Abstract
Canine vector-borne diseases (CVBDs) are caused by a range of pathogens transmitted to dogs by arthropods. The present study investigates Ehrlichia canis, Anaplasma spp., Borrelia burgdorferi sensu lato, and Dirofilaria immitis seroprevalences in hunting dogs from southern Italy. Dogs (no. 1335) were tested using a commercial in-clinic enzyme-linked immunosorbent assay kit. Odds ratios (ORs) were calculated by logistic regression analysis to identify risk factors. Overall, 138/1335 dogs (10.3%) were seroreactive to at least one CVBD pathogen. E. canis, Anaplasma spp., B. burgdorferi s.l., and D. immitis seroprevalences were 7.6, 4.4, 0.3, and 0.2%, respectively. E. canis and Anaplasma spp. co-exposures were found in 30 dogs (2.2%), compared with Anaplasma spp. and B. burgdorferi s.l. co-exposures in 2 dogs (0.1%). Adult age was a risk factor for E. canis (OR 2.35) seroreactivity whereas hunting fur-bearing animals for E. canis (OR 4.75) and Anaplasma spp. (OR 1.87), respectively. The historical presence of tick infestation was identified as a risk factor for positivity to E. canis (OR 2.08) and Anaplasma spp. (OR 2.15). Finally, larger dog pack size was significantly associated with E. canis (OR 1.85) and Anaplasma spp. (OR 2.42) exposures. The results of the present survey indicated that hunting dog populations are at relative risk of CVBDs in southern Italy. Further studies are needed to evaluate the role of hunting dogs in the epidemiology of vector-borne organisms due to sharing common environments with wild, sympatric animal populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Canine vector-borne diseases (CVBDs) are caused by a range of pathogens transmitted to dogs by arthropods including ticks and insects, many of which pose a zoonotic risk for human infection, with dogs potentially serving as reservoirs.
Canine monocityc ehrlichiosis (CME) caused by Ehrlichia canis, a Gram-negative obligate intracellular bacterium with a tropism for mononuclear leukocytes, is a widespread tick-borne infection, transmitted by Rhipicephalus sanguineus, the most common tick species found in the Mediterranean basin (Sainz et al. 2015). Human infection with E. canis has been reported in Latin America (Venezuela and Costa Rica) (Perez et al. 2006; Bouza-Mora et al. 2017). In dogs, ehrlichiosis can vary in severity from minimally symptomatic to fatal illness in chronic stages (Cardoso et al. 2012). Clinical presentation in dogs is typically characterized by fever, depression, myalgia, anorexia, lymphadenomegaly, anemia, and thrombocytopenia (Mircean et al. 2012).
Anaplasma phagocythophilum (formerly known as E. phagocytophila or E. equi) is an obligate intracellular Gram-negative bacterium that has tropism for neutrophilic granulocytes and is recognized as the causative agent of granulocytic anaplasmosis in dogs, cats, horses, sheep, and humans. In Europe, Ixodes ricinus is the only known vector for A. phagocythophilum (Sainz et al. 2015). Although there is an overlap of the clinical features with E. canis infection, many A. phagocytophilum-infected dogs have a subclinical and self-limiting disease, as suggested by the high number of healthy seropositive dogs in I. ricinus endemic regions (Kohn et al. 2011).
A. platys (formerly known as E. platys), the cause of canine infectious cyclic thrombocytopenia (CICT), is an obligate intracellular Gram-negative bacterium that infects platelets and megakaryocytes (Latrofa et al. 2016). Clinical signs associated with CICT include lethargy, fever, anorexia, and bleeding disorders. A. platys may be transmitted by R. sanguineus, but vector competence has not conclusively demonstrated for this tick species (Ramos et al. 2014). In dogs, A. platys is found in co-infection with other vector-borne agents. Human infection with A. platys has been reported from the USA and Venezuela (Arraga-Alvarado et al. 2014; Breitschwerdt et al. 2014).
Borrelia burgdorferi sensu lato spirochetes infect a wide range of mammals, including dogs and humans. I. ricinus is the most important vector in Europe (Hovius 2013). Borreliosis, commonly referred as Lyme disease, is the most common tick-borne human infection in Europe and North America (Little et al. 2010, Rizzoli et al. 2011). In dogs, B. burgdorferi most often causes mild and non-specific clinical signs, such as fever, anorexia, lethargy, and lymphadenomegaly (Hovius 2013). More severe clinical manifestations, such as arthritis with lameness, neurologic disorders, and glomerulonephritis occur occasionally in dogs, although the specific role of B. burgdorferi in the development of renal failure remains unclear (Greene et al. 2012).
In Italy, E. canis, A. phagocytophilum, and A. platys infections have been reported in dogs in previous surveys (Antognoni et al. 2014; Pennisi et al. 2012; de Caprariis et al. 2011; Otranto et al. 2010; Trotta et al. 2009; Ebani et al. 2008; Torina et al. 2008; Torina et al. 2007; Corrain et al. 2007; de la Fuente et al. 2006; Solano-Gallego et al. 2006; Torina and Caracappa 2006). Due to use of different CVBD diagnostic techniques, varying geographical regions studied, and different selection criteria for inclusion of dogs in various studies, it is difficult to compare historical prevalence data generated throughout Italy. In a recent survey, E. canis was detected also in wild canids, as red foxes (Vulpes vulpes) and gray wolves (Canis lupus) (Santoro et al. 2016). Dogs in Italy are infrequently exposed to B. burgdorferi (Giudice et al. 2003; Mannelli et al. 1999).
