Abstract
Human infection with the nematode Strongyloides stercoralis, which may have a life-threatening course, primarily occurs in tropical settings. Epidemiological data on the occurrence of strongyloidiasis are scarce, and microscopic stool-based detection methods are insensitive. Polymerase chain reaction (PCR) assays have been developed, yet conflicting results have been reported. Our goal was to determine whether there was diagnostic agreement between an in-house PCR and two microscopic techniques, the Baermann funnel (BM) and the Koga agar plate culture (KAP) for the detection of S. stercoralis in stool samples. Eighty ethanol-fixed stool samples stemming from a cross-sectional survey in Maluku, Indonesia, were purposefully selected for PCR analysis. The final sample size comprised four groups, each with 20 samples: group 1, positive for S. stercoralis on both BM and KAP; group 2, positive only by BM; group 3, positive only by KAP; and group 4, negative on both BM and KAP. A Strongyloides-specific PCR targeting the internal transcribed spacer 2 (ITS2) region was carried out in an Indonesian reference laboratory. The overall agreement between PCR and microscopy was 61% (49/80 samples), being highest in group 1 (15/20, 75%) and lowest in group 3 (9/20, 45%). PCR revealed eight additional S. stercoralis infections in group 4. Future studies should elucidate the ‘true’ infection status of samples that are negative by PCR, but positive upon microscopy. Taken together, there is a lack of agreement between microscopy and PCR results for the diagnosis of human S. stercoralis infection in Indonesia. ClinicalTrials.gov (identifier: NCT02105714)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human strongyloidiasis is a parasitic disease caused by an infection with the nematodes Strongyloides stercoralis and, much less frequently, Strongyloides fuelleborni. These nematode species occur primarily in tropical and subtropical countries (Becker et al. 2011; Keiser and Nutman 2004; Schär et al. 2013b). However, strongyloidiasis is also increasingly being reported from temperate areas such as Italy (Buonfrate et al. 2016), Slovakia (Strkolcova et al. 2017) and Spain (Belhassen-García et al. 2017). Infections are usually asymptomatic with eosinophilia being sometimes the only laboratory finding, while larvae excretion fluctuates at very low levels (Concha et al. 2005). Larvae of S. stercoralis hatch inside the host’s gastrointestinal tract and may cause auto-infection so that infections may persist asymptomatically for decades (Vadlamudi et al. 2006). If the host undergoes immunosuppression, the larvae may replicate rapidly (hyperinfection), which can lead to disseminated strongyloidiasis with a fatal course (Buonfrate et al. 2013). Hence, it is recommended that every detected human S. stercoralis infection be treated to avoid such complications, even though recent research suggests that complete parasitological cure may not be achieved (Repetto et al. 2018).
As opposed to the major soil-transmitted helminth species (i.e., Ascaris lumbricoides, hookworm and Trichuris trichiura) (Jourdan et al. 2017), estimates on the global number of individuals infected with S. stercoralis lack accuracy, ranging between 100 and 370 million (Albonico et al. 2016; Bisoffi et al. 2013). People living in rural areas with inadequate access to clean water, protective footwear, sanitation and hygiene are at highest risk of strongyloidiasis and other parasitic worm infections (Utzinger et al. 2012). Hence, strongyloidiasis is highly endemic in many parts of Latin America, sub-Saharan Africa and Southeast Asia (Buonfrate et al. 2015; Schär et al. 2013b).
The diagnosis of S. stercoralis in human faecal samples is complex and the sensitivity of conventional microscopic techniques is low. Because only larvae, but no eggs, can be found in stool samples, microscopic techniques that are often used in laboratories (e.g., direct faecal smear) and epidemiological surveys and clinical studies (e.g., Kato-Katz technique) fail to detect the infection. More sensitive, but also more laborious parasitological methods have been developed, namely the Baermann funnel (BM) and a nutrient agar plate culture technique (Koga agar plate; KAP) (Requena-Méndez et al. 2013). Both techniques show higher diagnostic accuracy for detection of S. stercoralis compared to standard methods, but controversy remains as to which of the two is more sensitive (Campo Polanco et al. 2014). A challenge for comparative diagnostic studies on human strongyloidiasis is the absence of a sensitive diagnostic ‘gold’ standard. In recent years, molecular diagnostic techniques have been developed (Verweij et al. 2009), and a stool-based, S. stercoralis-specific polymerase chain reaction (PCR) assay was reported to be the single most sensitive diagnostic technique (Becker et al. 2015).
