Abstract
A large-scale cross-sectional epidemiological study was conducted to evaluate prevalence, species diversity, and associated risk factors of Eimeria infections in 55 cattle farms across seven states of Colombia, including subtropical and tropical regions. In total, 1333 fecal samples from young animals (< 1 year of age) were examined at a single sampling date from August 2016 to December 2016. Flotation and McMaster techniques were conducted for parasitological investigation. Excreted Eimeria oocysts were allowed to sporulate in vitro and thereafter identified to species level based on morphological and morphometric characteristics. The overall Eimeria prevalence was 75.5% (1006/1333), with no difference observed between age categories. In total, 13 different Eimeria species were identified. The most prevalent species was E. bovis (33.5%), followed by E. auburnensis (12.5%) and E. zuernii (11.9%). Analysis of extrinsic associated risk factors revealed the floor type, feeding system, watering system, and herd size as significant (p < 0.05) risk factors for Eimeria spp. infections. Based on these data, it can be assumed that bovine coccidiosis infections occur ubiquitously in the country and might play an important role especially in its subclinical form by affecting production parameters in conventional cattle management systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eimeria species are common gastrointestinal parasites and the etiological cause of bovine coccidiosis which mainly affects young animals worldwide. Bovine coccidiosis is known as a limiting factor of cattle production causing economic losses mainly by subclinical infections (Fox 1985; Lassen and Østergaard 2012). Typically, when few calves show clinical signs (e.g., diarrhea, straining, dehydration), the majority of calves in the same environment are likely to be subclinically infected and undiagnosed, and, hence, associated costs are easily overlooked. Thus, all calves/young animals in the group should be treated to reduce losses due to reduced growth rates and the following production parameters: time to weaning, finishing, delayed onset of puberty, suboptimal weight at first calving, and increased feeding costs (Daugschies and Najdrowki 2005). In this respect, metaphylactic approaches applying toltrazuril or diclazuril have now become common in both dairy and beef industries (Enemark et al. 2013; Philippe et al. 2014) and showed to ameliorate negative effects of subclinical coccidiosis on growth performance (Daugschies et al. 2007; Veronesi et al. 2011).
A recent study on cattle industry from the Colombian Cattle Producers Association (Fedegan 2017) cited a population of approximately 22.5 million heads accounting for 950,000 tons of meat and 3200 million liters of milk produced annually. The annual consumption of meat and milk for an average Colombian person is 19 kg and 140 L, respectively (Fedegan 2017). With 33% of the Colombian population currently estimated to live on < 3 US$ per day, the capacity of cattle industry to reduce poverty may depend on the ability of poor households to participate in this economy sector. By number of animals per farm, Colombian farms present as following: 81% (˂ 50 heads), 10% (50–100 heads), 8% (101–500 heads), and 1% (˃ 500 heads) (Fedegan 2017).
Despite the fact that bovine Eimeria infections occur globally, little is still known on infection dynamics and associated risk factors influencing the outcome of subclinical and/or clinical coccidiosis in subtropical/tropical regions. In order to improve prevention and control of cattle coccidiosis (eimeriosis), large-scale epidemiological studies reporting on prevalence, risk factors, and infection pressure of pathogenic Eimeria species are urgently needed. So far, there are no reports available on the prevalence and species diversity of Eimeria with respect to Colombian cattle. For cattle, more than 20 Eimeria species are described (Daugschies and Najdrowki 2005), which differ in terms of pathogenicity and endogenous development. Amongst these, E. bovis, E. zuernii, and E. alabamensis are the most pathogenic ones (Deplazes et al. 2016). Due to the development of E. bovis and E. zuernii macromeronts in host endothelial cells of lymph capillaries of the ileum (> 120.000 merozoites I per meront stage) (Taubert et al. 2010), massive infections of cecum/colon host epithelial cells resulting in second generation meronts and gamonts will lead to severe hemorrhagic typhlocolitis (Hermosilla et al. 2012). Historically, identification of Eimeria species was based on clinical features (i.e., hemorrhagic vs. catarrhalic diarrhea) and on morphological criteria of sporulated oocysts. More recently, molecular (PCR) techniques have been developed since the former morphological parameters are not fully accurate due to overlapping Eimeria morphological characteristics (Kawaharaa et al. 2010).
Several studies identified management practices and other risk factors enhancing the likelihood of clinical eimeriosis for non-tropical cattle-rearing systems (Lassen et al. 2009a, b; Bangoura et al. 2011; Rehman et al. 2011). In general, such conditions which allow fecal contamination of feed and water are considered as high risk, thereby rendering overcrowding and poor sanitation as optimal conditions for infection transmission (Daugschies and Najdrowki 2005). Consequently, the detailed identification of risk factors for clinical and subclinical coccidiosis in calves of different tropical/subtropical management systems is essential to establish preventive and control measures.