Dirofilaria immitis, the cause of canine heartworm disease (CHD), is a filarial nematode that lives as adult in the right ventricle of the heart, extending into the pulmonary arteries. CHD is associated with exercise intolerance, dyspnoea, cough, and right-sided congestive heart failure (McCall et al. 2008). In Italy, mosquitoes of genera Culex, Aedes, and Anopheles transmit D. immitis (Otranto and Dantas -Torres 2010). Although there is a risk of zoonotic transmission, human heartworm infection is uncommon. In Europe, CHD occurs in Mediterranean basin countries, with the largest endemic area located along the Po River Valley in northern Italy (Otranto et al. 2013). Recent epidemiological data suggests a geographic expansion for D. immitis transmission throughout southern Italy and the surrounding islands (Del Prete et al. 2015; Pipia et al. 2014; Otranto et al. 2009).
Due to closer contact with wooded and rural areas, cohabitation in outdoor kennels and potentially less consistent use of acaricide products, hunting dogs are more likely to be exposed to CVBDs compared to other dog populations (e.g., household dogs) (Kordick et al. 1999; Solano-Gallego et al. 2006); however, few comparative studies from the same region have been published (Ebani et al. 2014). In Italy, serological and molecular data for E. canis and A. phagocythophilum were reported in a small number of hunting dogs from Central Italy by Ebani et al. (2013, 2015). The aim of the present study was to determine the CVBD seroprevalences in a large number of hunting dogs from southern Italy and to assess exposure risk factors.
Materials and methods
Study area
Avellino (40°54′55″N–14°47′22″E) and Salerno (40°41′00″N–14°47′00″E) provinces belong to the Campania region in southern Italy. The territory of the two provinces is contiguous and that of Salerno overlooks the Tyrrhenian Sea. It has a typical Mediterranean temperate climate along the coast that becomes progressively continental in the inland and mountainous areas. The study area has surface of 4527.81 square km, including the hunting district—Ambito Territoriale Caccia—of Avellino (ATC AV) and one of the two hunting districts of Salerno (ATC SA 1).
Study animals and sample size
This study included 1335 hunting dogs from 114 municipalities. The study was conducted as a component of the hunting dog’s health assistance program of University of Naples Federico II, which was supported by the Italian management committees of the respective hunting districts (ATCs).
Blood samples were collected in 37 private veterinary hospitals located in the study area between March and October 2015. Animal sampling was performed by different veterinary operators (DP, BN, MS, LP, VV) during a routine health check.
The study was approved by the Ethical Animal Care and Use Committee of the University of Naples Federico II (number of approval 0039904; date of approval 20 October 2014), and a written consent was obtained from the owners of the hunting dogs.
After an overnight fast, 5 ml of blood withdrawn from the cephalic vein was collected into K3-EDTA anticoagulant tubes and immediately tested using in-clinic CVBD ELISA assay defined below.
The sample size was calculated using the formula proposed by Thrustfield (1995) for a theoretically “infinite” population inserting the following data: expected prevalence of 2.1% for E. canis based on the results of a similar large-scale study in canine population from Romania using the same in-clinic enzyme-linked immunosorbent assay (ELISA) (Mircean et al. 2012); confidence interval (99%) and desired absolute precision (1%).
A questionnaire was submitted to each owner to obtain information about dog’s size (small, medium, large), type of coat (short hair or long hair), age (< 3 years, ≥ 3–8 years, ≥ 8 years), gender, cohabitation with other dogs (pack size), type of housing (indoor or outdoor), contact with other pet or farm animals (dogs, cats, horses, and ruminants), hunted animal species (game birds, wild boars, foxes, and hares), hunting periods in foreign countries, history of tick infestation, and general ectoparasite control practices.
Serological assay
Serological analyses were performed using an in-clinic assay test system (SNAP® 4Dx® Plus, IDEXX Laboratories Inc., Westbrook, ME, USA) based on enzyme immunoassay technique and following the manufacturer’s instructions for use. The device consists of a coated membrane matrix with five spots in the reaction area (result window). Three spots are impregnated respectively with peptide antigens from p30 and p30–1 outer membrane immunodominant proteins of E. canis, a peptide from the immunodominant major outer surface protein (p44/MSP2) of A. phagocytophilum and the synthetic C6 peptide derived from IR6 region within the membrane protein of B. burgdorferi. Regarding the European Borrelia species, Krupka et al. (2009) in mice experimentally infected by B. burgdorferi and B. garinii have detected antibodies against C6 peptide. D. immitis analyte is derived from polyclonal antibodies specific for a carbohydrate antigen of the adult female nematodes. The fifth spot serves as a positive control. According to Stillman et al. (2014), sensitivity and specificity of the in-clinic ELISA were for detection of antibodies against A. phagocytophilum (93.2 and 99.2%, respectively), A. platys (89.2 and 99.2%, respectively), B. burgdorferi (96.7 and 98.8%, respectively), E. canis (97.8 and 92.3%, respectively), and E. ewingii (96.5 and 93.9%, respectively). Sensitivity of the assay for detection of D. immitis was 98.9%, with 99.3% specificity.
The SNAP® 4Dx® Plus test uses a peptide from a major outer surface protein (p28) of E. ewingii on the Ehrlichia portion of the test. In addition to the SNAP® 4Dx® Plus, a Knott’s test was performed on D. immitis seropositive dogs. Furthermore, the seropositive samples were qualitatively tested for antibodies to Leishmania infantum using the SNAP® Leishmania in order to rule out a possible coinfection with this protozoan. The locations of seropositive dogs were mapped using a Geographical Information System (ARC GIS 10.1, ESRI Corporation, USA).
Clinical examination and complete blood cell count
For each SNAP® 4Dx® Plus seropositive dog, a complete clinical examination was performed by participating veterinarians (DP, BN, NDA, FDP, VV). A complete blood cell count was done using a semi-automatic cell counter (HM5, Abaxis, USA). The body condition score (BCS) was assessed using a nine-point system (Laflamme 1997).
