Abstract
Globalisation and climate change are the main drivers of invasion of non-endemic regions by mosquitoes. Mass transportation of people, animals and goods facilitate accidental long-distance displacement while climate warming supports active spread and establishment of thermophilic species. In the framework of a mosquito-monitoring programme, eight non-indigenous culicid species have been registered in Germany since 2011, with four of them being more or less efficient vectors of disease agents and another four now considered established. The eight newly emerged species include Aedes albopictus, Ae. japonicus, Ae. aegypti, Ae. koreicus, Ae. berlandi, Ae. pulcritarsis, Anopheles petragnani and Culiseta longiareolata. We here review recent findings and at the same time present new findings of specimens of non-native mosquito species in Germany.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After the eradication of endemic malaria from Europe around the mid-twentieth century (Bruce-Chwatt and de Zulueta 1980), research on the indigenous mosquito fauna had been largely neglected in many European countries since endemic mosquitoes were not considered relevant vectors of disease agents anymore. In Germany, mosquitoes were subsequently monitored only sporadically and on local or regional scales, as demonstrated by a very limited body of reports. Thus, after decades of ignorance, basic data on the occurrence and distribution of mosquito species became outdated.
Since the late twentieth century, Europe has been facing increasing importation of invasive mosquitoes, including efficient vectors, mainly due to globalisation. In particular, the yellow fever mosquito Aedes aegypti, the Asian tiger mosquito Ae. albopictus, the Asian bush mosquito Ae. japonicus and Ae. koreicus have repeatedly been introduced and succeeded in colonising parts of Europe. Following the establishment and spread of Ae. aegypti and Ae. albopictus, mosquito-borne diseases, such as chikungunya and dengue, (re)emerged in southern Europe (Schaffner et al. 2013).
Before the background of the unexpected outbreak of bluetongue disease in 2006 (Saegerman et al. 2009), a ceratopogonid-borne viral disease of ruminants which demonstrated a dire lack of expertise and data on European insect vectors, these developments prompted a nationwide mosquito-monitoring programme in Germany in 2011 which is still ongoing. The programme is based on an active and a passive approach. In the active part, adults and larvae have been targetedly sampled during field surveys since 2011. Passively, mosquito occurrence and distribution data have been collected since 2012 by analysing adult specimens submitted to the citizen science project ‘Mueckenatlas’ (mosquito atlas) (Werner et al. 2014; Kampen et al. 2015).
Using both approaches, more than 360,000 mosquitoes collected from 2011 to 2016 all over Germany were identified, among them numerous specimens of non-indigenous species. We here give an account of these which, in part, are considered efficient vectors of disease agents and may pose public health risks in the case of establishment. In addition to published reports on the emergence of these species in Germany, recent and hitherto non-published findings are presented.
Materials and methods
Mosquito collection
Mosquitoes were collected throughout Germany from 2011 and 2012, respectively, to 2016 based on active and passive approaches. Active monitoring was done by trapping, netting and aspirating adults and by dipping for aquatic developmental stages. For trapping, BG Sentinels (up to 80 in 2013) and EVS (encephalitis virus surveillance) traps (up to 48 in 2013) were predominantly used, with traps operated from April to October for 24 h per week (Kampen et al. 2016a). Passively, mosquitoes were collected by the citizen science project ‘Mueckenatlas’ (www.mueckenatlas.de) where private persons are invited to capture and submit mosquito specimens for identification (Werner et al. 2014; Kampen et al. 2015).
Mosquito identification
Mosquitoes collected as larvae or pupae were generally brought to the laboratory and reared until adult emergence for easier morphological identification. Adults were morphologically identified to species or species group using the determination keys by Schaffner et al. (2001) and Becker et al. (2010). Exceptionally in the case of immature stages but in all cases of adults belonging to cryptic or other closely related species not reliably identifiable by morphological traits (An. claviger complex, An. maculipennis complex, Culex pipiens complex), as well as in the case of damaged specimens, genetic analyses by species-specific PCR assays (Kampen et al. 2003; Rudolf et al. 2013; Kronefeld et al. 2014) or by COI (cytochrome c oxidase subunit I)-barcoding (Folmer et al. 1994; Hébert et al. 2003) were performed. In the majority of cases, one leg of an adult mosquito and two to three terminal segments of the abdomen of larvae and pupae were used for genetic processing. Morphological identification of a newly emerging species was confirmed by barcoding at least one specimen per collection site.
Dry voucher specimens (pinned or in vials) of all species and from all collection sites and deep-frozen (− 80 °C) DNA samples of the individuals subjected to genetic identification are kept at ZALF and FLI, respectively, for long-term storage.
Results
Since 2011, specimens of eight non-indigenous culicid species (Aedes albopictus, Ae. japonicus, Ae. aegypti, Ae. koreicus, Ae. berlandi, Ae. pulcritarsis, Anopheles petragnani, Culiseta longiareolata) have been collected in the framework of the monitoring activities, four by active approaches (Ae. albopictus, Ae. japonicus, An. petragnani, Cs. longiareolata) and all but one passively by the citizen science approach. Only An. petragnani has not yet been among the species submitted to the ‘Mueckenatlas’ (Table 1).
Aedes (Stegomyia) albopictus (Skuse, 1895) (Asian tiger mosquito)
Aedes albopictus, once native to tropical and subtropical Asian-Pacific regions, can today be found on all continents (Paupy et al. 2009). In the USA, it is established in at least 26 states (Bonizzoni et al. 2013) while in Europe, it has been detected in 26 countries and is established in 19 (Medlock et al. 2015). It is a vector of numerous viruses, including dengue, West Nile, Japanese encephalitis, Zika, chikungunya and Rift Valley fever viruses, as well as of dirofilarial worms (Gratz 2004; Paupy et al. 2009; Heitmann et al. 2017).