This study was conducted in seven regions across the subtropical and tropical Colombian territory from August to December of 2016. It was performed on 1333 fecal samples from calves and young animals with the objective to analyze the prevalence, distribution, and risk factors associated with Eimeria infections in animals reared with conventional management systems and different production systems, i.e., dairy, meat, or dual purpose.
Materials and methods
Sample collection, study design, questionnaire, and animals
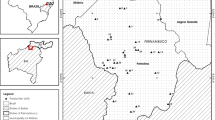
In total, 1333 rectally obtained fecal samples from young animals (< 1 year of age) were collected at a single time point between August and December 2016. Overall, 55 cattle farms allocated in seven states (i.e. Antioquia, Córdoba, Meta, Eje Cafertero, Arauca, Boyacá, Cundinamarca) of Colombia were sampled (Fig. 1). The distribution of the current samples by type of production system was as follows: 14.5% beef (8/55), 20% dual-purpose (11/55), and 65.5% dairy (36/55).
Exemplary illustration of typical Colombian cattle management systems (A–F) and illustration of sampling areas (G). (A) Milk production farm in Antioquia. (B) Milk production farm in Boyacá. (C) Beef production farm in Arauca. (D) Milk and beef production farm in Córdoba. (E) Milk and beef production farm in Arauca. (F) Milk and beef production farm in Meta. Map modified from Koppen climate Map (Beck et al. 2018)
Calves and young animals were assigned to five different age groups as follows: ≤ 1, 1–3, 3–6, 6–9, and 9–12 months of age. At least 10 g of fecal samples was processed within 24 h by flotation with saturated sodium chloride solution and examined with a modified McMaster chamber for quantitative determination of Eimeria spp. oocysts (sensitivity of 16 oocysts/g). The oocyst number per gram of feces (OPG) were counted and arbitrarily classified as low (16–1000), medium (1000–5000), and high (> 5000). For species identification, oocysts from each individual sample were allowed to sporulate in 2.5% potassium dichromate under constant oxygenation (Hermosilla et al. 2002). Eimeria species were identified based on morphological/morphometric parameters of sporulated oocysts using the taxonomic key described by Florião et al. (2016).
Additionally, a questionnaire with closed (dichotomous and multiple choice) questions was developed for risk factor analysis. Therefore, information on animals, herd size, and herd management practices were collected to determine single risk factors which are associated with the presence of distinct Eimeria species as published elsewhere (Thrusfield 2008; Carrau et al. 2018). In detail, the questionnaire collected data on animal-related factors (e.g., age, breed, body weight, body condition, and consistence of feces), on herd management practices related to coccidiosis, such as general information on the farm (e.g., farm size, access to veterinary services, production type, cattle breed population), information on the occurrence of coccidiosis (e.g., actual cases of symptomatic animals and of clinical coccidiosis observed during the last 2 years), management factors affecting transmission between herds (e.g., cattle purchase, own animals grazing on foreign pastures, foreign animals grazing on own pastures), and factors regarding housing and hygiene conditions (i.e. existence of a calving area, type of calf housing before weaning, feeding of calves before weaning, spreading of manure on pastures).
The choice of farms depended on the willingness of cooperation of the farmers. The current sample size was estimated to represent an entire population of 1,614,906 calves (ICA 2016), with an expected Eimeria prevalence of 70.5%, a 3% standard error, and 95% confidence level. Table 1 shows the number of farms and animals, and the type of production system for each Colombian state.
All animal procedures were performed according to the guidelines of the Ethic Committee for Animal Experimentation, approved by the Institutional Committee for Care and Use of Animals of the University of Antioquia (Act No.105; 2016) in accordance to current Colombian Animal Protection Laws.
Statistical analysis
Differences on Eimeria prevalence in different age groups were evaluated using a chi square test (SPSS 12.0 for Windows, SPSS Inc. Chicago, IL) and p < 0.05 was considered as significant. A Kruskall-Wallis one-way analysis of variance was used to compare OPG counts between different age groups and variation is presented as standard error of means. Descriptive statistics were provided for other variables of interest: overall prevalence of infection, prevalence of different Eimeria species, coinfection rates, OPG, and demographic data. Furthermore, multiple logistic regression models (MLRM) were calculated using generalized mixed model analysis for hierarchical designs (animals were nested within the farm, within the strata as random factors) with the statistical program package R (Free Software Foundation’s GNU project, 2016). In the first step of the analysis for each of the independent variables, two-way frequency tables were built to describe rough relationships between these variables and different Eimeria prevalences. Quantitative independent variables were transformed by common logarithm (log). In that case, descriptive statistics was given by geometric means and dispersion factors. In the second step, regression analyses were performed to identify risk factors of extensity and intensity of Eimeria oocyst excretion. Due to the high number of independent variables, a stepwise procedure was applied. Rough association of the variables to Eimeria spp. prevalence was analyzed for each variable separately in order to filter the most conspicuous factors. Then, a multifactorial analysis was performed using these variables in a common model. For oocyst excretion intensity, the number of different Eimeria species found per sample and the log of total OPG number (without differentiating between species) was here considered. For both variables, a multiple linear regression model was adjusted, again using generalized mixed model analysis for hierarchical designs. Outcomes of statistical tests (Wald tests) were considered to indicate statistical significant effects when p ≤ 0.05.