Statistical analysis
The analysis was performed by coauthor LA with dedicated software (Prism® 6.0, GraphPad Software Inc., La Jolla, CA, USA). Data are reported as absolute number, percentage of the total and the relative 95% confidence interval (95% CI). A two-tailed chi-square test was applied to evaluate the effect of each risk factor, and the odds ratio (OR) and the relative 95% CI were calculated for the significant variable (Petrie and Watson 2013). Contingency tables were used to identify whether the distribution of clinical signs or hematological abnormalities varied among the different CVBDs or among dogs that were exposed to more than one CVBD. Significance was set at P < 0.05.
Results
Overall, the average age of this hunting dog population was 3.6 ± 2.4 years (ranging from 3 months to 14 years). There were 749 males and 586 females, including the following breeds: 493 Italian Segugio, 393 English Setter, 107 Epagneul Breton, 61 mixed-breeds, 46 Pointer, 43 Beagle, 33 Ariegeois, 32 Griffon Bleu de Gascogne, 21 Italian Bracco, 20 Kurzhaar, and 86 of other 14 hunting breeds. All of dogs lived in rural environments and the pack size varied from 2 to more than 13 dogs. The majority of dogs (1203/1335; 90.1%) were in contact with pets or farm animals. Regarding management practices, all the owners reported administration of ectoparasiticides to their dogs.
Overall, 138/1335 dogs (10.3%; CI 8.7–12.1%) were seropositive to one CVBD pathogen.
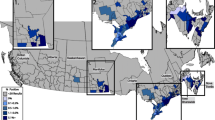
The seroprevalences for the pathogens were E. canis 7.6% (102/1335; CI 6.3–9.2%), Anaplasma spp. 4.4% (59/1335; CI 3.4–5.7%), B. burgdorferi s.l. 0.3% (4/1335; CI 0.1–0.8%), and D. immitis 0.2% (3/1335; CI 0.05–0.6%). Co-infection with E. canis and Anaplasma spp. was found in 30 dogs (2.2%; CI 1.5–3.2%), while co-infection with Anaplasma spp. and B. burgdorferi s.l. in only 2 animals (0.1%; CI 0.02–0.5%). The distribution of the ELISA positive dogs for the pathogens in the study area is shown in Fig. 1.
L. infantum antibodies were detected in 7 (5.1%; CI 2.5–11.1%) out of 138 CVBD seropositive dogs. Three Leishmania seropositive dogs had antibodies against E. canis, two against Anaplasma spp., and two against both pathogens.
Seroprevalence analyses in relation to the detailed characteristics of the hunting dog population hypothesized as potential risk factors associated with the exposure to any of the different CVBD pathogens are summarized in Table 1. Due to the very low prevalence in the study population, B. burgdorferi s.l. and D. immitis positivities were not included in the risk factor statistical analysis.
Adult age was a risk factor for E. canis exposure. Hunting fur-bearing animals (boars, hares, foxes), large dog pack size (> 10 animals), and a history of tick infestation(s) were significantly associated with E. canis and Anaplasma spp. exposures. Table 2 shows the significant risk factors for E. canis and Anaplasma spp., while B. burgdorferi s.l. and D. immitis risk factors were not calculated.
A clinical examination was performed on 124/138 seropositive dogs (89.9%). The clinical features observed in dogs infected by E. canis, Anaplasma spp., and B. burgdorferi s.l. are summarized in Fig. 2. Lymphadenomegaly (41.1%; CI 38.5–55.8%), elevated rectal temperature (45.6%; CI 37.1–54.3%), and splenomegaly (42.0%; CI 33.7–50.7%), as determined by abdominal palpation, represented the most frequent clinical findings among these hunting dogs. Clinical abnormalities were not found in three dogs that were D. immitis antigen positive, all of which were confirmed by identification of circulating microfilariae using Knott’s test (concentration ranged from 44 to 60 microfilariae/ml of blood).
Statistically, there were no differences in the frequency of clinical findings among dogs seroreactive to E. canis, Anaplasma spp., or B. burgdorferi s.l.; to E. canis, Anaplasma spp., and their respective co-infections; and between Anaplasma spp., B. burgdorferi s.l., and their respective co-infections.
Complete blood cell count (CBC) results for 120/138 (86.9%) CVBD seroreactors are depicted in Fig. 3. A normal CBC was detected in 61.8% (CI 49.2–73.3%) of dogs with E. canis, in 46.1% (CI 26.6–66.6%) with Anaplasma spp., in 50.0% (CI 1.3–98.7%) with B. burgdorferi s.l., in 36.0% (CI 18.0–57.5%) co-infected by E. canis and Anaplasma spp., and in all dogs with D. immitis and co-infected by Anaplasma spp. and B. burgdorferi s.l..
Hematological findings in the seropositive dogs. Co-infection E + A: co-infection with E. canis and Anaplasma spp. Co-infection A + B: co-infection with Anaplasma spp. and B. burdorferi s.l. Reference ranges from Bush (1991): red blood cells (RBCs)—5.5–8.5 × 1012/l; packed cell volume (PCV)—37–55%; hemoglobin (Hb)—12–18 g/dl; white blood cells (WBCs)—6–17 × 109/l; platelets (PLTs)—200–500 × 109/l
Thrombocytopenia was found in 38.2% (CI 26.7–50.8%) of dogs with E. canis, in 34.6% (CI 17.2–55.7%) with Anaplasma spp., in none of the dogs with B. burgdorferi s.l. or with B. burgdorferi s.l. and Anaplasma spp. co-infection. Anemia was documented in 22.1% (CI 12.9–33.8%) of dogs with E. canis, in 26.9% (CI 11.6–47.8%) with Anaplasma spp., in 50.0% (CI 1.3–98.7%) with B. burgdorferi s.l., and in 32.0% (CI 14.9–53.5%) with E. canis and Anaplasma spp. co-infection. Leukopenia was found in 5.9% (CI 1.6–14.4%) of dogs with E. canis, in 3.8% (CI 0.1–19.6%) with Anaplasma spp., and in 12.0% (CI 2.4–31.2%) of dogs co-infected by E. canis and Anaplasma spp. None of the dogs with single infection due to B. burgdorferi s.l. were leukopenic. The contingency tables applied to the distribution of the above-mentioned hematological abnormalities did not highlight any difference among the different types of CVBD infections.