Aedes albopictus was first detected in Europe in 1979 (Adhami and Reiter 1998) but only spread after successive importations of eggs by used tyres to Italy in 1990 (Knudsen et al. 1996). In Germany, the species was found first in 2007 in the form of eggs during a survey from 2005 to 2009 along the motorway A5 from the Swiss-German border to Heidelberg (ca. 240 km) (Pluskota et al. 2008). No specimens were recorded in 2008 and 2009, and the survey was interrupted for 2010. Since 2011, adults have regularly been trapped along the same and other South German motorways as well as on a South German cargo railway station where trucks arrive and continue their way on the road after railway transportation from Italy (Werner et al. 2012; Becker et al. 2013; Kampen et al. 2013a).
In 2014, the first Ae. albopictus individuals were submitted to the ‘Mueckenatlas’ surveillance scheme (Werner and Kampen 2015), followed by increasing numbers of specimens in 2015 and 2016 (Walther et al. 2017; Fig. 1). After the reappearance of Ae. albopictus developmental stages at several places where they had been found in the year before (e.g. Freiburg-East, Freiburg-West, Jena), overwintering and establishment in Germany must by now be considered accomplished (Pluskota et al. 2016; Walther et al. 2017).
Aedes (Hulecoeteomyia) japonicus Theobald, 1901 (Asian bush or rock pool mosquito)
Aedes japonicus originates from East Asia where it occurs in temperate climatic zones (Kampen and Werner 2014). It has successfully established in numerous states of the USA and several regions of Canada and Europe (Kampen and Werner 2014; Fielden et al. 2015; Jackson et al. 2016; Zielke et al. 2016). In the laboratory, it has been shown to be vector-competent for West Nile, Japanese encephalitis, La Crosse, St. Louis, eastern equine encephalitis and Rift Valley fever viruses as well as for dirofilarial worms, while—despite the demonstration of Japanese encephalitis, West Nile and La Crosse viruses in field-collected specimens—its vector potential under natural conditions is unclear (Kampen and Werner 2014; Harris et al. 2015; Silaghi et al. 2017).
Aedes japonicus emerged in Europe in 2000, in France, but was immediately controlled and successfully eliminated (Schaffner et al. 2003). In 2002, establishment was demonstrated in Belgium (Versteirt et al. 2009), followed by continuous colonisation until 2015, when eradication was achieved (Deblauwe et al. 2014; Versteirt et al. 2017). Since 2008, six further populations, several crossing country borders, were reported, affecting Austria, Croatia, France, Hungary, Italy, Liechtenstein, the Netherlands, Slovenia, Switzerland (Schaffner et al. 2009; Huber et al. 2012; Seidel et al., 2012, 2016a, b; Ibáñez-Justicia et al. 2014; Kalan et al. 2014; Krebs et al. 2014; Klobučar et al. 2017) and Germany, where ‘Mueckenatlas’ submissions led to the discovery of three populations in 2012 (western Germany), 2013 (northern Germany) and 2015 (southeastern Germany) (Kampen et al. 2012; Werner and Kampen 2013; Zielke et al. 2016). The fourth Ae. japonicus population had already been detected in southwestern Germany in 2008 (Schaffner et al. 2009; Huber et al. 2012). The excellent preadaptation of this species to the Central European climate and environment is demonstrated by an about fourfold increase of the area covered by the western German population between 2012 and 2015, as determined by field sampling (Kampen et al. 2016a).
In 2016, numerous Ae. japonicus specimens were collected in Germany far from the previously known distribution areas, e.g. in various parts of Bavaria, Thuringia, Saxony and Saxony-Anhalt, confirming the ongoing dispersal trend (Fig. 2). It remains to be clarified whether these findings were attributed to mere displacement of single specimens or new local colonisations. Due to its wide current distribution in Germany, its partly high regional abundances and its continuing spread, Ae. japonicus must, at any rate, now be considered belonging to the indigenous German, and Central European, mosquito fauna.
Aedes (Stegomyia) aegypti (Linnaeus, 1762) (yellow fever mosquito)
Aedes aegypti is an efficient vector of dengue, yellow fever and Zika viruses (Black et al. 2002; Roundy et al. 2017). It is a particularly thermophilic mosquito species not adapted to temperate climates, although populations historically occurred as far north as western France and southern Wales during summer (Coleman 1984; Smith and Gibson 1986). By contrast, the species was endemic in the European Mediterranean at least from the late seventeenth until the mid-twentieth century where it caused numerous outbreaks of yellow fever and dengue with high morbidity and mortality (Morillon et al. 2002; Schaffner and Mathis 2014). After it had disappeared from Europe, re-emergence was recently reported from the Portuguese island of Madeira and the eastern Black Sea coast (southern Russia, Abkhazia, Georgia, eastern Turkey) (Yunicheva et al. 2008; Almeida et al. 2007; Akiner et al. 2016; Ganushkina et al. 2016). Eggs and adults introduced into the Netherlands by the used tyre trade and by aircraft during the past few years were eliminated (Scholte et al. 2010; Ibáñez-Justicia et al. 2016).
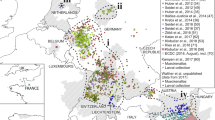
In Germany, the ‘Mueckenatlas’ helped detect the importation of Ae. aegypti eggs by exotic plant seedlings through a traveller and the successive development of a population in the household of this traveller in 2016 (Fig. 3). Altogether, 13 adult specimens were submitted but the house owner claimed to have killed and discarded at least the same number of adults as well as numerous larvae detected in the watering jar of the plants. Apparently, the species was not able to escape and establish outdoors, as suggested by the absence of stages during a small-scale monitoring around the infested house (Kampen et al. 2016b). Based on the adaptation of Ae. aegypti to warm climates, it is unlikely that reproduction is possible in Central Europe unless temporarily during unusually warm summers.