In Indonesia, soil-transmitted helminths are highly endemic (Dunn et al. 2016) and a national survey carried out in 2008 reported regional prevalences of up to 61% (Depkes 2009). However, no data on S. stercoralis were included in this report, although previous surveys had demonstrated the presence of S. stercoralis infection in parts of the country (Widjana and Sutisna 2000). Recently, a large multi-centric study on the aetiology of persistent digestive disorders was carried out by the NIDIAG research consortium (Becker et al. 2013; 2016; Polman et al. 2015). Here, we present a laboratory study to determine whether there was diagnostic agreement between BM, KAP and PCR for the diagnosis of S. stercoralis on ethanol-fixed stool samples stemming from the NIDIAG study in Maluku, Indonesia.
Material and methods
Location, study period and sample collection
As part of a NIDIAG study on persistent digestive disorders, a cross-sectional survey pertaining to intestinal parasitic infections and related symptoms was carried out in a rural community of Maluku Tengah Regency, Maluku, Indonesia, between August and October 2015. Stool containers were provided on the day before sampling and study participants were informed on how to dispose a fresh stool sample into these containers. A questionnaire was filled in and stool samples were collected the next morning and transferred to a nearby laboratory.
Parasitological analyses
A series of parasitological examinations, including BM and KAP, were carried out on the day of sampling. All analyses followed standard operating procedures (SOPs), which were developed by the NIDIAG research consortium and are freely available online (Alirol et al. 2016; Becker et al. 2016). In brief, ~ 2 g of stool were utilised for KAP. The faecal material was placed at the centre of a Koga agar plate and incubated at 26–33 °C for 48 h. Subsequently, the agar plate was visually analysed for larval tracks, and the agar surface washed with sodium acetate-acetic acid-formalin (SAF). The washings were collected and centrifuged, and the sediment subjected to further microscopic analysis. For BM, ~ 20 g of faecal sample was scooped onto a medical gauze, which was submerged in the water of the BM funnel. The sample was exposed to artificial light for 3 h, and the water was drained into a centrifuge tube. Following centrifugation, the sediment was analysed by light microscopy for the presence of helminth larvae. Approximately 1 g of each sample was preserved in 96% ethanol for additional investigations. These samples were stored in a freezer at − 20 °C and transferred to the Centre for Tropical Medicine, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada in Yogyakarta, Indonesia. From a total of 907 faecal samples obtained during the main study, 80 samples were purposefully chosen according to their diagnostic test results; i.e., group 1, 20 samples with a positive test result for S. stercoralis in both BM and KAP; group 2, 20 samples with a positive test result for S. stercoralis in BM only; group 3, 20 samples with a positive test result for S. stercoralis in KAP only; and group 4, 20 samples with a negative test result for S. stercoralis in both BM and KAP. All researchers carrying out the PCR analyses were blinded to the results of the parasitological methods.
Nucleic acid extraction from stool samples
For the extraction of nucleic acids from ~ 100 mg of each stool specimen, the Favor Prep Stool DNA Isolation Mini Kit (Favorgen Biotech Corporation; Ping-Tung, Taiwan) was used according to the manufacturer’s instructions. This extraction method includes the use of glass beads, proteinase K, SDE 1, SDE 2, SDE 3, SDE 4, wash buffer, elution buffer and 96% ethanol. Isolated nucleic acids were stored at − 20 °C.