Discussion and conclusion
This study documents that hunting dogs in southern Italy are exposed to five organisms that cause CVBDs. Our results are consistent with a recent study that found an increasing gradient of CME incidence risk from northern towards southern areas, particularly in Italy (Renè-Martellet et al. 2015). In addition to E. canis, the Ehrlichia peptides in the in-clinic rapid ELISA assay used in this study detects E. chaffeensis, E. ewingii, and E. muris antibodies (Stillman et al. 2014). In a study from North America that used Ehrlichia species-specific peptides, E. ewingii was the most seroprevalent Ehrlichia spp. infecting dogs (Qurollo et al. 2014). To date, tick transmission of E. chaffeensis, E. ewingii, and E. muris has not been reported in European dogs; for this reason, the Ehrlichia spp. seropositivity found in this study was attributed exclusively to E. canis. The E. canis seroprevalence (7.6%) in hunting dogs living in Campania region is similar to the value reported by Ebani et al. (2014) in a dog population living in a rural environment in Central Italy (no. 721; 7.12%). No seroprevalence data are available for comparative purposes on the general dog population of Campania region. In Apulia and Sicily regions of southern Italy, higher E. canis seroprevalences were reported in stray dogs co-housed in kennels (from 14.9 to 46.0%) that were infested with large numbers of R. sanguineus ticks, and these findings may be explained by irregular and partially effective metaphylactic treatment schemes against ectoparasites (Otranto et al. 2008; Pennisi et al. 2012).
The overall Anaplasma spp. seroprevalence in the present study (4.4%) was lower than the A. phagocytophilum seroprevalence, determined by an indirect fluorescent antibody test (IFAT) with a low cut-off value (1:40), reported in hunting dogs from central Italy (no. 215; 14.8%) (Ebani et al. 2013). Differences in the serological methods used in these two studies most likely account for the substantial seroprevalence differences among the two dog populations. When testing for exposure to A. phagocytophilum, the SNAP® 4Dx® Plus test was calibrated by the manufacturer to be positive at an IFAT titer of approximately 1:100 or greater (O’Connor 2015).
It is important to underline that A. platys infection in dogs has been previously described in southern Italy (de Caprariis et al. 2011; de la Fuente et al. 2006; Sparagano et al. 2003). The Anaplasma peptide in the SNAP® 4Dx® Plus ELISA platform detects A. platys and A. phagoctyophilum antibodies. Therefore, we report our results as indicative of exposure to Anaplasma spp. in hunting dogs, but specific identification at species level would require organism visualization or PCR amplification of organism-specific DNA sequences.
There are limited B. burgdorferi s.l. seroprevalence data for the general dog population in Italy. Ebani et al. (2014) reported a seroprevalence of 1.47% in dogs living in central Italy, and Mannelli et al. (1999) did not find serological evidence of B. burgdorferi s.l. exposure in dogs on the Thyrrenian coast of central Italy. Our results also document a low B. burgdorferi seroprevalence (0.3%) in the study area, which is consistent with other serosurveys conducted in southern European countries, such as Portugal (0.2%) (Cardoso et al. 2012).
In the seropositive clinically sick dogs, there was an overlap of symptoms and hematological changes for the different investigated CVBDs, either as a single infection or as co-infections. Previously, de Caprariis et al. (2011) in a longitudinal study in young dogs naturally infected by vector-borne pathogens emphasized the clinical challenge associated with assigning a specific clinical sign or hematological abnormality to a particular CVBD. Due to substantial overlap in both clinical and hematological abnormalities, an etiological diagnosis requires species-specific serological assays or molecular assays that confirm the infecting species on the basis of organism-specific DNA sequences.
A substantial number of hunting dogs in this study (32/138; 23.2%) were exposed to more than one CVBD pathogen. Although it has been hypothesized that the presence of two, or more CVBDs, is responsible for alteration, and worsening, of clinical manifestations, typically associated with singular infections, the pathogenic consequences of vector-borne co-infections are minimally documented (De Tommasi et al. 2013). However, veterinarians should keep in mind that CVBDs co-infections may make diagnosis and treatment more difficult, as well as adversely affect the prognosis. Pennisi et al. (2012) in a serological survey in southern Italy (Stretto di Messina) found seropositivity to at least two tick-borne pathogens in 57% of examined dogs, suggesting the possibility that a single tick species may be a vector for multiple pathogens. In our study, two dogs were co-exposed to Anaplasma spp. and B. burgdorferi s.l., and this is not surprising because both organisms has the same vector (I. ricinus), and A. phagocytophilum DNA has been detected in ticks from central and northern Italy (Carpi et al. 2009; Veronesi et al. 2011). Dual infection with A. phagocytophilum and B. burgdorferi s.l. has been reported for I. ricinus (8.3% out of 303 examined adults ticks) in northern Poland (Stańczak et al. 2004). In Europe, CME is transmitted by R. sanguineus and in our study, 30 dogs were co-exposed to E. canis and Anaplasma spp.
Otranto et al. (2010) found in dogs living in a shelter in Apulia region of southern Italy a high prevalence of A. platys and Babesia vogeli coinfections, supporting the suspicion that R. sanguineus ticks are likely vectors for both pathogens. A. platys DNA has been PCR amplified from R. sanguineus ticks in southern Italy, further supporting that this tick species a putative vector of A. platys (Ramos et al. 2014). On the basis of our findings, we speculate that hunting dogs in southern Italy may become co-infected with A. platys and E. canis by R. sanguineus tick bites.
