG adjuvant during profilin vaccination against experimental coccidiosis. PLoS One 8(4). https://doi.org/10.1371/journal.pone.0059786.t001
Jiang L, Lin J, Han H, Zhao Q, Dong H, Zhu S, Huang B (2012) Identification and partial characterization of a serine protease inhibitor (serpin) of Eimeria tenella. Parasitol Res 110(2):865–874. https://doi.org/10.1007/s00436-011-2568-0
Jin YM, et al. (2018) Overexpression of a new zinc finger protein transcription factor OsCTZFP8 improves cold tolerance in rice.5480617. https://doi.org/10.1155/2018/5480617
Kehrl JH, Roberts AB, Wakefield LM, Jakowlew S, Sporn MB, Fauci AS (1986) Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol 137(12):3855–3860
Kundu K, Garg R, Kumar S, Mandal M, Tomley FM, Blake DP, Banerjee PS (2017) Humoral and cytokine response elicited during immunisation with recombinant immune mapped protein-1 (EtIMP-1) and oocysts of Eimeria tenella. Vet Parasitol 244:44–53. https://doi.org/10.1016/j.vetpar.2017.07.025
Laurent F, Mancassola R, Lacroix S, Menezes R, Naciri M (2001) Analysis of chicken mucosal immune response to Eimeria tenella and Eimeria maxima infection by quantitative reverse transcription-PCR. Infect Immun 69(4):2527–2534. https://doi.org/10.1128/IAI.69.4.2527-2534.2001
Lee KW, Lillehoj HS, Jang SI, Lee SH, Bautista DA, Donald Ritter G, Lillehoj EP, Siragusa GR (2013) Comparison of live Eimeria vaccination with in-feed salinomycin on growth and immune status in broiler chickens. Res Vet Sci 95(1):110–114. https://doi.org/10.1016/j.rvsc.2013.02.005
Lillehoj HS, Ding X, Quiroz MA, Bevensee E, Lillehoj EP (2005) Resistance to intestinal coccidiosis following DNA immunization with the cloned 3-1E Eimeria gene plus IL-2, IL-15, and IFN-gamma. Avian Dis 49(1):112–117. https://doi.org/10.1637/7249-073004r
Lin RQ, Lillehoj HS, Lee SK, Oh S, Panebra A, Lillehoj EP (2017) Vaccination with Eimeria tenella elongation factor-1alpha recombinant protein induces protective immunity against E. tenella and E. maxima infections. Vet Parasitol 243:79–84. https://doi.org/10.1016/j.vetpar.2017.06.003
Liu L, Heermann DW (2015) The interaction of DNA with multi-Cys2His2 zinc finger proteins. J Phys Condens Matter 27(6):064107. https://doi.org/10.1088/0953-8984/27/6/064107
Liu T, Huang J, Li Y, Ehsan M, Wang S, Zhou Z, Song X, Yan R, Xu L, Li X (2018) Molecular characterisation and the protective immunity evaluation of Eimeria maxima surface antigen gene. Parasit Vectors 11(1):325. https://doi.org/10.1186/s13071-018-2906-5
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Lu H, Zhong G (1999) Interleukin-12 production is required for chlamydial antigen-pulsed dendritic cells to induce protection against live Chlamydia trachomatis infection. Infect Immun 67(4):1763–1769
Miller J, McLachlan AD, Klug A (1985) Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J 4(6):1609–1614
Miska KB, Fetterer RH, Barfield RC (2004) Analysis of transcripts expressed by Eimeria tenella oocysts using subtractive hybridization methods. J Parasitol 90(6):1245–1252. https://doi.org/10.1645/ge-309r
Morehouse NF, Baron RR (1970) Coccidiosis: evaluation of coccidiostats by mortality, weight gains, and fecal scores. Exp Parasitol 28(1):25–29
Muh F et al (2018) In vitro invasion inhibition assay using antibodies against Plasmodium knowlesi Duffy binding protein alpha and apical membrane antigen protein 1 in human erythrocyte-adapted P. knowlesi A1-H.1 strain. Malar J 17(1):272. https://doi.org/10.1186/s12936-018-2420-4
Mukhopadhyay A, Vij S, Tyagi AK (2004) Overexpression of a zinc-finger protein gene from rice confers tolerance to cold, dehydration, and salt stress in transgenic tobacco. Proc Natl Acad Sci U S A 101(16):6309–6314. https://doi.org/10.1073/pnas.0401572101
Orditura M, De Vita F, Roscigno A, Auriemma A, Infusino S, Catalano G (1998) Soluble interleukin-2 receptor and soluble CD8 antigen levels in serum from patients with solid tumors. Int J Mol Med 2(1):75–79
Papageorgiou FT, Soteriadou KP (2002) Expression of a novel Leishmania gene encoding a histone H1-Like protein in Leishmania major modulates parasite infectivity in vitro. Infect Immun 70(12):6976–6986. https://doi.org/10.1128/iai.70.12.6976-6986.2002
Pastor-Fernandez I et al (2018) Development of cross-protective Eimeria-vectored vaccines based on apical membrane antigens. Int J Parasitol 48(7):505–518. https://doi.org/10.1016/j.ijpara.2018.01.003
Peek LJ, Middaugh CR, Berkland C (2008) Nanotechnology in vaccine delivery. Adv Drug Deliv Rev 60(8):915–928. https://doi.org/10.1016/j.addr.2007.05.017
Plattner F, Yarovinsky F, Romero S, Didry D, Carlier MF, Sher A, Soldati-Favre D (2008) Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe 3(2):77–87. https://doi.org/10.1016/j.chom.2008.01.001