Primers used and PCR protocol
Previously developed primers (Moghaddassani et al. 2011) were used to amplify part (114 bp) of the internal transcribed spacer 2 (ITS2) rDNA of S. stercoralis. The sequence of the forward primer (SSF) was 5′ ATC GTG TGG GTG GAT CAT TC 3′, and the sequence of the reverse primer (SSR) was 5′ CTA TTA GCG CCA TTT GCA TTC 3′. The PCR reaction was performed using the following reaction mix: 15 μl of PCR mastermix, 2 μl primers, 4 μl sample DNA, and up to 9 μl of nuclease-free water so that the final volume reached 30 μl. The PCR conditions comprised an initial denaturation/enzyme activation step at 95 °C for 5 min, followed by 30 cycles of 30 s at 94 °C (denaturation), 58 °C for 50 s (annealing) and 72 °C for 50 s (extension) (Ghasemikhah et al. 2017; Moghaddassani et al. 2011). Positive (a known positive sample of S. stercoralis) and negative controls (PCR mix without DNA template) were included in all PCR runs. A gel electrophoresis was carried out after the PCR to visualise amplification products. Of note, no quantitative PCR analyses were performed.
Results
Diagnostic comparison of BM, KAP and PCR
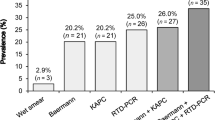
The experimental design used in this study is shown in Fig. 1. In total, 37 out of 60 microscopically positive samples (groups 1, 2 and 3) were confirmed by PCR, and molecular diagnostics revealed an additional eight positive results in samples that tested negative on both BM and KAP (group 4). The overall diagnostic agreement between PCR and the combination of BM and KAP was 61% (49/80 specimens) with considerable variation from one group to another. The highest agreement was found for the BM- and KAP-positive samples in group 1 (75%), whereas only 45% of the KAP-positive, but BM-negative samples (group 3) were confirmed by PCR. An agarose gel displaying several ITS2 amplicons of S. stercoralis is displayed in Fig. 2.
Infection intensity analysis
According to BM and KAP techniques, most samples contained a low number of microscopically detected S. stercoralis larvae, i.e., ≤ 10 larvae were seen in 46/60 samples (77%). An infection intensity of 11–50 larvae was observed in nine specimens (15%), whereas a high larval load with > 50 larvae was only seen in five samples (8%). The PCR confirmation rate in the groups with a low, medium and high number or S. stercoralis larvae was 52%, 89% and 100%, respectively (Table 1).
Co-infections between hookworm and S. stercoralis
While the BM technique detected exclusively S. stercoralis, KAP revealed additional hookworm infections in 31 of the 80 stool samples (39%). Co-infection with hookworm was observed in 43% (29/68) of all S. stercoralis-infected individuals (Table 2). Among the microscopy-positive, but PCR-negative samples for S. stercoralis (23 samples from groups 1–3), nine samples were also positive for hookworm.
Discussion
The current study revealed relatively poor agreement between microscopic methods (BM and KAP) and a specific PCR for the detection of S. stercoralis infection in human stool samples obtained from central Maluku, Indonesia. Of note, diagnostic agreement was highest when both BM and KAP techniques gave a positive result and when the number of microscopically detected larvae was high. Co-infection with hookworm was observed in almost half of all S. stercoralis-positive participants.
In contrast to the major soil-transmitted helminths, epidemiological and clinical data pertaining to S. stercoralis infection are scarce, and this also holds true for the present study area in Maluku island (Salakory et al. 2013). This issue is partially explained by the lack of a readily available diagnostic tool that can identify S. stercoralis with high fidelity at low cost. Indeed, direct faecal smears and Kato-Katz thick smears, which are the most commonly employed techniques in resource-constrained settings, fail to detect S. stercoralis. The more laborious BM and KAP techniques are rarely performed on a routine basis, and their sensitivities are estimated at approximately 75% if only a single stool sample is examined (Campo Polanco et al. 2014). Hence, PCR techniques were developed with the goal to enhance diagnostic accuracy. Several protocols targeting either subunits of the 18S (Janwan et al. 2011; Verweij et al. 2009) or the 28S ribosomal RNA gene of S. stercoralis (Kramme et al. 2011) have been applied in clinical settings. While studies from Côte d’Ivoire (Becker et al. 2015), Ethiopia (Amor et al. 2016), Iran (Sharifdini et al. 2015), Mozambique (Meurs et al. 2017) and Spain (Saugar et al. 2015) reported PCR to be the single most sensitive diagnostic technique for S. stercoralis, discouraging results have been observed elsewhere, both in endemic (e.g., Tanzania) (Knopp et al. 2014) and non-endemic settings (e.g., Italy) (Buonfrate et al. 2017). Of note, some studies observed a higher rate of ‘false-negative’ PCR results in cases with low infection intensity. In Cambodia, the diagnostic accuracy of PCR for S. stercoralis was low when it was used for an analysis of stool samples from asymptomatic individuals who usually have low-intensity infections (Schär et al. 2013a). These findings are in line with our observations; the PCR confirmation rate dropped from 100% in high-intensity infections to 53% in samples characterised by few microscopically detected larvae. Additionally, the diagnostic agreement between PCR and microscopy was highest when both BM and KAP were positive, which is more likely the case in samples with a higher pathogen quantity. Hence, our findings support those of a recently published systematic review and meta-analysis, which concluded that current PCR assays for S. stercoralis might not (yet) be suitable for screening purposes (Buonfrate et al. 2018). However, the observation that eight samples were exclusively positive by PCR underscores the lack of diagnostic accuracy of stool microscopy, unless multiple consecutive samples are examined.