Results
Sampled farms were located in different climate regions, including subtropical and tropical regions, as illustrated in Fig. 1. The elevation in meters above sea level (masl) of these 55 cattle farms was as follows: 100–500 masl (44%), 1001–2000 masl (6%), and ˃ 2000 masl (45%). The management practices within single farms differed according to their type of production system. For beef cattle, management practice was predominated by “low input-low output systems” in which calves were allowed to graze freely with their dams on premises or were stocked together and dams brought in for lactation twice a day. Specialized dairy cattle farms occurred in highland tropics (≥ 2000 masl) in which calves were either individually raised or stocked together on pasture and exclusively given milk replacers and supplemental feed for 1–2 months. Dual-purpose farm systems occurred in the low tropics (≤ 1200 masl) and in these farms, calves were allowed to suck residual milk and grazed with their dams until midday.
Overall, diarrhea-related calf deaths were reported in 63% of all farms. Furthermore, only 7% of farm owners reported on never observing clinical coccidiosis, while 62% of the farmers recognized clinical coccidiosis outbreaks in last 2 years and 31% had recently observed some sporadic cases of clinical coccidiosis. Regarding housing conditions, hutches were used in 85% of the farms with the ground being composed of grass (49%), cement (15%), soil (6%), straw (4%), or combinations of these forms (26%). In 70% of the farms, the drinking water supply for calves originated from own properties thereby lacking any water treatments (non-potable).
Prevalence of Eimeria spp. infections
Eimeria oocysts were detected in 75.5% (1006/1333) of fecal samples, with at least one positive animal in each cattle farm. Taxonomic identification of bovine Eimeria species revealed the presence of 13 species in farms from two states Antioquia and Arauca, of 12 species in Córdoba, Cundinamarca, Boyacá, and Eje Cafetero, and of 11 species in Meta (Fig. 2). The most prevalent species was E. bovis (33.5%), followed by E. auburnensis (12.5%) and E. zuernii (11.9%). Less prevalent species were E. pellita (7.4%), E. ellipsoidalis (5.4%), E. canadensis (5.3%), E. wyomingensis (5.3%), E. bukidnonensis (5.3%), E. brasiliensis (3.4%), E. alabamensis (3.1%), E. subspherica (2.9%), E. cylindrica (1.6%), and E. illinoiensis (0.8%) (Fig. 2). Exemplary images on sporulated Eimeria spp. oocysts found in this survey are depicted in Fig. 3.
The Colombian state–related distribution of different Eimeria species is shown in Fig. 2A, and the overall diversity in all samples in Fig. 2B. Infections with a single Eimeria species were most frequently detected (26.9%, 358/1333), followed by mixed infections with two (15.6%, 208/1333) or three species (6.5%, 86/1333) (Table 2). When related to age groups, a significant relationship between age and infection rates could be stated with lower infection rates in the neonate group (≤ 1 month, χ2 (4 df, n = 1333) = 70.2, p < 0.01) (Table 3). However, when Eimeria prevalence was related to age categories of 3-month intervals > 1 month of age, no significant differences were observed. Given that the overall prevalence of Eimeria spp. infections was quite similar in cattle farms of the same Colombian state, data were pooled by state for ease of data presentation and are shown in Table 4 and Fig. 2A.
Analyses on oocyst shedding showed a wide range from 16–360,000 OPG in each farm, with an overall median value of 100–200 OPG (Table 4). The distribution of low-, medium- and high-OPG levels for animals assigned by age classes is presented in Table 5. Overall, in all age groups, oocyst shedding mainly occurred in the range of 16–1000 OPG. Nevertheless, chi-square analysis showed that there were significant differences of OPG levels among different age groups (χ2 (9 df, n = 1333) = 98.1, p < 0.01) since higher OPG were observed in younger animals (< 6 months) when compared with older ones (> 6 months). For animals aged ≥ 6 months, only 1–2% showed high OPG levels, few (4–6%) demonstrated medium OPG values, and most (70%) showed low OPG values. In total, 23% of animals ≥ 6 months of age were Eimeria spp. negative. However, when overall OPG counts were compared between age groups using Kruskall-Wallis analysis of variance, no significant differences were observed in spite of the apparent large differences in means (± S. E.) (Table 5).