Three dogs without a travel history to heartworm endemic areas were positive to D. immitis, confirming the appearance of autochthonous foci of this filarial infection in previously non-endemic areas, such as Campania and Apulia (Del Prete et al. 2015; Giangaspero et al. 2013). This is potentially a consequence of the environmental changes that have an impact on the vector’s geographical distribution, density, and activity pattern (Genchi et al. 2011). A serological approach for detection of CHD in this specific subpopulation of dogs is very suited, because many hunters in southern Italy had the habit to extra-label use of macrocyclic lactones (mainly ivermectin) on their dogs giving rise to occult infections. Courtney and Zeng (2001) have reported that the sensitivity of rapid assay tests for D. immitis is dependent on the number of adult female worms. The infected dogs in our study had a low degree of microfilaremia, and thus it cannot be ruled out that the prevalence may be underestimated in a non-hyperendemic area, such as southern Italy.
In these hunting dogs, adult age was a risk factor for E. canis seroreactivity, probably due to a longer duration of exposure to R. sanguineus, the vector for this pathogen that induces long-lasting infections in some dogs, in accordance to what was previously observed (Costa et al. 2007). Hunting fur-bearing animals (hares, foxes, boars) was a risk factor for exposure to E. canis, Anaplasma spp., and B. burgdorferi s.l., respectively; these data can be explained by the closer contact with vegetation and wild mammals that are reservoirs for tick-borne pathogen agents, request by this type of hunting (Santoro et al. 2016; Westmoreland et al. 2016). Furthermore, E. canis and Anaplasma spp. infections were significantly more frequent in hunting dogs with a history of previous tick infestation. Finally, a large dog pack size was significantly associated with E. canis and Anaplasma spp. exposures, being probably related to the sharing of the arthropod vectors and could be correlated to the monotropic three-host life cycle of R. sanguineus.
Overall, the prevalence of the examined CVBDs was relatively low considering the likelihood of frequent environmental exposure to ticks and mosquitoes. In the interpretation of these data, it must be considered that all the hunters treated their dogs with ectoparasiticide molecules, as a result of being informed of the risks of pathogen transmission by ticks and other vectors. The mean number of treatments per year was 6.1 (range 1–12). Ectoparasiticide treatments were performed monthly in 27% of hunting dogs (Veneziano, personal communication). A more accurate assessment of the effect of preventive measures on the development of CVBDs is not obtainable, due to a number of variables regarding the ectoparasite treatment modalities recorded at questionnaire survey (variability of the treatment schemes during the year and the hunting season, empirical dosage calculation, molecules used, association of more molecules, route of administration, extra-label use of macrocyclic lactones) and was beyond the aim of the study design.
To our knowledge, this is the first large-scale serological survey on hunting dogs in southern Italy. Over the last few decades, changes in ecosystems due to urbanization occurred, resulting in thinning of the boundaries between domestic animals and wildlife. For example, some rural landscapes have become periurban areas, providing very attractive food sources for adaptable species (such as the red fox) (Mackenstedt et al. 2015). This new scenario has created a changing dynamic interaction between wildlife, domestic animals, and humans. Understanding these changes will be crucial for epidemiological studies and preventive strategies for CVBDs in hunting and pet dogs in this area. Some specific ecological variants, such as hunting practice, may facilitate parasite circulation from domestic canids to wildlife and vice versa (Otranto et al. 2015). Recently, many studies have investigated the role of wild animals as epidemiological reservoir for many parasites that may infect other susceptible species sharing the same habitats, including humans (Piantedosi et al. 2016; Tolnai et al. 2015; Duscher et al. 2015; Hodžić et al. 2015; Cardoso et al. 2013). Using PCR on tissue samples from red foxes in southern Italy, Santoro et al. (2016) amplified E. canis DNA from 55 (52%) out of 105 animals. Epidemiologically, monitoring the circulation of CVBDs in a region by testing serum samples from hunting dogs may represent a useful sentinel population, as well as a compromise between difficulties associated in testing wild animals. In this way, hunting dogs can act as sentinel animals for monitoring wildlife zoonotic infections, as recently demonstrated by Gómez-Morales et al. (2016) for Trichinella spp.
In conclusion, the results of the present survey indicate that the hunting dog population in southern Italy is at low risk for contracting CVBDs. Further studies are needed to evaluate the role of hunting dogs in monitoring the epidemiology of zoonotic vector-borne agents, as these dogs are exposed to wild animals and vectors residing in the same environments.