There are several reasons for the low sensitivity of helminth PCR assays in low-intensity infections. First, the low quantity of stool used for DNA extraction plays an important role (Wongratanacheewin et al. 2002) because only ≤ 0.1 g of stool are utilised for the extraction procedure, whereas several grams are used in the BM funnel and for KAP (Paula et al. 2015). Hence, the amount of stool used for the nucleic acid extraction may simply not contain any helminth larvae. Second, the effectiveness of the actual nucleic acid extraction procedure may also have an influence on the results obtained by subsequent helminth PCR examinations (Barda et al. 2018), e.g., the addition of a washing step to remove the fixative (ethanol) might have improved the diagnostic yield of PCR in our study. Third, we cannot exclude that some samples, which were only positive for S. stercoralis on KAP, but negative on BM and PCR were actually ‘false-positives’ due to a microscopic misidentification of morphologically similar hookworm larvae as S. stercoralis. Such observations have been previously made in PCR-based studies (Becker et al. 2015) and emphasise the need for detailed training of laboratory technicians and quality assurance schemes in medical laboratories. A clear distinction between hookworm and S. stercoralis is particularly warranted in settings of high co-endemicity, such as the one reported here. Fourth, the presence of inhibitors in the faecal samples (e.g., bacterial proteases, nucleases, cell debris and bile acids) and/or partial nucleic acid degradation despite sample preservation in ethanol may have led to some ‘false-negative’ PCR results.
Our study has several limitations. First, examination of single stool samples is not sufficient to exclude strongyloidiasis, regardless of the diagnostic technique employed. A higher sensitivity is achieved if consecutive samples are being analysed. Second, the absence of a diagnostic ‘gold’ standard did not allow for a clear judgement of the true infection status in samples with discrepant BM, KAP and PCR results. The addition of a serological test, which is the single most sensitive screening test for detection of an individual’s previous exposure to S. stercoralis, would have been very valuable in this regard (Bisoffi et al. 2014). However, blood sampling was not done in the NIDIAG study on persistent digestive disorders. Third, the prevalence of hookworm co-infection was probably higher than reported, because KAP is not the most accurate laboratory test for the diagnosis of hookworm infection. Fourth, it would have been interesting to compare different published primers for detection of S. stercoralis in order to comparatively evaluate the accuracy of different PCR protocols for this nematode species. Such studies are warranted, so that the most promising assays can be further optimised.
In conclusion, our study provides the first comparative molecular and parasitological study on the occurrence of S. stercoralis in Indonesia. We observed a high confirmation rate of PCR when a high number of S. stercoralis larvae were present in the stool sample and when both BM and KAP were positive. PCR identified additional infections in microscopically negative specimens. However, PCR failed to confirm infections with low larval load. There is a need for well-designed comparative evaluation studies in different settings, which should include quantitative PCR and serology, to accurately assess the suitability and diagnostic accuracy of specific molecular diagnostic techniques for S. stercoralis.