Collections of Ae. aegypti (black circle: 2016), Ae. koreicus (black square: 2016), Ae. berlandi (white square: 2016), Ae. pulcritarsis (white circle: 2016), Ae. koreicus (black square: 2016) and An. petragnani (white triangle: 2015, black triangle: 2016) in Germany. Map: German Federal Agency for Cartography and Geodesy
Aedes (Hulecoeteomyia) koreicus (Edwards, 1917)
In many respects, Ae. koreicus is very similar to its close relative Ae. japonicus. Its native distribution area being in China, Japan, Korea and eastern Russia (Knight 1968), it is well adapted to temperate climates (Tanaka et al. 1979) and its eggs are regarded as similarly cold- and drought-resistant as those of Ae. japonicus. Its emergence in Europe, most probably via the same transportation routes (trade of tyres, plants, and water-holding gardening equipment and machinery) as Ae. albopictus and Ae. japonicus, is therefore not surprising.
Also, morphologically, Ae. koreicus highly resembles Ae. japonicus (Cameron et al. 2010; Versteirt et al. 2012). Historically, the two species had once been considered two varieties of the same species (Gutsevich et al. 1971), and it has been suggested to put them into the same species group again (Cameron et al. 2010).
The vector potential of Ae. koreicus is not clear. Already decades ago, Feng (1930) demonstrated transmission of Dirofilaria immitis, and Montarsi et al. (2015) recently confirmed those findings using Ae. koreicus reared in the laboratory from specimens that had been collected in the field in Italy. The species also appears to be able to transmit Brugia malayi (KCDC 2007) and Japanese encephalitis virus (Miles 1964). It is suggested to be a vector of further arboviruses (Cameron et al. 2010; Capelli et al. 2011), although there is no evidence for that.
Aedes koreicus was found in Europe for the first time in 2008 in Belgium (Versteirt et al. 2012) and was still present there in 2015 (Versteirt, pers. comm.). Populations were also detected in Italy in 2011 and Switzerland in 2013 (Capelli et al. 2011; Suter et al. 2015). In southern Germany (federal state of Bavaria), a female specimen was captured in summer 2015 and submitted to the ‘Mueckenatlas’ (Werner et al. 2016; Fig. 3), but local reproduction could not be verified, according to larval monitoring conducted immediately after the identification of the specimen and again in summer 2016. Aedes koreicus also emerged in Slovenia in 2013 (Kalan et al. 2017), on the eastern Baltic Sea coast in southern Russia in 2013 (Ganushkina et al. 2016) and in Hungary in 2016 (Kurucz et al. 2016). Due to the continuing existence of the Belgian population and several others in Europe as well as the climatic preferences of the species, it can be reasonably assumed that Ae. koreicus will keep spreading in Central Europe and reappear in Germany in the future.
Aedes (Ochlerotatus) berlandi Séguy, 1921
Aedes berlandi is a tree-hole breeder endemic in the Mediterranean. It feeds on both animals and humans, but does not appear to be a vector of disease agents (Ribeiro et al. 1988).
A single adult female of Ae. berlandi collected in Freiburg, southwestern Germany, was submitted to the ‘Mueckenatlas’ surveillance scheme in mid-2016 (Fig. 3). The area surrounding the collection site had had records of Ae. japonicus and Ae. albopictus occurrence and has therefore regularly been monitored for larval stages and Aedes eggs. Notwithstanding, additional evidence for Ae. berlandi has not been found. Similar to numerous Ae. albopictus findings in that area (Becker et al. 2013; Kampen et al. 2013a), the species could well have been imported by vehicle transport from southern Europe.
Aedes (Ochlerotatus) pulcritarsis (Rondani, 1872)
Aedes pulcritarsis is closely related to Ae. berlandi and similar in many respects. It also uses tree-holes for larval development and has its major distribution area in the Mediterranean. However, in contrast to Ae. berlandi, it occurs further to the east, as far as Central Asia and probably India (Horsfall 1955). Some decades ago, Ryba (1979) described the species for Bohemia, i.e. the part of the Czech Republic adjacent to Germany, but recent documentations from that region are missing. The species is zoo- and anthropophilic (Becker et al. 2010), but there is no information available on pathogen transmission.
Similar to Ae. berlandi, a female caught in central western Germany (Fig. 3) was submitted to the ‘Mueckenatlas’ in mid-2016. It had been collected far from major roads in a rural area adjacent to a fragmented forest. Local monitoring was not conducted.
Anopheles (Anopheles) petragnani Del Vecchio, 1939
Anopheles petragnani is another thermophilic species, native to the western Mediterranean (Ramsdale and Snow 2000). It is strongly zoophilic and therefore, in contrast to its sibling An. claviger, not considered a malaria vector.
Quite unexpectedly, larval collections from rock pools in a Black Forest mountain stream (southwestern Germany) made in 2015 and 2016 did not only produce the target species, Ae. japonicus, but also included An. petragnani specimens (Becker et al. 2016; own unpublished findings, Table 1). Furthermore, two An. petragnani adults were trapped at another site in a more central part of southern Germany by a BG Sentinel in 2015 (Fig. 3). Findings of numerous larvae in the above Black Forest rock pools in 2017 suggest establishment of the species. A more in-depth monitoring for geographical distribution in Germany has not yet been carried out.
Culiseta (Allotheobaldia) longiareolata (Macquart, 1838)
Culiseta longiareolata is a thermophilic mosquito species widely distributed in various parts of Africa, central and southern Asia, the Near East and southern Europe. Exceptional findings were reported from more northern parts of Europe, such as northern France and southern England (Moussiegt 1986; Cranston et al. 1987). Specimens were also found in close proximity to southwestern Germany, in French Alsace (Moussiegt 1986) and northern Switzerland (Schaffner et al. 2009).
Since Cs. longiareolata is thought to preferentially feed on birds and rarely on humans (Roubaud and Colas-Belcour 1933; Cranston et al. 1987), it is not considered a vector of medical importance. Vector competence for avian pathogens (e.g. plasmodia), however, has been described (Corradetti and Scanga 1968).