References
Antognoni MT, Veronesi F, Morganti G, Mangili V, Fruganti G, Miglio A (2014) Natural infection of Anaplasma platys in dogs from Umbria region (Central Italy). Vet Ital 50:45–56
Arraga-Alvarado CM, Qurollo BA, Parra OC, Berrueta MA, Hegarty BC, Breitschwerdt EB (2014) Case report: molecular evidence of Anaplasma platys infection in two women from Venezuela. Am J Trop Med Hyg 91:1161–1165
Bouza-Mora L, Dolz G, Solórzano-Morales A, Romero-Zuñiga JJ, Salazar-Sánchez L, Labruna MB, Aguiar DM (2017) Novel genotype of Ehrlichia canis detected in samples of human blood bank donors in Costa Rica. Ticks Tick Borne Dis 8:36–40
Breitschwerdt EB, Hegarty BC, Qurollo BA, Saito TB, Maggi RG, Blanton LS, Bouyer DH (2014) Intravascular persistence of Anaplasma platys, Ehrlichia chaffeensis, and Ehrlichia ewingii DNA in the blood of a dog and two family members. Parasit Vectors 7:298
Bush BM (1991) Part 1: Haematology. In: Interpretation of laboratory results for small animal clinicians, 1st edn. Blackwell Scientific Publications, Oxford, UK, pp. 31–220
de Caprariis D, Dantas-Torres F, Capelli G, Mencke N, Stanneck D, Breitschwerdt EB, Otranto D (2011) Evolution of clinical, haematologicl and biochemical findings in young dogs naturally infected by vector-borne pathogens. Vet Microbiol 149:206–212
Cardoso L, Mendão C, Madeira de Carvalho L (2012) Prevalence of Dirofilaria immitis, Ehrlichia canis, Borrelia burgdorferi sensu lato, Anaplasma spp. and Leishmania infantum in apparently healthy and CVBD-suspect dogs in Portugal—a National serological study. Parasit Vectors 5:62
Cardoso L, Cortes HCE, Reis A, Rodrigues P, Simoes M, Lopes AP, Vila-Vicosa MJ, Talmi-Frank D, Eyal O, Solano-Gallego L, Baneth G (2013) Prevalence of Babesia microti-like infection in red foxes (Vulpes vulpes) from Portugal. Vet Parasitol 19:90–95
Carpi G, Bertolotti L, Pecchioli E, Cagnacci F, Rizzoli A (2009) Anaplasma phagocytophilum groEL gene heteroneity in Ixodes ricinus larvae feeding on roe deer in northeastern Italy. Vector Borne Zoonotic Dis 9:179–184
Corrain R, Di Francesco A, Bolognini M, Ciucci P, Baldelli R, Guberti V (2007) Serosurvey for CPV-2, distemper virus, ehrlichiosis and leishmaniosis in free-ranging dogs in Italy. Vet Rec 160:91–92
Costa LM Jr, Rembeck K, Ribeiro MF, Beelitz P, Pfister K, Passos LM (2007) Sero-prevalence and risk indicators for canine ehrlichiosis in three rural areas of Brazil. Vet J 174:673–676
Courtney CH, Zeng QY (2001) Comparison of heartworm antigen test kit performance in dogs having low heartworm burdens. Vet Parasitol 96:317–322
De la Fuente J, Torina A, Naranjo V, Nicosia S, Alongi A, La Mantia F, Kocan KM (2006) Molecular characterization of Anaplsma platys strains from dogs in Sicily, Italy. BMC Vet Res 2:24
De Tommasi AS, Otranto D, Dantas-Torres F, Capelli G, Breitschwerdt EB, de Caprariis D (2013) Are vector-borne pathogen co-infections complicating the clinical presentation in dogs? Parasit Vectors 6:97
Del Prete L, Maurelli MP, Pennacchio S, Bosco A, Musella V, Ciuca L, Cringoli G, Rinaldi L (2015) Dirofilaria immitis and Angiostrongylus vasorum: the contemporaneous detection in kennels. BMC Vet Res 11:305
Duscher GG, Leschnik M, Fuehrer HP, Joachim A (2015) Wildlife reservoirs for vector-borne canine, feline and zoonotic infections in Austria. Int J Parasitol Wild 4:88–89
Ebani VV, Cerri D, Fratini F, Ampola M, Andreani E (2008) Seroprevalence of Anaplasma phagocytophilum in domestic and wild animals from central Italy. New Microbiol 31:371–375
Ebani VV, Bertelloni F, Turchi B, Cerri D (2013) Serological and molecular survey of Anaplasma phagocytophilum in Italian hunting dogs. Ann Agric Environ Med 20:289–292
Ebani VV, Bertelloni F, Torracca B, Cerri D (2014) Serological survey of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Ehrlichia canis infections in rural and urban dogs in Central Italy. Ann Agric Environ Med 21:671–675
Ebani VV, Nardoni S, Fognani G, Mugnaini L, Bertelloni F, Rocchigiani G, Papini RA, Stefani F, Mancianti F (2015) Molecular detection of vector-borne bacteria and protozoa in healthy hunting dogs from Central Italy. Asian Pac J Trop Biomed 5:108–112
Genchi C, Kramer LH, Rivasi F (2011) Dirofilarial infections in Europe. Vector Borne Zoonotic Dis 10:1307–1317
Giangaspero A, Marangi M, Latrofa MS, Martinelli D, Traversa D, Otranto D, Genchi C (2013) Evidences of increasing risk of dirofilarioses in southern Italy. Parasitol Res 12:1357–1361
Giudice E, Domina F, Britti D, Di Pietro S, Pugliese A (2003) Clinical findings associated with Borrelia burgdorferi infection in the dog. Vet Res Commun 27:767–770
Gómez-Morales MA, Selmi M, Ludovisi A, Amati M, Fiorentino E, Breviglieri L, Poglayen G, Pozio E (2016) Hunting dogs as sentinel animals for monitoring infections with Trichinella spp. in wildlife. Parasit Vectors 16:154
Greene CE, Straubinger RK, Levy SA (2012) Chapter 43: Borreliosis in: Greene CE, infectious diseases of the dog and cat, 4th edn. Saunders, Elsevier Inc, pp 453–455
Hodžić A, Alic A, Fuehrer HP, Harl J, Wille-Piazzai W, Duscher GG (2015) A molecular survey of vector-borne pathogens in red foxes (Vulpes vulpes) from Bosnia and Herzegovina. Parasit Vectors 8:88