Identification of risk factors associated with Eimeria infections
Logistic regression analysis on the presence of Eimeria spp., excretion intensity, and qualitative risk factors was also performed by MLRM analysis. Respective data on the level of Eimeria species are summarized in Table 6. Overall, the presence of Eimeria stages and corresponding OPG counts showed significant association to the herd size in terms of animal numbers (total number of cows = OR 0.7; C.I. 0.517 to 1.014), which indicates that a reduced number of animals per farm was considered as protective factor for Eimeria infections. For pathogenic Eimeria species, we found that E. bovis–related OPG counts were significantly associated with the type of housing ground (grass = OR 1.81; 95% confidence interval (C.I.): 1.138–2.88; cement and straw = OR 2.648; C.I.: 1.382–5.075) and the mode of drinking water supply (i.e., potable and non-potable = OR 2.816; C.I.: 1.338–5.923). In the case of E. zuernii, the factors associated with its presence were the size of pasture premises (OR 3.038; C.I:. 1.344–6.867) and the presence of floodable zones on pastures (OR 2.226; C.I.: 1.087 to 4.558). This suggests that the environment was the main factor influencing the presence of bovine coccidiosis for both pathogenic Eimeria species. For non-pathogenic Eimeria species, some of the factors influencing the occurrence of coccidiosis were the type of food used (i.e., grass, cut grass, concentrated feed) and presence of a veterinarian in the farm.
Discussion
Most studies on bovine coccidiosis focus on E. bovis, E. zuernii, and E. alabamensis as they are the most dominant and pathogenic of Eimeria species. Nonetheless, several other Eimeria species frequently occur and thus should not be ignored since they may contribute to subclinical coccidiosis. The biodiversity of bovine Eimeria species affecting cattle often differs between geographic regions of a country (Daugschies and Najdrowki 2005; Tomczuk et al. 2015) as also described for small ruminant coccidian infections (Catchpole and Gregory 1985; Carrau et al. 2018). To date, no epidemiological data are available on bovine coccidiosis in tropical and subtropical regions of Colombia. To our best knowledge, this is the first large-scale epidemiological survey on bovine Eimeria spp. infections and related risk factors.
Mean prevalence data confirmed Eimeria spp. infections as a frequent intestinal disease of cattle in subtropical/tropical regions of seven states of Colombia, with an overall herd prevalence of 75.5% was estimated. This is in line with other epidemiological studies on bovine coccidiosis worldwide which also stated an Eimeria prevalence of > 70% thereby emphasizing the relevance of coccidiosis in dairy and beef cattle industry (Faber et al. 2002; Daugschies and Najdrowki 2005; von Samson-Himmelstjerna et al. 2006; Koutny et al. 2012; Tomczuk et al. 2015). However, some studies from other tropical/subtropical regions showed lower prevalences, such as 33.2% in India (Das et al. 2015) and 60.7% in Pakistan (Rehman et al. 2011). Since fecal samples (n = 1333) were taken from a randomly selected young animal population of 55 farms in seven Colombian states, it can be assumed that Eimeria infections are widely spread in the country and may play important role as underestimated subclinical or clinical disease affecting growth rate performance. We found 26.9% (n = 358) samples with single Eimeria species infections and 26.45% (n = 352) with mixed infections. Surprisingly, the actual impact of Eimeria spp. co-infections on calf performance remains uncertain since diarrhea was only found associated with either total OPG counts or single E. zuernii or E. bovis infections (Hermosilla et al. 1999; Bangoura and Daugschies 2007; Bangoura et al. 2011; Enemark et al. 2013). This is consistent with experimental infections with E. zuernii consistently causing diarrhea accompanied by reduced body weight gains and hemoconcentration in calves (Bangoura and Daugschies 2007). Although oocyst shedding did not correlate well to the degree of clinical disease, a correlation between diarrhea and OPG ≥ 500 was demonstrated in case of E. bovis and E. zuernii infection (Bangoura et al. 2011), which are the two most commonly reported species worldwide (Faber et al. 2002; Dong et al. 2012; Rehman et al. 2011; Kennedy and Kralka 1987; Lucas et al. 2014; Tomczuk et al. 2015). Consequently, the differentiation between pathogenic and non-pathogenic Eimeria species is necessary to better predict the outcome of infection (Daugschies and Najdrowki 2005). In total, 16.9% of all examined animals were infected with one or both of these species and were excreted ≥ 500 OPG. It is therefore very likely that these animals would be suffering from production losses due to clinical or subclinical coccidiosis as postulated elsewhere (Hermosilla et al. 2002; Faber et al. 2002; Daugschies and Najdrowki 2005; Taubert et al. 2010).
The number of different Eimeria species present in mixed infections ranged from two to eight. This finding correlates with data from Ethiopia (Abebe et al. 2008), USA (Ernst et al. 1987), The Netherlands (Cornelissen et al. 1995), and in Turkey (Arslan and Tuzer 1998). In line with data from the Czech Republic (Chroust 2000), the current study also showed that E. bovis (33.5%) was the most prevalent species followed by E. auburnensis (12.5%) and E. zuernii (11.9%) in Colombian animals. Eimeria bovis and E. zuernii are the most frequently reported species during outbreaks of clinical coccidiosis (Deplazes et al. 2016; Waruiru et al. 2000; Faber et al. 2002; Speer 1999; López-Osorio et al. 2018) and previously been reported in South America (Rebouças et al. 1994). Consistent with current findings, > 30% and 7–23% of Brazilian cattle herds were found infected with E. bovis and E. zuernii, respectively (Rebouças et al. 1994; Almeida et al. 2011; Bruhn et al. 2011, 2012; Tosi Cardim et al. 2018).