While Maslov (1967) listed the southern part of the Federal Republic of Germany among the distribution areas of Cs. longiareolata already in 1967, unfortunately without citing original literature, the species had not been included in Germany’s mosquito inventories (Mohrig 1969; Dahl et al. 1999). Nowadays, it is generally accepted that the first German specimens of Cs. longiareolata were collected in 2011 in the Upper Rhine Valley (Becker and Hoffmann 2011; Werner et al. 2012), which is characterised by relatively warm summers and mild winters. Since that time, the species was repeatedly demonstrated in that part of Germany but also in central and even northern areas, both as larvae (southern and central Germany) and as adults (single specimens; as far north as Berlin) (Kampen et al. 2013b; Fig. 4). Establishment is deduced from repeated findings over the years but has not yet been shown by demonstrating the species to occur at exactly the same places in subsequent years.
Discussion
Thanks to extensive monitoring activities, several non-indigenous mosquito species have been encountered in Germany since 2011. Some of them appear to have established only recently (An. petragnani, Ae. albopictus, Ae. japonicus, Cs. longiareolata) as an ongoing increase in numbers of annual detections and/or geographical spread are observable, or occurrence at exactly the same places in subsequent years, i.e. most likely overwintering, was demonstrated. Other species (Ae. aegypti, Ae. berlandi, Ae. koreicus, Ae. pulcritarsis) were found only once without further evidence of occurrence suggesting that they had been imported on one single occasion and have not succeeded in establishing.
Except for Ae. albopictus which is known to be regularly imported by vehicles from southern Europe (Becker et al. 2013; Kampen et al. 2013a) and Ae. aegypti which was introduced in the form of eggs attached to plants (Kampen et al. 2016b), it is unclear how mosquito introduction took place. Culiseta longiareolata may have initially immigrated actively from neighbouring France or Switzerland into southern Germany where it was first documented in 2011. In the cases of the other Aedes species, importation of eggs attached to humid surfaces, of larvae in water containers or of single females by vehicles is conceivable, although Ae. berlandi and Ae. pulcritarsis females are not known to be as highly anthropophilic and aggressive as to enter cars for blood-feeding. Active immigration can be excluded in all cases as the German collection sites are too distant from documented natural distribution areas. Least explicable is probably the occurrence of An. petragnani which is neither anthropophilic nor prone to be displaced by eggs or larvae.
While there is no doubt that Cs. longiareolata has established in southern Germany, the finding of specimens of this species in more northern German areas may be incidental. As Cs. longiareolata is not known to be a vector of human pathogens, targeted monitoring activities were not carried out yet. The opposite is the case with Ae. japonicus which seems to pop up everywhere in Germany. Aedes japonicus can easily be displaced by vehicles so that it might emerge at new places far from known distribution areas but also seems to have a high tendency to spread actively (Kampen et al. 2016a). Based on data from repeated monitoring activities in identical areas, it must be assumed that Ae. japonicus is much more distributed than demonstrated so far as areas apparently not colonised may just have population densities below detection limit.
The ninth mosquito species recently described for Germany but not dealt with, An. daciae (Kronefeld et al. 2012), is neither an invasive species, nor has its detection to do with changing climatic or changing environmental conditions. This species has probably been present in Germany as long as its An. maculipennis complex siblings but was just not recognised as a separate species.
The different contributions of globalisation, climate change, continuing adaptation of mosquito species to moderate climates and intensified monitoring activities to the finding of new mosquito species in Germany are unclear. Globalisation definitely contributes to the continental and intercontinental displacement of mosquitoes, particularly aedine species such as Ae. albopictus, Ae. japonicus and Ae. koreicus, e.g. through the transport of eggs with used tyres and of larvae with ornamental plants (Medlock et al. 2012). Within Europe, vehicle transport of adult females seems to be of major importance (Kampen et al. 2013a; Zielke et al. 2016).
The influence of climate on the dispersal and establishment is dependent on the mosquito species. For thermophilic species, development and propagation are the easier and more efficient the warmer it gets, sufficient precipitation provided. According to recent models, Europe has been predicted to become climatically suitable for Ae. albopictus in the near future mainly in its western parts but far up to the north, including Germany (e.g. Fischer et al. 2011; Caminade et al. 2012). In line with ongoing climate warming, a continuing northward spread of Ae. albopictus has been observed in more southern parts of Europe for many years (e.g. Knudsen et al. 1996; Wymann et al. 2008; Flacio et al. 2015) and, more recently, also in Germany (Becker et al. 2013; Kampen et al. 2013a; Walther et al. 2017). Although Ae. albopictus doubtlessly benefits from a warmer climate, these developments can also, at least in part, be attributed to population pressure (continuous northward conquest of territory) or cold acclimation (Hanson and Craig 1994; Romi et al. 2006). A certain influence of a warming climate facilitating the establishment of more thermophilic mosquito species in Germany seems likely, however, as Cs. longiareolata and An. petragnani, which are endemic in southern Europe, have appeared in Germany more or less at the same time, while a third thermophilic species, Uranotaenia unguiculata, which had previously been restricted to southwestern Germany, was coincidentally found in northern Germany (Tippelt et al. 2017).
Although the endemic mosquito fauna was scarcely studied in Germany after the disappearance of malaria, it has thoroughly been monitored in the southwestern part of the country for several decades linked to extensive control efforts (Becker and Ludwig 1983). The Upper Rhine Valley is the only German region where mosquito control is routinely carried out as floodwater species cause serious pest situations several times per year. Despite the concomitant monitoring activities in this region, which is one of the warmest ones in Germany and therefore offers best conditions for the establishment of thermophilic mosquito species, Ae. albopictus and other invasive species had never been encountered before 2007, with the exception of Ur. unguiculata which was found for the first time in 1994 (Becker and Kaiser 1995). Rather than the new species having remained unnoticed due to a lack of monitoring activities, this argues for an actual recent increase in the occurrence and spread of thermophilic species and an impact of climate change.
As some of the invasive species are vectors of disease agents, mosquito monitoring ought to be continued in Germany so as to be able to implement adequate and targeted control activities in due time when necessary in order to prevent the species from establishing or keep them at minimum population densities.