Hovius KE (2013) Canine borreliosis. In: Beugnet F. Guide to vector borne diseases of pets. Merial, Lyon, pp 219–229
Kohn B, Silaghi C, Galke D, Arndt G, Pfister K (2011) Infection with Anaplasma phagocytophilum in dog in Germany. Res Vet Sci 91:71–76
Kordick SK, Breitschwerdt EB, Hegarty BC, Southwick KL, Colitz CM, Hancock SI, Bradley JM, Rumbough R, Mcpherson JT, MacCormack JN (1999) Coinfection with multiple tick-borne pathogens in a walker hound kennel in North Carolina. J Clin Microbiol 37:2631–2638
Krupka I, Knauer J, Lorentzen L, O’Connor TP, Saucier J, Straubinger RK (2009) Borrelia burgdorferi sensu lato species in Europe induce diverse immune responses against C6 peptides in infected mice. Clin Vaccine Immunol 16:1546–1562
Laflamme DP (1997) Development and validation of a body condition score system for dogs. Canine Pract 22:10–15
Latrofa MS, Dantas-Torres F, de Caprariis D, Cantacessi C, Capelli G, Lia RP, Breitschwerdt EB, Otranto D (2016) Vertical transmission of Anaplasma platys and Leishmania infantum in dogs during the first half of gestation. Parasit Vectors 9:269
Little SE, Heise SR, Blagburn BL, Callister SM, Mead PS (2010) Lyme borreliosis in dogs and humans in the USA. Trends Parasitol 26:213–218
Mackenstedt U, Jenkins D, Roming T (2015) The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int J Parasitol Wildl 4:71–79
Mannelli D, Cerri D, Buffrini L, Rossi S, Arata T, Innocenti M, Grignolo MC, Bianchi G, Iori A, Tolari F (1999) Low risk of Lyme borreliosis in a protected area on the Thyrrhenian coast, in central Italy. Eur J Epidemiol 15:371–377
McCall JW, Genchi C, Kramer LH, Guerrero J, Venco L (2008) Heartworm disease in animals and humans. Adv Parasitol 66:193–285
Mircean V, Dumitrache MO, Gyorke A, Pantchev N, Jodies R, Mihalca AD, Cozma V (2012) Seroprevalence and geographic distribution of Dirofilaria immitis and tick-borne infections (Anaplasma phagocytophilum, Borrelia burgdorfei sensu lato, and Ehrlichia canis ) in dogs from Romania. Vector Borne Zoonotic Dis 7:595–604
O’Connor TP (2015) SNAP assay technology. Top Companion Anim Med 30:132–138
Otranto D, Dantas -Torres F (2010) Canine and feline vector-borne disease in Italy: current situation and perspectives. Parasit Vectors 3:2
Otranto D, Paradies P, Testini G, Latrofa MS, Weigl S, Cantacessi C, Mencke N, de Caprariis D, Parisi V, Capelli G, Stanneck V (2008) Application of 10% imidacloprid 50% permethrin to prevent Ehrlichia canis exposure in dogs under natural conditions. Vet Parasitol 153:320–328
Otranto D, Capelli G, Genchi C (2009) Changing distribution patterns of canine vector borne disease in Italy. Leishmaniosis vs. Dirofilariosis. Parasit Vectors 2(Suppl 1):S2
Otranto D, Testini G, Dantas-Torres F, Latrofa MS, Vissotto de Paiva Diniz PP, de Caprariis D, Lia RP, Mencke N, Stanneck D, Capelli G, Breitschwerdt EB (2010) Diagnosis of canine vector-borne disease in young dogs: a longitudinal study. J Clin Microbiol 48:3316–3324
Otranto D, Dantas-Torres F, Brianti E, Traversa D, Petric D, Genchi C, Capelli G (2013) Vector-borne helminths of dogs and humans in Europe. Parasit Vectors 6:16
Otranto D, Cantacessi C, Pfeffer M, Dantas-Torres F, Brianti E, Deplazes P, Genchi C, Guberti V, Capelli G (2015) The role of wild canids and felids in spreading parasite to dogs and cats in Europe. Part I: protozoa and tick-borne agents. Vet Parasitol 213:12–23
Pennisi MG, Caprì A, Solano-gallego L, Lombardo G, Torina A, Masucci M (2012) Prevalence of antibodies against Rickettsia conorii, Babesia canis, Ehrlichia canis and Anaplasma phagocytophilum antigens in dogs from the Stretto di Messina area (Italy). Ticks Tick Borne Dis 3:314–317
Perez M, Bodor M, Zhang C, Xiong Q, Rikihisa Y (2006) Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann N Y Acad Sci 1078:110–117
Petrie A, Watson P (2013) Experimental design and clinical trials. In: Petrie A, Watson P (eds) Statistics for veterinary and animal science, 3rd edn. John Wiley & Sons, Ltd, Oxford, pp 77–103
Piantedosi D, Veneziano V, Di Muccio T, Foglia Manzillo V, Fiorentino E, Scalone A, Neola B, Di Prisco F, D’Alessio N, Gradoni L, Oliva G, Gramiccia M (2016) Epidemiological survey on Leishmania infection in red fox (Vulpes vulpes) and hunting dogs sarin the same rural area in southern Italy. Acta Parasitol 61(4):769–775
Pipia AP, Varcasia A, Tosciri G, Seu S, Manunta ML, Mura MC, Sanna G, Tamponi C, Brianti E, Scala A (2014) New insights onto cardiopulmonary nematodea of dogs in Sardinia, Italy. Parasitol Res 113:1505–1059
Qurollo BA, Chandrashekar R, Hegarty BC, Beall MJ, Stillman BA, Liu J, Thatcher B, Pultorak E, Cerrito B, Walsh M, Breitschwerdt EB (2014) A serological survey of tick-borne pathogens in dogs in North America and the Caribbean as assessed by Anaplasma phagocytophilum, A. platys, Ehrlichia canis, E. chaffeensis, E. ewingii, and Borrelia burgdorferi species-specific peptides. Infect Ecol Epidemiol 20:4