It is well known that young animals are more susceptible to Eimeria infections than adults due to a lack of protective immunity at young age (Hermosilla et al. 1999, 2002, 2012; Taubert et al. 2008, 2009, 2010). Moreover, calves exposed to low doses of oocysts are reported to develop protective adaptive immunity against homologous Eimeria species (Hermosilla et al. 1999; Sühwold et al. 2010; Taubert et al. 2010) resulting in reduced oocyst shedding and clinical manifestations (Sanchez et al. 2008; Rind et al. 2007). In contrast to other reports (Dong et al. 2012; Daugschies and Najdrowki 2005), the large variation in OPG counts present in all age categories in this study prevented any age-related statistical differences of OPG counts. In general, current OPG counts were within the range recorded for sub-clinically infected animals (Lucas et al. 2014; Rehman et al. 2011; Klockiewicz et al. 2007). Furthermore, two surveys have shown age-related differences in Eimeria prevalence (Gorsich et al. 2014; Rehman et al. 2011), a finding which we could only confirm for the comparison of neonate (< 1 month) vs. animals older than 2 months.
The identification of risk factors related to the occurrence of cattle coccidiosis is important to prevent clinical infections. In this study, we identified factors related to management and husbandry practices that influence the occurrence of infections, such as non-potable water (for E. bovis, E. canadensis, E. subspherica), floor type (for E. bovis), herd size (for E. subspherica), type of food (for E. alabamensis, E. canadensis, E. cylindrica), source of colostrum (for E. alabamensis), and floodable zones and size of the pastures (for E. zuernii). More importantly, the herd size represented a key factor for Eimeria spp. infections in calves, meaning that small herds (< 50 animals) were less affected by these parasites than large herds. Consistent with this finding, Klockiewicz et al. (2007) reported that highly pathogenic Eimeria species occurred more frequently in large than in small cattle farms. Additionally, Kusiluka et al. (1998) concluded that small herd sizes show lower environmental contamination than larger ones. Furthermore, McKellar (2008) stated that clinical coccidiosis was more prevalent under poor sanitation conditions, bad nutrition, and overcrowding. In the current study, the type of flooring had a marked influence on the presence of E. bovis which was in agreement to former findings (Gräfner et al. 1978, 1985). Thus, keeping calves on cement floors with embedded straw revealed critical for coccidiosis outbreaks (Gräfner et al. 1978, 1985). Generally higher oocyst contamination of shed floors compared with smooth floors are most likely due to suboptimal cleaning options and optimal humidity and temperature conditions for oocyst sporulation and survival in strawbeds. In line, Gulliksen et al. (2009) determined a lower incidence of diarrhea in calves kept on slatted floors (which are easy to clean) and Rehman et al. (2011) recorded higher Eimeria prevalence in animals kept on non-cemented floors (difficult for sanitizing). Ernst et al. (1987) concluded that clinical coccidiosis bovine is more common in housed animals than in those on pastures. However, in the case of E. zuernii, Colombian animals kept on pastures with floodable zones had a higher probability for coccidiosis, which may be due to higher oocyst contamination at point sources, such as around limited food and water sources, leading to host and oocyst concentration at restricted areas (Rehman et al. 2011). Identified risk factors associated with clinical/subclinical coccidiosis outbreaks include higher risk of infection when calves feed at ground level and/or drink from pond water (Rehman et al. 2011). Apart from husbandry practices mentioned above, other factors were reported to increase Eimeria prevalence, such as season (wet ˃ dry season; Waruiru et al. 2000), temperature (warm ˃ cold; Makau et al. 2017), size of herds (larger ˃ smaller; Klockiewicz et al. 2007; Chibunda et al. 1997), and stocking density (Sanchez et al. 2008).
In conclusion, this study revealed that Eimeria infections frequently occur in Colombian calves/young animals regardless of the type of production system. Given that clinical and subclinical Eimeria infections are well-known to dampen bovine production parameters, regular monitoring, including diagnosis of species biodiversity, and metaphylactic treatments could help to prevent in future Eimeria-induced economic losses in Colombian cattle rearing.