Rafiqi SI, Garg R, KR K, Ram H, Singh M, Banerjee PS (2018) Immune response and protective efficacy of Eimeria tenella recombinant refractile body protein, EtSO7, in chickens. Vet Parasitol 258:108–113. https://doi.org/10.1016/j.vetpar.2018.06.013
Rose ME, Mockett AP (1983) Antibodies to coccidia: detection by the enzyme-linked immunosorbent assay (ELISA). Parasite Immunol 5(5):479–489
Samso M, Daban JR, Hansen S, Jones GR (1995) Evidence for sodium dodecyl sulfate/protein complexes adopting a necklace structure. Eur J Biochem 232(3):818–824
Sharma S, Azmi S, Iqbal A, Nasirudullah N, Mushtaq I (2015) Pathomorphological alterations associated with chicken coccidiosis in Jammu division of India. J Parasit Dis 39(2):147–151. https://doi.org/10.1007/s12639-013-0302-9
Shirley MW, Smith AL, Blake DP (2007) Challenges in the successful control of the avian coccidia. Vaccine 25(30):5540–5547. https://doi.org/10.1016/j.vaccine.2006.12.030
Smith NC, Bucklar H, Muggli E, Hoop RK, Gottstein B, Eckert J (1993) Use of IgG- and IgM-specific ELISAs for the assessment of exposure status of chickens to Eimeria species. Vet Parasitol 51(1-2):13–25
Song H, Yan R, Xu L, Song X, Shah MA, Zhu H, Li X (2010) Efficacy of DNA vaccines carrying Eimeria acervulina lactate dehydrogenase antigen gene against coccidiosis. Exp Parasitol 126(2):224–231. https://doi.org/10.1016/j.exppara.2010.05.015
Tomley F (1997) Techniques for isolation and characterization of apical organelles from Eimeria tenella sporozoites. Methods 13(2):171–176
Vij S, Tyagi AK (2008) A20/AN1 zinc-finger domain-containing proteins in plants and animals represent common elements in stress response. Funct Integr Genomics 8(3):301–307. https://doi.org/10.1007/s10142-008-0078-7
Wallach M (2010) Role of antibody in immunity and control of chicken coccidiosis. Trends Parasitol 26(8):382–387. https://doi.org/10.1016/j.pt.2010.04.004
Williams RB (1973) Effects of different infection rates on the oocyst production of Eimeria acervulina or Eimeria tenella in the chicken. Parasitology 67(3):279–288
Williams RB (2001) Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl: its importance for experimental designs and the production of oocyst stocks. Int J Parasitol 31(10):1056–1069
Willsie SK, Herndon BL, Miller L, Dew M (1996) Soluble versus cell-bound CD4, CD8 from bronchoalveolar lavage: correlation with pulmonary diagnoses in human immunodeficiency virus-infected individuals. J Leukoc Biol 59(6):813–816
Xie MQ, Gilbert JM, Fuller AL, McDougald LR (1990) A new method for purification of Eimeria tenella merozoites. Parasitol Res 76(7):566–569
Yin G, Qin M, Liu X, Suo J, Tang X, Tao G, Han Q, Suo X, Wu W (2013) An Eimeria vaccine candidate based on Eimeria tenella immune mapped protein 1 and the TLR-5 agonist Salmonella typhimurium FliC flagellin. Biochem Biophys Res Commun 440(3):437–442. https://doi.org/10.1016/j.bbrc.2013.09.088
Zajkowska J, Hermanowska-Szpakowicz T, Swierzbinska R (2001) Concentration of soluble CD4, CD8 and CD25 receptors in early localized and early disseminated Lyme borreliosis. Infection 29(2):71–74
Zhai Q, Huang B, Dong H, Zhao Q, Zhu S, Liang S, Li S, Yang S, Han H (2016) Molecular characterization and immune protection of a new conserved hypothetical protein of Eimeria tenella. PLoS One 11(6):e0157678. https://doi.org/10.1371/journal.pone.0157678
Zhu H, Xu L, Yan R, Song X, Tang F, Wang S, Li X (2012) Identification and characterization of a cDNA clone-encoding antigen of Eimeria acervulina. Parasitology 139(13):1711–1719. https://doi.org/10.1017/S0031182012001163
Acknowledgments
We would like to thank all organizations which funded this work and all the teachers who cooperated in technical assistance.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 31572266) and National Sharing Service Platform for Parasite Resource (No. TDRC-22) and Shanghai Minhang District talent development special funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animal experiments, which involved animal immune protection experiments, were performed in accordance with the Institutional Animal Care and Use Committee of Shanghai Veterinary Research Institute, the Chinese Academy of Agricultural Sciences (Permit Number:SHVRI-SZ-20180106-3), and were conducted in strict follow the recommendations outlined in the Guide for the Care and Use of Laboratory Animals.
Additional information
Section Editor: Berit Bangoura
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, H., Zhao, Q., Zhu, S. et al. Molecular characterization and immune protection of an AN1-like zinc finger protein of Eimeria tenella. Parasitol Res 119, 623–635 (2020). https://doi.org/10.1007/s00436-019-06545-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06545-x