Ramos RA, Latrofa MS, Giannelli A, Lacasella V, Campbell BE, Dantas-Torres F, Otranto D (2014) Detection of Anaplasma platys in dogs and Rhipicephalus sanguineus group ticks by a quantitative real-time PCR. Vet Parasitol 205:285–288
Renè-Martellet M, Lebert I, Chêne J, Massot R, Leon M, Leal A, Badavelli S, Chalvet-Monfray K, Ducrot C, Abrial D, Chabanne L, Halos L (2015) Diagnosis and incidence risk of clinical canine monocytic ehrlichiosis under field conditions in southern Europe. Parasit Vectors 8:3
Rizzoli A, Hauffe HC, Carpi G, Vourc HG, Neteler M, Rosa R (2011) Lyme borreliosis in Europe. Euro Surveill 16(27):19906
Sainz Á, Roura X, Miró G, Estrada-Peña A, Kohn B, Harrus S, Solano-Gallego L (2015) Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit Vectors 8:75
Santoro M, Veneziano V, D'Alessio N, Di Prisco F, Lucibelli MG, Borriello G, Cerrone A, Dantas-Torres F, Latrofa MS, Otranto D, Galiero G (2016) Molecular survey of Ehrlichia canis and Coxiella burnetii infections in wild mammals of southern Italy. Parasitol Res 115:4427–4431
Solano-Gallego L, Trotta M, Razia L, Furlanello T, Caldin M (2006) Molecular survey of Ehrlichia canis and Anaplasma phagocytophilum from blood of dogs in Italy. Ann N Y Acad Sci 1078:515–518
Sparagano OA, de Vos AP, Paoletti B, Cammà C, de Santis P, Otranto D, Giangaspero A (2003) Molecular detection of Anaplasma platys in dogs using polymerase chain reaction and reverse line blot hybridization. J Vet Diagn Investig 15:527–534
Stańczak J, Gabre RM, Kruminis-Łozowska W, Racewicz M, Kubica-Biernat B (2004) Ixodes ricinus as a vector of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti in urban and suburban forests. Ann Agric Environ Med 11:109–114
Stillman BA, Monn M, Liu J, Thatcher B, Foster P, Andrews B, Little S, Eberts M, Breitschwerdt EB, Beall MJ, Chandrashekar R (2014) Performance of a commercially available in-clinic ELISA for detection of antibodies against Anaplasma phagocytophilum, Anaplasma platys, Borrelia burgdorferi, Ehrlichia canis, and Ehrlichia ewingii and Dirofilaria immitis antigen in dogs. JAVMA 245:80–86
Thrustfield M (1995) Veterinary epidemiology. Blackwell Science Ltd, London, pp 138–188
Tolnai Z, Sreter-Lancz Z, Sreter T (2015) Spatial distribution of Anaplasma phagocytophilum and Hepatozoon canis in red foxes (Vulpes vulpes) in Hungary. Ticks Tick Borne Dis 6:645–648
Torina A, Caracappa S (2006) Dog tick-borne disease in Sicily. Parassitologia 48:145–147
Torina A, Vicente J, Alongi A, Scimeca S, Turlà R, Nicolsia S, Di Marco V, Caracappa S, de la Fuente J (2007) Observed prevalence of tick-borne pathogens in domestic animals in Sicily, Italy during 2003-2005. Zoonoses Public Health 54:8–15
Torina A, Alongi A, Naranjo V, Scimeca S, Nicosia S, Di Marco V, Caracappa S, Kocan KM, de la Fuente J (2008) Characterization of Anaplasma infections in Sicily, Italy. Ann N Y Acad Sci 1149:90–93
Trotta M, Fogliazza A, Furlanello T, Solano-Gallego L (2009) A molecular and serological study of exposure to tick-borne pathogens in sick dogs from Italy. Clin Microbiol Infect 15(Suppl 2):62–63
Veronesi F, Galuppi R, Tampieri MP, Botoli C, Mammoli R, Piergili Fioretti D (2011) Prevalence of Anaplasma phagocytophilum in fallow deer (Dama dama) and feeding ticks from an Italy preserve. Res Vet Sci 90:40–43
Westmoreland LS, Stoskopf MK, Maggi RG (2016) Prevalence of Anaplasma phagocytophilum in North Carolina eastern black bears (Ursus americanus ). J Wildl Dis 52:968–970
Acknowledgements
The study was supported by the management committees of the hunting districts of Salerno (ATC SA 1) and Avellino (ATC AV) and partially funded by the grant from the Ministry of Health of the Italian Republic (IZSME 01/14 RC).
The authors thank the following veterinaries for their cooperation: Claudio Amore, Pasquale Apicella (ASL Salerno), Loredana Avallone, Gennaro Barra, Raffaele Bevilacqua, Annalisa Bianco, Gaetano Bove (ASL Salerno), Antonio Bufalo, Giacomo Calabria, Vincenzo Cardamone, Francesco Celano, Domenico Crocetta, Cristina Cucciniello, Susanna De Luca, Giovanni De Lucia, Lucio De Maria, Anna Fiorella Desiderio, Enrico Di Blasi, Antonio Fimiani, Alfonso Gallo, Saverio Giordano, Enrico Lanaro, Cosimo Manna, Paola Napolitano, Carmine Palo, Gerardo Paraggio (ASL Salerno), Agnese Parrilli, Luca Pericolo, Gaetano Petta, Simona Pisano, Giovanni Russo (ASL Salerno), Antonio Raffaele, Vincenzo Raimondi, Enrico Renzulli, Antonio Ricco, Giampiero Sepe, Pierluigi Vitale, and Antonio Zotti.
The authors thank Eng. Tullio Panico (CIRAM) for his technical assistance.
This manuscript is dedicated to the memory of Dr. Enrico Lanaro, friend and colleague of many of the coauthors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that the research comply with the current Italian laws.
Conflict interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Piantedosi, D., Neola, B., D’Alessio, N. et al. Seroprevalence and risk factors associated with Ehrlichia canis, Anaplasma spp., Borrelia burgdorferi sensu lato, and D. immitis in hunting dogs from southern Italy. Parasitol Res 116, 2651–2660 (2017). https://doi.org/10.1007/s00436-017-5574-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5574-z