References
Abebe R, Kumesa B, Wessene A (2008) Epidemiology of Eimeria infections in calves in Addis Ababa and Debre Zeit Dairy Farms, Ethiopia. Intern J Appl Res Vet Med 6:24–30
Almeida VA, Magalhães VCS, Muniz ESN, Munhoz AD (2011) Frequency of species of the Genus Eimeria in naturally infected cattle in Southern Bahia, Northeast Brazil. Rev Bras Parasitol Vet 20(1):78–81
Arslan M, Tuzer E (1998) Prevalence of bovine eimeridosis in Thracia, Turkey. Turk J Vet Anim Sci 22:161–164
Bangoura B, Daugschies A (2007) Parasitological and clinical parameters of experimental Eimeria zuernii infection in calves and influence on weight gain and haemogram. Parasitol Res 100:1331–1340
Bangoura B, Mundt HC, Schmaschke R, Wesphal B, Daugschies A (2011) Prevalence of Eimeria bovis and Eimeria zuernii in German cattle herds and factors influencing oocyst excretion. Parasitol Res 109:S129–S138
Beck HE, Zimmermann NE, McVicar TR, Vergopolan N, Berg A, Wood EF (2018) Present and future Köppen-Geiger climate classification maps at 1-km resolution. Nat Sci Data. https://doi.org/10.1038/sdata.2018.214
Bruhn FRP, Lopes MA, Demeu FA, Perazza CA, Pedrosa MF, Guimarães AM (2011) Frequency of species of Eimeria in females of the Holstein-Friesian breed at the post-weaning stage during autumn and winter. Rev Bras Parasitol Vet 20(4):303–307
Bruhn FRP, Silva FA Jr, Carvalho AHO, Orlando DR, Rocha CMBM, Guimarães AM (2012) Occurrences of Eimeria spp. and gastrointestinal nematodes in dairy calves in southern Minas Gerais, Brazil. Rev Bras Parasitol Vet 21(2):171–175
Carrau T, Silva LMR, Pérez D, Failing K, Martínez-Carrasco C, Macías J, Taubert A, Hermosilla C, Ruiz de Ybáñez R (2018) Associated risk factors influencing ovine Eimeria infections in southern Spain. Vet Parasitol 263:54–58
Catchpole J, Gregory MW (1985) Pathogenicity of the coccidium Eimeria crandallis in laboratory lambs. Parasitology 91(1):45–52
Chibunda RT, Muhaiwa AP, Kambarage DM, Mtambo MMA, Kusiluka LJM, Kazwala RR (1997) Eimeriosis in dairy cattle farms in Morogoro municipality of Tanzania. Prev Vet Med 31:191–197
Chroust K (2000) Parazitozy u masnych plemen skotu v marginalnich oblastech a jejich tlumeni. Veterinarstvi 56:430–437
Cornelissen AWCA, Verstegen R, van den Brand H et al (1995) An observational study of Eimeria species in housed cattle on Dutch dairy farms. Vet Parasitol 56:7–16
Das M, Deka DK, Sarmah PC, Islam S, Sarma S (2015) Diversity of Eimeria spp. in dairy cattle of Guwahati, Assam, India. Vet World 8(8):941–945
Daugschies A, Najdrowki M (2005) Eimeriosis in cattle: current understanding. J Veterinary Med Ser B 52:417–427
Daugschies A, Agneessens J, Goosens L, Mengel H, Veys P (2007) The effect of a metaphylactic treatment with diclazuril (Vecoxan®) on the oocyst excretion and growth performance of calves exposed to a natural Eimeria infection. Vet Parasitol 149:199–206
Deplazes P, Eckert J, Mathis A, von Samson-Himmelstjerna G, Zahner H (2016) Parasitology in Veterinary Medicine. Wageningen Academic Publishers, The Netherlands, p 650
Dong H, Zhao Q, Han H, Jiang L, Zhu S, Li T, Kong C, Huang B (2012) Prevalence of coccidial infection in dairy cattle in Shanghai, China. J Parasitol 98:963–966
Enemark HL, Dahl J, Enemark JMD (2013) Eimeriosis in Danish dairy calves – correlation between species, oocyst excretion and diarrhea. Parasitol Res 112:S169–S179
Ernst JV, Stewart TB, Witlock DR (1987) Quantitative determination of coccidian oocysts in beef calves from the coastal plain area of Georgia (USA). Vet Parasitol 23:1–10
Faber JE, Kollmann D, Heise A, Bauer C, Failing K, Burger HJ, Zahner H (2002) Eimeria infections in cows in the periparturient phase and their calves: oocyst excretion and levels of specific serum and colostrum antibodies. Vet Parasitol 104:1–17 32
Fedegan (2017) Federación Colombiana de Ganaderos: Censo pecuario Nacional. IOP https://www.fedegan.org.co/noticias/conozca-el-censo-pecuario-nacional-del-ica-2017. Accessed 21 March 2019
Florião MM, Bomfim LB, Pereira BB, Gomes-Lopes GW (2016) New approaches for morphological diagnosis of bovine Eimeria species: a study on a subtropical organic dairy farm in Brazil. Trop Anim Health Prod 48:577–584
Fox JE (1985) Coccidiosis in cattle. Mod Vet Pract 66:113–116