References
Albonico M, Becker SL, Odermatt P, Angheben A, Anselmi M, Amor A, Barda B, Buonfrate D, Cooper P, Gétaz L, Keiser J, Khieu V, Montresor A, Muñoz J, Requena-Méndez A, Savioli L, Speare R, Steinmann P, van Lieshout L, Utzinger J, Bisoffi Z, StrongNet Working Group (2016) StrongNet: an international network to improve diagnostics and access to treatment for strongyloidiasis control. PLoS Negl Trop Dis 10:e0004898. https://doi.org/10.1371/journal.pntd.0004898
Alirol E, Horie NS, Barbé B, Lejon V, Verdonck K, Gillet P, Jacobs J, Büscher P, Kanal B, Bhattarai NR, El Safi S, Phe T, Lim K, Leng L, Lutumba P, Mukendi D, Bottieau E, Boelaert M, Rijal S, Chappuis F (2016) Diagnosis of persistent fever in the tropics: set of standard operating procedures used in the NIDIAG febrile syndrome study. PLoS Negl Trop Dis 10:e0004749. https://doi.org/10.1371/journal.pntd.0004749
Amor A, Rodriguez E, Saugar JM, Arroyo A, Lopez-Quintana B, Abera B, Yimer M, Yizengaw E, Zewdie D, Ayehubizu Z, Hailu T, Mulu W, Echazú A, Krolewieki AJ, Aparicio P, Herrador Z, Anegagrie M, Benito A (2016) High prevalence of Strongyloides stercoralis in school-aged children in a rural highland of north-western Ethiopia: the role of intensive diagnostic work-up. Parasit Vectors 9:617. https://doi.org/10.1186/s13071-016-1912-8
Barda B, Wampfler R, Sayasone S, Phongluxa K, Xayavong S, Keoduangsy K, Schindler C, Keiser J (2018) Evaluation of two DNA extraction methods on the detection of Strongyloides stercoralis infection. J Clin Microbiol 56:e01941–e01917. https://doi.org/10.1128/JCM.01941-17
Becker SL, Sieto B, Silué KD, Adjossan L, Koné S, Hatz C, Kern WV, N’Goran EK, Utzinger J (2011) Diagnosis, clinical features, and self-reported morbidity of Strongyloides stercoralis and hookworm infection in a co-endemic setting. PLoS Negl Trop Dis 5:e1292. https://doi.org/10.1371/journal.pntd.0001292
Becker SL, Vogt J, Knopp S, Panning M, Warhurst DC, Polman K, Marti H, von Müller L, Yansouni CP, Jacobs J, Bottieau E, Sacko M, Rijal S, Meyanti F, Miles MA, Boelaert M, Lutumba P, van Lieshout L, N’Goran EK, Chappuis F, Utzinger J (2013) Persistent digestive disorders in the tropics: causative infectious pathogens and reference diagnostic tests. BMC Infect Dis 13:37. https://doi.org/10.1186/1471-2334-13-37
Becker SL, Piraisoody N, Kramme S, Marti H, Silué KD, Panning M, Nickel B, Kern WV, Herrmann M, Hatz CF, N’Goran EK, Utzinger J, von Müller L (2015) Real-time PCR for detection of Strongyloides stercoralis in human stool samples from Côte d'Ivoire: diagnostic accuracy, inter-laboratory comparison and patterns of hookworm co-infection. Acta Trop 150:210–217. https://doi.org/10.1016/j.actatropica.2015.07.019
Becker SL, Yap P, Horié NS, Alirol E, Barbé B, Bhatta NK, Bhattarai NR, Bottieau E, Chatigre JK, Coulibaly JT, Fofana HKM, Jacobs J, Karki P, Khanal B, Knopp S, Koirala K, Mahendradhata Y, Mertens P, Meyanti F, Murhandarwati EH, N’Goran EK, Peeling RW, Pradhan B, Ravinetto R, Rijal S, Sacko M, Saye R, Schneeberger PHH, Schurmans C, Silué KD, Steinmann P, van Loen H, Verdonck K, van Lieshout L, von Müller L, Yao JA, Boelaert M, Chappuis F, Polman K, Utzinger J (2016) Experiences and lessons from a multicountry NIDIAG study on persistent digestive disorders in the tropics. PLoS Negl Trop Dis 10:e0004818. https://doi.org/10.1371/journal.pntd.0004818
Belhassen-García M, Alonso-Sardón M, Martinez-Perez A, Soler C, Carranza-Rodriguez C, Pérez-Arellano JL, Muro A, Salvador F, Soil-Transmitted Helminths Study Group of the SEMTSI (2017) Surveillance of strongyloidiasis in Spanish in-patients (1998-2014). PLoS One 12:e0189449. https://doi.org/10.1371/journal.pone.0189449