References
Adhami J, Reiter P (1998) Introduction and establishment of Aedes (Stegomyia) albopictus skuse (Diptera: Culicidae) in Albania. J Am Mosq Control Assoc 14:340–343
Akiner MM, Demirci B, Babuadze G, Robert V, Schaffner F (2016) Spread of the invasive mosquitoes Aedes aegypti and Aedes albopictus in the Black Sea region increases risk of chikungunya, dengue, and Zika outbreaks in Europe. PLoS Negl Trop Dis 10:e0004664
Almeida AP, Gonçalves YM, Novo MT, Sousa CA, Melim M, Grácio AJ (2007) Vector monitoring of Aedes aegypti in the Autonomous Region of Madeira, Portugal. Euro Surveill 12:e3311
Becker N, Geier M, Balczun C, Bradersen U, Huber K, Kiel E, Krüger A, Lühken R, Orendt C, Plenge-Bönig A, Rose A, Schaub GA, Tannich E (2013) Repeated introduction of Aedes albopictus into Germany, July to October 2012. Parasitol Res 112:1787–1790
Becker N, Hoffmann D (2011) First record of Culiseta longiareolata (Macquart) for Germany. Eur Mosq Bull 29:143–150
Becker N, Kaiser A (1995) Die Culicidenvorkommen in den Rheinauen des Oberrheingebiets mit besonderer Berücksichtigung von Uranotaenia (Culicidae, Diptera)—einer neuen Stechmückengattung für Deutschland. Mitt Dtsch Ges Allg Angew Entomol 10:407–413
Becker N, Ludwig HW (1983) Mosquito control in West Germany. Bull Soc Vector Ecol 8:85–93
Becker N, Petrić D, Zgomba M, Boase C, Madon M, Kaiser A (2010) Mosquitoes and their control, 2nd edn. Springer, Heidelberg
Becker N, Pfitzner WP, Czajka C, Kaiser A, Weitzel T (2016) Anopheles (Anopheles) petragnani Del Vecchio 1939—a new mosquito species for Germany. Parasitol Res 115:2671–2677
Black WC 4th, Bennett KE, Gorrochótegui-Escalante N, Barillas-Mury CV, Fernández-Salas I, de Lourdes MM, Farfán-Alé JA, Olson KE, Beaty BJ (2002) Flavivirus susceptibility in Aedes aegypti. Arch Med Res 33:379–388
Bonizzoni M, Gasperi G, Chen X, James AA (2013) The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol 29:460–468
Bruce-Chwatt LJ, de Zulueta J (1980) The rise and fall of malaria in Europe. Oxford University Press, Oxford
Cameron EC, Wilkerson RC, Mogi M, Myiagi I, Toma T, Kim HC, Fonseca D (2010) Molecular phylogenetics of Aedes japonicus, a disease vector that has recently invaded western Europe, North America, and the Hawaiian islands. J Med Entomol 47:527–535
Caminade C, Medlock JM, Ducheyne E, McIntyre KM, Leach S, Baylis M, Morse AP (2012) Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. J R Soc Interface 9:2708–2717
Capelli G, Drago A, Martini S, Montarsi F, Soppelsa M, Delai N, Ravagnan S, Mazzon L, Schaffner F, Mathis A, Di Luca M, Romi R, Russo F (2011) First report in Italy of the exotic mosquito species Aedes (Finlaya) koreicus, a potential vector of arboviruses and filariae. Parasit Vectors 4:e188
Coleman W (1984) Epidemiological method in the 1860s: yellow fever at Saint Nazaire. Bull Hist Med 58:145–163
Corradetti A, Scanga M (1968) Sporogony of Plasmodium (Giovannolaia) polare in Culiseta longiareolata. Parassitologia 10:1–9
Cranston PS, Ramsdale C, Snow KR, White GB (1987) Adults, larvae and pupae of British mosquitoes (Culicidae). Freshw Biol Assoc 48:1–152
Dahl CI, Kaiser A, Becker N (1999) Culicidae. In: Schumann H, Bährmann R, Stark A (eds) Checkliste der Dipteren Deutschlands. Studia Dipterol, Suppl 2:51–52
Deblauwe I, Sohier C, Schaffner F, Marrama Rakotoarivony L, Coosemans M (2014) Implementation of surveillance of invasive mosquitoes in Belgium according to the ECDC guidelines. Parasit Vectors 7:e201
Feng LC (1930) Experiments with Dirofilaria immitis and local species of mosquitoes in Peiping, North China, with a note on Lankesteria culicis in Ae. koreicus. Ann Trop Med Parasitol 24:347–366
Fielden MA, Chaulk AC, Bassetta K, Wiersma YF, Erbland M, Whitney H, Chapman TW (2015) Aedes japonicus japonicus (Diptera, Culicidae) arrives at the most easterly point of North America. Can Entomol 147:737–740
Fischer D, Thomas SM, Niemitz F, Reineking B, Beierkuhnlein C (2011) Projection of climatic suitability for Aedes albopictus Skuse (Culicidae) in Europe under climate change conditions. Glob Planet Chang 78:54–64
Flacio E, Engeler L, Tonolla M, Lüthy P, Patocchi N (2015) Strategies of a thirteen year surveillance programme on Aedes albopictus (Stegomyia albopicta) in southern Switzerland. Parasit Vectors 8:e208
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Ganushkina LA, Patraman IV, Rezza G, Migliorini L, Litvinov SK, Sergiev VP (2016) Detection of Aedes aegypti, Aedes albopictus, and Aedes koreicus in the area of Sochi, Russia. Vector Borne Zoonotic Dis 16:58–60
Gratz NG (2004) Critical review of the vector status of Aedes albopictus. Med Vet Entomol 18:215–227
Gutsevich AV, Monchadskii AS, Shtakel’berg AA (1971) Fauna of the USSR, Diptera Vol. 3, Mosquitoes. Academy of Sciences of the USSR, Zoological Institute, Leningrad [English translation by the ‘Israel Program for Scientific Translations’, Jerusalem 1974]