Gorsich EE, Ezenwa VO, Jolles AE (2014) Nematode-coccidia parasite co-infections in African-buffalo: epidemiology and associations with host condition and pregnancy. Int J Parasitol Parasites Wildl 3:124–134
Gräfner G, Graubmann HD, Kron A (1978) About the epizoology of cattle coccidiosis in raising and fattening farms. Mh Vet Med 33:910–912
Gräfner G, Graubmann HD, Schwartz K, Hiepe T, Kron A (1985) Further studies on the incidence, epidemiology and control of bovine Eimeria coccidiosis under intensive-rearing conditions. (Article in German) Mh. Vet Med 40:41–44
Gulliksen SM, Jor E, Lie KI, Hamnes IS, Løken T, Akerstedt J, Osterås O (2009) Enteropathogens and risk factors for diarrhea in Norwegian dairy calves. J Dairy Sci 92(10):5057–5066
Hermosilla C, Bürger HJ, Zahner H (1999) T cell responses in calves to a primary E. bovis infection: phenotypical and functional changes. Vet Parasitol 84:49–64
Hermosilla C, Barbisch B, Heise A et al (2002) Development of Eimeria bovis in vitro: suitability of several bovine, human and porcine endothelial cell lines, bovine fetal gastrointestinal, Madin–Darby bovine kidney (MDBK) and African green monkey kidney (VERO) cells. Parasitol Res 88:301
Hermosilla C, Ruiz A, Taubert A (2012) Eimeria bovis: an update on parasite-host cell interactions. Int J Med Microbiol 302(4-5):210–215
ICA (2016) Censo pecuario nacional. https://www.ica.gov.co/areas/pecuaria/servicios/epidemiologia-veterinaria/censos-2016/censo-2018.aspx. Accessed August 2016
Kawaharaa F, Zhanga G, Mingalab CN, Tamurac Y, Koiwac M, Onumaa M, Nunoyaa T (2010) Genetic analysis and development of species-specific PCR assays based on ITS-1 region of rRNA in bovine Eimeria parasites. Vet Parasitol 174:49–57
Kennedy MJ, Kralka RA (1987) A survey of Eimeria spp. in cattle in Central Canada. Can Vet J 28(3):124–125
Klockiewicz M, Kaba J, Tomczuk K, Janecka E, Sadzikowski AB, Rypuła K, Studzinska M, Małecki-Tepicht J (2007) The Epidemiology of Calf Coccidiosis (Eimeria spp.) in Poland. Parasitol Res 101:S121–S128
Koutny H, Joachim A, Tichy A, Baumgartner W (2012) Bovine Eimeria species in Austria. Parasitol Res 110(5):1893–1901
Kusiluka LJM, Kambaragea DM, Harrisonb LJS, Dabornb CJ, Matthewman RW (1998) Prevalence and seasonal patterns of coccidial infections in goats in two ecoclimatic areas in Morogoro, Tanzania. Small Rumin Res 30:85–91
Lassen B, Østergaard B (2012) Estimation of the economical effects of Eimeria infections in Estonian dairy herds using a stochastic model. Prev Vet Med 106:258–265
Lassen B, Viltrop A, Raaperi K, Järvis T (2009a) Eimeria and Cryptosporidium in Estonian dairy farms in regard to age, species, and diarrhoea. Vet Parasitol 166(3-4):212–219
Lassen B, Viltrop A, Järvis T (2009b) Herd factors influencing oocyst production of Eimeria and Cryptosporidium in Estonian dairy cattle. Parasitol Res 105(5):1211–1222
López-Osorio S, Silva LMR, Taubert A, Chaparro-Gutiérrez JJ, Hermosilla CR (2018) Concomitant in vitro development of Eimeria zuernii- and Eimeria bovis-macromeronts in primary host endothelial cells. Parasitol Int 67(6):742–750
Lucas AS, Swecker WS, Lindsay DA, Scaglia G, Neel JPS, Elvinger FC, Zajac AM (2014) A study of the level and dynamics of Eimeria populations in naturally infected, grazing beef cattle at various stages of production in the Mid-Atlantic USA. Vet Parasitol 202:201–206
Makau DN, Gitau GK, Muchemi GK, Thomas LF, Cook EAJ, Wardrop NA, Févre EM, de Glanville WA (2017) Environmental predictors of bovine Eimeria infection in western Kenya. Trop Anim Health Prod 49:409–416
McKellar AQ (2008) Gastrointestinal parasites of ruminants. In: Kahn CM, Line S, Aiello SE (eds) The Merck Veterinary Manual. Whitehouse Station, NJ
Philippe P, Alzieu JP, Taylor MA, Dorchies P (2014) Comparative efficacy of diclazuril (Vecoxan®) and toltrazuril (Baycox bovis®) against natural infections of Eimeria bovis and Eimeria zuernii in French calves. Vet Parasitol 206:3–4
Rebouças MM, Grasso LMPS, Spósito Filha E, Amaral V, Santos SM, Silva DM (1994) Prevalência e distribuição de protozoários do gênero Eimeria (Apicomplexa: Eimeriidae) em bovinos nos municípios de Altinópolis, Taquaritinga, São Carlos e Guaíra – Estado de São Paulo, Brasil. Rev Bras Parasitol Vet 3(2):125–130
Rehman TU, Khan MN, Sajid MS, Abbas RZ, Arshad M, Igbal Z, Igbal A (2011) Epidemiology of Eimeria and associated risk factors in cattle of district Toba Tek Singh, Pakistan. Parasitol Res 108(5):1171–1177