Bisoffi Z, Buonfrate D, Montresor A, Requena-Méndez A, Munoz J, Krolewiecki AJ, Gotuzzo E, Mena MA, Chiodini PL, Anselmi M Moreira J, Albonico M (2013) Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis 7:e2214. https://doi.org/10.1371/journal.pntd.0002214
Bisoffi Z, Buonfrate D, Sequi M, Mejia R, Cimino RO, Krolewiecki AJ, Albonico M, Gobbo M, Bonafini S, Angheben A, Requena-Mendez A, Muñoz J, Nutman TB (2014) Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis 8:e2640. https://doi.org/10.1371/journal.pntd.0002640
Buonfrate D, Requena-Méndez A, Angheben A, Muñoz J, Gobbi F, Van Den Ende J, Bisoffi Z (2013) Severe strongyloidiasis: a systematic review of case reports. BMC Infect Dis 13:78. https://doi.org/10.1186/1471-2334-13-78
Buonfrate D, Mena MA, Angheben A, Requena-Méndez A, Muñoz J, Gobbi F, Albonico M, Gotuzzo E, Bisoffi Z (2015) Prevalence of strongyloidiasis in Latin America: a systematic review of the literature. Epidemiol Infect 143:452–460. https://doi.org/10.1017/S0950268814001563
Buonfrate D, Baldissera M, Abrescia F, Bassetti M, Caramaschi G, Giobbia M, Mascarello M, Rodari P, Scattolo N, Napoletano G, Bisoffi Z, on behalf of the CCM Strongyloides Study Group (2016) Epidemiology of Strongyloides stercoralis in northern Italy: results of a multicentre case-control study, February 2013 to July 2014. Euro Surveill 21. https://doi.org/10.2807/1560-7917.ES.2016.21.31.30310
Buonfrate D, Perandin F, Formenti F, Bisoffi Z (2017) A retrospective study comparing agar plate culture, indirect immunofluorescence and real-time PCR for the diagnosis of Strongyloides stercoralis infection. Parasitology 144:812–816. https://doi.org/10.1017/S0031182016002559
Buonfrate D, Requena-Méndez A, Angheben A, Cinquini M, Cruciani M, Fittipaldo A, Giorli G, Gobbi F, Piubelli C, Bisoffi Z (2018) Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection – a systematic review and meta-analysis. PLoS Negl Trop Dis 12:e0006229. https://doi.org/10.1371/journal.pntd.0006229
Campo Polanco L, Gutiérrez LA, Cardona Arias J (2014) Diagnosis of Strongyloides stercoralis infection: meta-analysis on evaluation of conventional parasitological methods (1980-2013). Rev Esp Salud Publica 88:581–600 [in Spanish]. https://doi.org/10.4321/S1135-57272014000500004
Concha R, Harrington W Jr, Rogers AI (2005) Intestinal strongyloidiasis: recognition, management, and determinants of outcome. J Clin Gastroenterol 39:203–211
Depkes RI (Indonesian Ministry of Health) (2009) Pencapaian Kegiatan Pengendalian Penyakit dan Penyehatan Lingkungan di dalam Profil Pemberantasan Penyakit Menular dan Penyehatan lingkungan 2008, hal 19-173. Jakarta, Indonesia. [in Bahasa Indonesia]
Dunn JC, Turner HC, Tun A, Anderson RM (2016) Epidemiological surveys of, and research on, soil-transmitted helminths in Southeast Asia: a systematic review. Parasit Vectors 9:31. https://doi.org/10.1186/s13071-016-1310-2
Ghasemikhah R, Tabatabaiefar MA, Shariatzadeh SA, Shahbazi A, Hazratian T (2017) A PCR-based molecular detection of Strongyloides stercoralis in human stool samples from Tabriz City, Iran. Sci Pharm 85. https://doi.org/10.3390/scipharm85020017
Janwan P, Intapan PM, Thanchomnang T, Lulitanond V, Anamnart W, Maleewong W (2011) Rapid detection of Opisthorchis viverrini and Strongyloides stercoralis in human fecal samples using a duplex real-time PCR and melting curve analysis. Parasitol Res 109:1593–1601. https://doi.org/10.1007/s00436-011-2419-z
Jourdan PM, Lamberton PHL, Fenwick A, Addiss DG (2017) Soil-transmitted helminth infections. Lancet 391:252–265. https://doi.org/10.1016/S0140-6736(17)31930-X