Hanson SM, Craig GB Jr (1994) Cold acclimation, diapause, and geographic origin affect cold hardiness in eggs of Aedes albopictus (Diptera: Culicidae). J Med Entomol 31:192–201
Harris MC, Dotseth EJ, Jackson BT, Zink SD, Marek PE, Kramer LD, Paulson SL, Hawley DM (2015) La Crosse virus in Aedes japonicus japonicus mosquitoes in the Appalachian Region, United States. Emerg Infect Dis 21:646–649
Hébert PDN, Cywinska A, Ball SL, deWaard JR (2003) Biological identifications through DNA barcodes. Proc R Soc Lond B 270:313–321
Heitmann A, Jansen S, Lühken R, Leggewie M, Badusche M, Pluskota B, Becker N, Vapalahti O, Schmidt-Chanasit J, Tannich E (2017) Experimental transmission of Zika virus by mosquitoes from central Europe. Euro Surveill 22:e30437
Horsfall WR (1955) Mosquitoes—their bionomics and relation to disease. Ronald Press Co., New York
Huber K, Pluskota B, Jöst A, Hoffmann K, Becker N (2012) Status of the invasive species Aedes japonicus japonicus (Diptera: Culicidae) in Southwest Germany in 2011. J Vector Ecol 37:462–465
Ibáñez-Justicia A, den Hartog W, Gloria-Soria A, Powell JR, Dik M, Jacobs F, Stroo CJ (2016) Accidental introductions of yellow fever mosquitoes (Aedes aegypti) to the Netherlands with human trade and transport. 20th E-SOVE Conference, 3-7 Oct 2016, Lisbon, Portugal, Abstracts:41
Ibáñez-Justicia A, Kampen H, Braks M, Schaffner F, Steeghs M, Werner D, Zielke D, den Hartog W, Brooks M, Dik M, van de Vossenberg B, Scholte E-J (2014) First report of established population of Aedes japonicus japonicus (Theobald, 1901) (Diptera, Culicidae) in the Netherlands. J Eur Mosq Control Assoc 32:9–13
Jackson M, Belton P, McMahon S, Hart M, McCann S, Azevedo D, Hurteau L (2016) The first record of Aedes (Hulecoeteomyia) japonicus (Diptera: Culicidae) and its establishment in western Canada. J Med Entomol 53:241–244
Kalan K, Buzan VE, Ivović V (2014) Distribution of two invasive mosquito species in Slovenia in 2013. Parasit Vector 7(Suppl 1):P9
Kalan K, Šušnjar J, Ivović V, Buzan E (2017) First record of Aedes koreicus (Diptera, Culicidae) in Slovenia. Parasitol Res 116:2355–2358
Kampen H, Kronefeld M, Zielke D, Werner D (2013a) Further specimens of the Asian tiger mosquito Aedes albopictus (Diptera, Culicidae) trapped in southwest Germany. Parasitol Res 112:905–907
Kampen H, Kronefeld M, Zielke D, Werner D (2013b) Three rarely encountered and one new Culiseta species (Diptera, Culicidae) in Germany. J Eur Mosq Control Assoc 31:36–39
Kampen H, Jansen S, Schmidt-Chanasit J, Werner D (2016a) Indoor development of Aedes aegypti in Germany, 2016. Euro Surveill 21:e30407
Kampen H, Kuhlisch C, Fröhlich A, Scheuch D, Werner D (2016b) Occurrence and spread of the invasive Asian bush mosquito Aedes japonicus japonicus (Diptera: Culicidae) in West and North Germany since detection in 2012 and 2013, respectively. PLoS One 11:e0167948
Kampen H, Medlock JM, Vaux AG, Koenraadt CJ, van Vliet AJ, Bartumeus F, Oltra A, Sousa CA, Chouin S, Werner D (2015) Approaches to passive mosquito surveillance in the EU. Parasit Vectors 8:e9
Kampen H, Sternberg A, Proft J, Bastian S, Schaffner F, Maier WA, Seitz HM (2003) Polymerase chain reaction-based differentiation of the mosquito sibling species Anopheles claviger s.s. and Anopheles petragnani (Diptera: Culicidae). Am J Trop Med Hyg 69:195–199
Kampen H, Werner D (2014) Out of the bush: the Asian bush mosquito Aedes japonicus japonicus (Theobald, 1901) (Diptera, Culicidae) becomes invasive. Parasit Vectors 7:e59
Kampen H, Zielke D, Werner D (2012) A new focus of Aedes japonicus japonicus (Diptera, Culicidae) distribution in western Germany: rapid spread or a further introduction event? Parasit Vectors 5:e284
KCDC = Korean Centres for Disease Control and Prevention (2007) Elimination of lymphatic filariasis in Korea. National Documentation for Certification. National Institute of Health, Ministry of Health and Welfare, Republic of Korea
Klobučar A, Lipovac I, Žagar N, Hamzić-Mitrović S, Tešić V, Vilibić-Čavlek T, Merdić E (2017) Aedes japonicus—the new invasive mosquito species spreading in Croatia. 2nd Croatian Symposium on Invasive Species, Zagreb, 21–22 November 2016, Abstracts: 47
Knight KL (1968) Contributions to the mosquito fauna of Southeast Asia. IV. Species of subgroup Chrysolineatus of group D, genus Aedes, subgenus Finlaya Theobald. Contrib Am Entomol Inst 2:1–45
Knudsen AB, Romi R, Majori G (1996) Occurrence and spread in Italy of Aedes albopictus, with implications for its introduction into other parts of Europe. J Am Mosq Control Assoc 12:177–183
Krebs T, Bindler P, L’Ambert G, Toti C, Perrin Y, Jourdain F (2014) First establishment of Aedes japonicus japonicus (Theobald, 1901) (Diptera: Culicidae) in France in 2013 and its impact on public health. J Vector Ecol 39:437–440
Kronefeld M, Dittmann M, Zielke D, Werner D, Kampen H (2012) Molecular confirmation of the occurrence in Germany of Anopheles daciae (Diptera, Culicidae). Parasit Vectors 5:e250