Rind R, Probert AJ, Kamboh AA (2007) The incidence of Eirmeria species in naturally infected calves. Int J Agric Biol 9(5):741–745
Sanchez RO, Romero JR, Founroge RD (2008) Dynamics of Eimeria oocyst excretion in dairy calves in the Province of Buenos Aires (Argentina), during their first 2 months of age. Vet Parasitol 151:133–138
Speer CA (1999) Coccidiosis. In: Howard JL, Smith RA (eds) Current veterinary therapy, food animal practice, 4th edn. W.B. Saunders, Philadelphia, pp 411–420
Sühwold A, Hermosilla C, Seeger T et al (2010) T cell reactions of Eimeria bovis primary- and challenge-infected calves. Parasitol Res 106:595
Taubert A, Hermosilla C, Suhwold A, Zahner H (2008) Antigen-induced cytokine production in lymphocytes of Eimeria bovis primary and challenge infected calves. Vet Immunol Immunopathol 126:309–320
Taubert A, Behrendt JH, Sühwold A, Zahner H, Hermosilla C (2009) Monocyte- and macrophage-mediated immune reactions against Eimeria bovis. Vet Parasitol 164:141–153
Taubert A, Wimmers K, Ponsuksili S, Arce Jimenez C, Zahner H, Hermosilla C (2010) Microarray-based transcriptional profiling of Eimeria bovis-infected bovine endothelial host cells. Vet Res 41(5):70
Thrusfield M (2008) Veterinary Epidemiology. Blackwell Publishing, London, p 178
Tomczuk K, Grzybek M, Szczepaniak K et al (2015) Analysis of intrinsic and extrinsic factors influencing the dynamics of bovine Eimeria spp. from central-eastern Poland. Vet Parasitol 214(1-2):22–28
Tosi Cardim S, Seixas M, Dutra Tabacow VB, Taroda A, Gomes Carneiro P, Martins TA, de Barros LD, Minutti AF, Lazaros Chryssafidis A, Vidotto O, Garcia JL (2018) Prevalence of Eimeria spp. in calves from dairy farms in northern Paraná state, Brazil. Braz J Vet Parasitol 27:119–123
Veronesi F, Diaferia M, Viola O, Piergili Fioretti D (2011) Long-term effect of toltrazuril on growth performances of dairy heifers and beef cattle exposed to natural Eimeria zuernii and Eimeria bovis infections. Vet J 190:296–299
von Samson-Himmelstjerna G, Epe C, Wirtherle N, von der Heyden V, Welz C, Radeloff I, … Krieger K (2006) Clinical and epidemiological characteristics of Eimeria infections in first-year grazing cattle. Vet Parasitol 136(3-4):215–21
Waruiru RMK, Yvsgaard NC, Thamsborg SM, Nansen P, Bogh HO, Munyua WK, Gathuma JM (2000) The prevalence and intensity of helminth and coccidial infections in dairy cattle in central Kenya. Vet Res Commun 24:39–51
Acknowledgments
Authors wish to acknowledge to Dr. Nicolas Martinez (University of Córdoba, Colombia), Dr. Agustín Góngora (Unillanos, Colombia), Dr. Arlex Rodríguez (Nacional University of Colombia), Dr. Laura Hortua (UPTC, Colombia) and Dr. Genaro Contreras which helped and coordinated sampling in different Colombian states. We also extend our gratitude to all staff members of the Special Veterinary Parasitology Laboratory, Faculty of Agrarian Sciences of the University of Antioquia, Medellin, Colombia, which helped with processing of samples. And last, to all cattle farm owners willing to collaborate with this epidemiological study. Thanks to the Strategy for supporting research groups in the process of consolidation 2018-2019, CODI, Universidad de Antioquia.
Funding
This study was exclusively funded by the Special Veterinary Parasitology Laboratory, Faculty of Agrarian Sciences of the University of Antioquia, Medellin, Colombia, and the Institute of Parasitology, Faculty of Veterinary Medicine of the Justus Liebig University (JLU) Giessen, Giessen, Germany.
Author information
Authors and Affiliations
Contributions
SL, JC, and DV designed, planed, and coordinated study. SL carried out the sampling and processing of the samples; SL, DV, JC, CH, and AT drafted and edited the manuscript. FK carried out the statistical analysis of the data. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Larissa Howe
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lopez-Osorio, S., Villar, D., Failing, K. et al. Epidemiological survey and risk factor analysis on Eimeria infections in calves and young cattle up to 1 year old in Colombia. Parasitol Res 119, 255–266 (2020). https://doi.org/10.1007/s00436-019-06481-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06481-w