Keiser PB, Nutman TB (2004) Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev 17:208–217
Knopp S, Salim N, Schindler T, Karagiannis Voules DA, Rothen J, Lweno O, Mohammed AS, Singo R, Benninghoff M, Nsojo AA, Genton B, Daubenberger C (2014) Diagnostic accuracy of Kato-Katz, FLOTAC, Baermann, and PCR methods for the detection of light-intensity hookworm and Strongyloides stercoralis infections in Tanzania. Am J Trop Med Hyg 90:535–545. https://doi.org/10.4269/ajtmh.13-0268
Kramme S, Nissen N, Soblik H, Erttmann K, Tannich E, Fleischer B, Panning M, Brattig N (2011) Novel real-time PCR for the universal detection of Strongyloides species. J Med Microbiol 60:454–458. https://doi.org/10.1099/jmm.0.025338-0
Meurs L, Polderman AM, Vinkeles Melchers NV, Brienen EA, Verweij JJ, Groosjohan B, Mendes F, Mechendura M, Hepp DH, Langenberg MC, Edelenbosch R, Polman K, van Lieshout L (2017) Diagnosing polyparasitism in a high-prevalence setting in Beira, Mozambique: detection of intestinal parasites in fecal samples by microscopy and real-time PCR. PLoS Negl Trop Dis 11:e0005310. https://doi.org/10.1371/journal.pntd.0005310
Moghaddassani H, Mirhendi H, Hosseini M, Rokni M, Mowlavi G, Kia E (2011) Molecular diagnosis of Strongyloides stercoralis infection by PCR detection of specific DNA in human stool samples. Iran J Parasitol 6:23–30
Paula FM, Malta Fde M, Marques PD, Sitta RB, Pinho JR, Gryschek RC, Chieffi PP (2015) Molecular diagnosis of strongyloidiasis in tropical areas: a comparison of conventional and real-time polymerase chain reaction with parasitological methods. Mem Inst Oswaldo Cruz 110:272–274. https://doi.org/10.1590/0074-02760140371
Polman K, Becker SL, Alirol E, Bhatta NK, Bhattarai NR, Bottieau E, Bratschi MW, Burza S, Coulibaly JT, Doumbia MN, Horié NS, Jacobs J, Khanal B, Landouré A, Mahendradhata Y, Meheus F, Mertens P, Meyanti F, Murhandarwati EH, N’Goran EK, Peeling RW, Ravinetto R, Rijal S, Sacko M, Saye R, Schneeberger PHH, Schurmans C, Silué KD, Thobari JA, Traoré MS, van Lieshout L, van Loen H, Verdonck K, von Müller L, Yansouni CP, Yao JA, Yao PK, Yap P, Boelaert M, Chappuis F, Utzinger J (2015) Diagnosis of neglected tropical diseases among patients with persistent digestive disorders (diarrhoea and/or abdominal pain ≥ 14 days): a multi-country, prospective, non-experimental case-control study. BMC Infect Dis 15:338. https://doi.org/10.1186/s12879-015-1074-x
Repetto SA, Ruybal P, Batalla E, López C, Fridman V, Sierra M, Radisic M, Bravo PM, Risso MG, González Cappa SM, Alba Soto CD (2018) Strongyloidiasis outside endemic areas: long-term parasitological and clinical follow-up after ivermectin treatment. Clin Infect Dis 66:1558–1565. https://doi.org/10.1093/cid/cix1069
Requena-Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Muñoz J (2013) The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis 7:e2002. https://doi.org/10.1371/journal.pntd.0002002
Salakory M, Soeyoko, Mardihusodo SJ, Sutanto (2013) Penggunaan teknologi remote sensing dan SIG untuk pengendalian dinamika populasi soil-transmitted helminths di satuan lahan endemis Pulau Ambon. J Manusia dan Lingkungan 20:339–352 [in Bahasa Indonesia]
Saugar JM, Merino FJ, Martin-Rabadan P, Fernandez-Soto P, Ortega S, Garate T, Rodriguez E (2015) Application of real-time PCR for the detection of Strongyloides spp. in clinical samples in a reference center in Spain. Acta Trop 142:20–25. https://doi.org/10.1016/j.actatropica.2014.10.020