Kronefeld M, Werner D, Kampen H (2014) PCR identification and distribution of Anopheles daciae (Diptera, Culicidae) in Germany. Parasitol Res 113:2079–2086
Kurucz K, Kiss V, Zana B, Schmieder V, Kepner A, Jakab F, Kemenesi G (2016) Emergence of Aedes koreicus (Diptera: Culicidae) in an urban area, Hungary, 2016. Parasitol Res 115:4687–4689
Maslov AV (1967) Krovososushchie komary podtriby Culisetina (Diptera, Culicidae) mirovoĭ fauny. Akadem Nauk SSSR. [English translation: Blood-sucking Mosquitoes of the Subtribe Culisetina (Diptera, Culicidae) in World Fauna. Smithsonian Institution Libraries and the National Science Foundation, Washington, DC, 1989]
Medlock J, Hansford KM, Versteirt V, Cull B, Kampen H, Fontenille D, Hendrickx G, Zeller H, van Bortel W, Schaffner F (2015) An entomological review of invasive mosquitoes in Europe. Bull Entomol Res 105:637–663
Medlock JM, Hansford KM, Schaffner F, Versteirt V, Hendrickx G, Zeller H, van Bortel W (2012) A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne Zoonotic Dis 12:435–447
Miles JAR (1964) Some ecological aspects of the problems of arthropodborne animal viruses in the Western Pacific and South East Asia regions. Bull World Health Org 30:197–210
Mohrig W (1969) Die Culiciden Deutschlands: Untersuchungen zur Taxonomie, Biologie und Ökologie der einheimischen Stechmücken. Gustav Fischer Verlag, Jena. Parasitol Schriftenr 18:1–260
Montarsi F, Ciocchetta S, Devine G, Ravagnan S, Mutinelli F, Frangipane di Regalbono A, Otranto D, Capelli G (2015) Development of Dirofilaria immitis within the mosquito Aedes (Finlaya) koreicus, a new invasive species for Europe. Parasit Vectors 8:e177
Morillon M, Mafart B, Matton T (2002) Yellow fever in Europe in 19th century. In: Bennike P, Bodzsar EB, Suzanne C (eds) Ecological Aspects of Past Settlement in Europe. European Anthropological Association, Biennal Yearbook. Eötvös University Press, Budapest, pp 211–222
Moussiegt O (1986) Moustiques de France: Bibliographie et Répartition. Musée National d’Histoire Naturelle, Paris. Invent Faune Flore 30:1–184
Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D (2009) Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect 11:1177–1185
Pluskota B, Jöst A, Augsten X, Stelzner L, Ferstl I, Becker N (2016) Successful overwintering of Aedes albopictus in Germany. Parasitol Res 115:3245–3247
Pluskota B, Storch V, Braunbeck T, Beck M, Becker N (2008) First record of Stegomyia albopicta (Skuse) (Diptera: Culicidae) in Germany. Eur Mosq Bull 26:1–5
Ramsdale C, Snow K (2000) The distribution of the genus Anopheles in Europe. Eur Mosq Bull 7:1–26
Ribeiro H, da Cunha RH, Pires CA, Antunes Capela R (1988) An annotated checklist of the mosquitoes of continental Portugal. Actas III Congr Ibér Entomol, Grenada, pp 233–253
Romi R, Severini F, Toma L (2006) Cold acclimation and overwintering of female Aedes albopictus in Roma. J Am Mosq Control Assoc 22:149–151
Roubaud PE, Colas-Belcour J (1933) Notes sur un culicide méditerranéen, Theobaldia longiareolata Macq. Bull Soc Pathol Exot 26:934–937
Roundy CM, Azar SR, Rossi SL, Huang JH, Leal G, Yun R, Fernandez-Salas I, Vitek CJ, Paploski IA, Kitron U, Ribeiro GS, Hanley KA, Weaver SC, Vasilakis N (2017) Variation in Aedes aegypti mosquito competence for Zika virus transmission. Emerg Infect Dis 23:625–632
Rudolf M, Czajka C, Börstler J, Melaun C, Jöst H, von Thien H, Badusche M, Becker N, Schmidt-Chanasit J, Krüger A, Tannich E, Becker S (2013) First nationwide surveillance of Culex pipiens complex and Culex torrentium mosquitoes demonstrated the presence of Culex pipiens biotype pipiens/molestus hybrids in Germany. PLoS One 8:e71832
Ryba J (1979) Faunistic record from Czechoslovakia. Diptera, Culicidae. Acta Entomol Bohemoslov 76:143
Saegerman C, Berkvens D, Mellor PS (2009) Bluetongue epidemiology in the EU. Emerg Infect Dis 14:539–544
Schaffner F, Angel G, Geoffroy B, Hervy JP, Rhaiem A, Brunhes J (2001) The mosquitoes of Europe. An identification and training programme (CD-Rom). IRD Éditions & EID Méditerrannée, Montpellier
Schaffner F, Chouin S, Guilloteau J (2003) First record of Ochlerotatus (Finlaya) japonicus japonicus (Theobald, 1901) in metropolitan France. J Am Mosq Control Assoc 19:1–5
Schaffner F, Kaufmann C, Hegglin D, Mathis A (2009) The invasive mosquito Aedes japonicus in Central Europe. Med Vet Entomol 23:448–451
Schaffner F, Mathis A (2014) Dengue and dengue vectors in the WHO European region. Lancet Infect Dis 14:1271–1280
Schaffner F, Medlock JM, Van Bortel W (2013) Public health significance of invasive mosquitoes in Europe. Clin Microbiol Infect 19:685–692
Scholte E, den Hartog W, Dik M, Schoelitsz B, Brooks M, Schaffner F, Foussadier R, Braks M, Beeuwkes J (2010) Introduction and control of three invasive mosquito species in the Netherlands, July–October 2010. Euro Surveill 15:e19710
Seidel B, Duh D, Nowotny N, Allerberger F (2012) First record of the mosquitoes Aedes (Ochlerotatus) japonicus japonicus (Theobald, 1901) in Austria and Slovenia 2011 and for Aedes (Stegomyia) albopictus (Skuse, 1895) in Austria. Entomol Zeitschr 122:223–226 [in German]
Seidel B, Montarsi F, Huemer HP, Indra A, Capelli G, Allerberger F, Nowotny N (2016a) First record of the Asian bush mosquito, Aedes japonicus japonicus, in Italy: invasion from an established Austrian population. Parasit Vectors 9:e284
Seidel B, Nowotny N, Bakonyi T, Allerberger F, Schaffner F (2016b) Spread of Aedes japonicus japonicus (Theobald, 1901) in Austria, 2011-2015, and first records of the subspecies for Hungary, 2012, and the principality of Liechtenstein, 2015. Parasit Vectors 9:e356
Silaghi C, Beck R, Capelli G, Montarsi F, Mathis A (2017) Development of Dirofilaria immitis and Dirofilaria repens in Aedes japonicus and Aedes geniculatus. Parasit Vectors 10:e94
Smith CEG, Gibson ME (1986) Yellow fever in South Wales, 1865. Med Hist 30:322–340
Suter T, Flacio E, Fariña BF, Engeler L, Tonolla M, Müller P (2015) First report of the invasive mosquito species Aedes koreicus in the Swiss-Italian border region. Parasit Vectors 8:e402
Tanaka K, Mizusawa K, Saugstad ES (1979) A revision of the adult and larval mosquitoes of Japan (including the Ryukyu Archipelago and the Ogasawara Islands) and Korea (Diptera: Culicidae). Contrib Am Entomol Inst 16:1–987
Tippelt L, Walther D, Kampen H (2017) The thermophilic mosquito species Uranotaenia unguiculata Edwards, 1913 (Diptera: Culicidae) moves north in Germany. Parasitol Res https://doi.org/10.1007/s00436-017-5652-2
Versteirt V, de Clercq EM, Fonseca DM, Pecor J, Schaffner M, Coosemans M, van Bortel W (2012) Bionomics of the established exotic mosquito species Aedes koreicus in Belgium, Europe. J Med Entomol 49:1226–1232
Versteirt V, Schaffner F, Garros C, Dekoninck W, Coosemans M, van Bortel W (2009) Introduction and establishment of the exotic mosquito species Aedes japonicus japonicus (Diptera: Culicidae) in Belgium. J Med Entomol 46:1464–1467
Versteirt V, Tack W, Schaffner F, Hendrickx G (2017) The successful elimination of a locally established population of Aedes japonicus in Belgium. Proc 8th Conf Eur Mosq Control Assoc, Bečići, Montenegro, 12–16 March 2017:74
Walther D, Scheuch DE, Kampen H (2017) The invasive Asian tiger mosquito Aedes albopictus (Diptera: Culicidae) in Germany: local reproduction and overwintering. Acta Trop 166:186–192
Werner D, Hecker S, Luckas M, Kampen H (2014) The citizen science project ‘Mückenatlas’ supports mosquito (Diptera, Culicidae) monitoring in Germany. Proc 8th Int Conf Urban Pests, Zurich, Switzerland. OOK Press Kft, Veszprém, pp 119–124
Werner D, Kampen H (2013) The further spread of Aedes japonicus japonicus (Diptera, Culicidae) towards northern Germany. Parasitol Res 112:3665–3668
Werner D, Kronefeld M, Schaffner F, Kampen H (2012) Two invasive mosquito species, Aedes albopictus and Aedes japonicus japonicus, trapped in south-west Germany, July to August 2011. Euro Surveill 17:e20067
Werner D, Kampen H (2015) Aedes albopictus breeding in southern Germany, 2014. Parasitol Res 114:831–834
Werner D, Zielke DE, Kampen H (2016) First record of Aedes koreicus (Diptera: Culicidae) in Germany. Parasitol Res 115:1331–1334
Wymann MN, Flacio E, Radczuweit S, Patocchi N, Luthy P (2008) Asian tiger mosquito (Aedes albopictus)—a threat for Switzerland? Euro Surveill 13:e8058
Yunicheva YV, Ryabova TY, Markovich NY, Bezzhonova OV, Ganushkina LA, Semenov VB, Tarkhov GA, Vasilenko LY, Guzyeva TM, Shevereva TV, Sergiev VP (2008) First evidence for breeding Aedes aegypti L. in the area of Greater Sochi and in some towns of Akhasia. Med Parazitol 3:40–43
Zielke DE, Walther D, Kampen H (2016) Newly discovered population of Aedes japonicus japonicus (Diptera: Culicidae) in Upper Bavaria, Germany, and Salzburg, Austria, is closely related to the Austrian/Slovenian bush mosquito population. Parasit Vectors 9:e163
Acknowledgments
This work was financially supported by the German Federal Ministry of Food and Agriculture (BMEL) through the Federal Office for Agriculture and Food (BLE), grant numbers 2810HS022, 2819104115 and 2819104615, and by the Robert Koch Institute, grant number 1362/1-982. We are also grateful to Brigitte Dannenfeld, Jutta Falland, Christin Henke, Juliane Horenk, Martina Pusch and Oliver Tauchmann for excellent technical assistance in the laboratory, and to all the people taking care of our mosquito traps and submitting mosquitoes to the citizen science project ‘Mueckenatlas’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kampen, H., Schuhbauer, A. & Walther, D. Emerging mosquito species in Germany—a synopsis after 6 years of mosquito monitoring (2011–2016). Parasitol Res 116, 3253–3263 (2017). https://doi.org/10.1007/s00436-017-5619-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5619-3