Schär F, Odermatt P, Khieu V, Panning M, Duong S, Muth S, Marti H, Kramme S (2013a) Evaluation of real-time PCR for Strongyloides stercoralis and hookworm as diagnostic tool in asymptomatic schoolchildren in Cambodia. Acta Trop 126:89–92. https://doi.org/10.1016/j.actatropica.2012.12.012
Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, Vounatsou P, Odermatt P (2013b) Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis 7:e2288. https://doi.org/10.1371/journal.pntd.0002288
Sharifdini M, Mirhendi H, Ashrafi K, Hosseini M, Mohebali M, Khodadadi H, Kia EB (2015) Comparison of nested polymerase chain reaction and real-time polymerase chain reaction with parasitological methods for detection of Strongyloides stercoralis in human fecal samples. Am J Trop Med Hyg 93:1285–1291. https://doi.org/10.4269/ajtmh.15-0309
Strkolcova G, Goldova M, Bockova E, Mojzisova J (2017) The roundworm Strongyloides stercoralis in children, dogs, and soil inside and outside a segregated settlement in Eastern Slovakia: frequent but hardly detectable parasite. Parasitol Res 116:891–900. https://doi.org/10.1007/s00436-016-5362-1
Utzinger J, Becker SL, Knopp S, Blum J, Neumayr AL, Keiser J, Hatz CF (2012) Neglected tropical diseases: diagnosis, clinical management, treatment and control. Swiss Med Wkly 142:w13727. https://doi.org/10.4414/smw.2012.13727
Vadlamudi RS, Chi DS, Krishnaswamy G (2006) Intestinal strongyloidiasis and hyperinfection syndrome. Clin Mol Allergy 4:8. https://doi.org/10.1186/1476-7961-4-8
Verweij JJ, Canales M, Polman K, Ziem J, Brienen EA, Polderman AM, van Lieshout L (2009) Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans R Soc Trop Med Hyg 103:342–346. https://doi.org/10.1016/j.trstmh.2008.12.001
Widjana DP, Sutisna P (2000) Prevalence of soil-transmitted helminth infections in the rural population of Bali, Indonesia. Southeast Asian J Trop Med Public Health 31:454–459
Wongratanacheewin S, Pumidonming W, Sermswan RW, Pipitgool V, Maleewong W (2002) Detection of Opisthorchis viverrini in human stool specimens by PCR. J Clin Microbiol 40:3879–3880
Acknowledgements
We thank all participants in Maluku Tengah Regency, Maluku, Indonesia, and the staff from the Province Health Office of Maluku who facilitated the field work. We are grateful to the laboratory technicians and research staff involved in the field work both from RSUD Tulehu, Maluku Tengah Regency, Maluku, Indonesia and the Department of Parasitology at Universitas Gadjah Mada, Yogyakarta, for laboratory analyses.
Funding
This work is part of the NIDIAG European research network (Collaborative Project), supported by the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no. 260260.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The protocol for the NIDIAG study on persistent digestive disorders was approved by the institutional review boards (IRBs) of the Institute of Tropical Medicine (ITM; Antwerp, Belgium) and Swiss Tropical and Public Health Institute (Swiss TPH; Basel, Switzerland) prior to external review. Approval in Indonesia was granted by the ethics committee of the Universitas Gadjah Mada (21 November 2013). The NIDIAG study is registered on ClinicalTrials.gov (identifier: NCT02105714). All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
Section Editor: Neil Bruce Chilton
Rights and permissions
About this article
Cite this article
Kristanti, H., Meyanti, F., Wijayanti, M.A. et al. Diagnostic comparison of Baermann funnel, Koga agar plate culture and polymerase chain reaction for detection of human Strongyloides stercoralis infection in Maluku, Indonesia. Parasitol Res 117, 3229–3235 (2018). https://doi.org/10.1007/s00436-018-6021-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-6021-5