Abstract
We present data on the species composition of helminths in brown bears (Ursus arctos) from the Murmansk Region, Russia. The absence of any information about helminths of brown bear in the region necessitated the conduct of these studies. Samples were collected in 2014 and 2015 in the southern part of the Kola Peninsula from the White Sea coastal habitats. Annually, in the study area, 1–3 bears are legally hunted and biological samples for examination are very difficult to obtain. Therefore, we used fecal samples. We studied 93 feces and identified parasite eggs identified in 43 of them by morphometric criteria. The surveys revealed eggs of the following helminths: Dicrocoelium sp., Diphyllobothrium sp., Anoplocephalidae, Capillariidae, Baylisascaris sp., Strongylida 1, and Strongylida 2. These results represent the first reconnaissance stage, which allowed characterizing the taxonomic diversity and prevalence of parasites of brown bears of the Kola Peninsula.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The brown bear (Ursus arctos Linnaeus, 1758) is a circumpolar species inhabiting the entire boreal zone of Eurasia and North America. The species spread throughout all taiga forests of Northwest Russia and is abundant in tundra zone of the arctic region along the river valleys until the Barents Sea coast. The brown bear is the common representative of the regional fauna and a popular game animal. Thus, most of research has been focused on the population dynamic and distribution but with a noticeable gap in ecological investigations and especially on the bear parasite fauna. Previous research has mainly aimed on species of high epidemiologic or epizootic significance (such as Trichinella spp.). The challenges for complete helminthological dissection of bears consist in the difficulty of both obtaining the animals and their examination in the laboratory. Accordingly, data on the parasite fauna of specific U. arctos populations were acquired from a limited number of individual animals (Ruhlyadev and Ruhlyadeva 1953; Worley et al. 1976; Rausch et al. 1979; Tranbenkova 2006; Catalano et al. 2015).
A universal and widely used non-invasive method for studying the helminth fauna of different groups of animals, including the brown bear, is fecal flotation of parasite eggs in salt solutions (Gau et al. 1999; Esaulova et al. 2012; Vavilova et al. 2015). In spite of the known shortcomings of this method (sensitivity, identification to species, problems with estimating quantitative indices), it does yield general information on the diversity of gastrointestinal helminths and the lower limit of prevalence of the most abundant parasites. It is, in fact, the only currently available method for studying the parasite fauna of the brown bear in this region, given the impossibility of complete helminthological dissections. At present, the problem of parasite species diagnosis can also be solved by the synthesis of flotation and genetic methods of animal feces analysis (De Ambrogi et al. 2011; Elmore et al. 2013; Aghazadeh et al. 2015).
Little is known about bear helminths in the Russian Northwest. Even in whole Russia, this subject has been studied briefly and randomly only. The results of studies of brown bear populations in the region have shown that in the coastal area exists a population displaying a considerable concentration pattern connected with local food conditions. Different sources such as seals carcasses in early spring, meadows with lush vegetation in summer, and berries in autumn attract bears to the relatively narrow line of sea coast (Tirronen et al. 2015). These concentrations may provide stable infection hotbed and reinvasion but on the other hand these places are of great value to collect samples and for systematic monitoring.
The aim of this study was to determine the species composition and prevalence of helminths in scats of brown bears inhabiting the White Sea coastal areas of the Kola Peninsula.
Material and methods
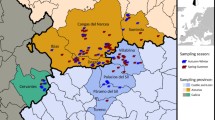
The study area (Tersky District, Murmansk Region) is situated in the northern taiga subzone with some forest tundra in the east. The landscape is uniform, appearing as a rolling plain. The subarctic climate is under the influence of the White Sea. Maritime tundra plant communities stretch in a narrow belt along the sea shore.
Sampling was carried out in 2014 (24.05–02.06; 31.08–02.09) and 2015 (03–12.07) in the south of the Kola Peninsula during transect to count brown bears in the White Sea coastal habitats (Fig. 1). The total length of transects was 453 km. The transects were located along the sea coast, river valleys, dirt roads, and animal paths. Tracks and scat sampling points were recorded by GPS devices.
Fecal samples (ca. 10 g) were fixed in 5% buffered formalin in Falcon tubes (30 ml). Sample preservation and DNA extraction for genetic analysis was performed as described in Andreassen et al. (2012) and followed the standard protocol for analyzing nonhuman forensic material (Linacre et al. 2011). The identity of the brown bears was determined by microsatellite analysis following a modified protocol (Andreassen et al. 2012) involving pre-PCR processing of the products and using eight markers (MU09, MU10, MU23, MU59, MU05, G10 L, MU51, MU50) (Taberlet et al. 1997).
Altogether, 93 fecal samples were studied by the benchtop (passive) flotation procedure (Ivashkin et al. 1971; Zajac and Conboy 2012). The flotation solution was sodium chloride (NaCl) (400 g/1000 ml water) with 1.2 specific gravity. Procedure: 4 g of feces were mixed with 50 ml of salt; the mixture was strained through cheesecloth and then left to stand for 60 min in Falcon tubes. Surface film was picked up by a wire loop (8 mm in diameter), mounted on a glass slide and placed under cover slip; three slides were made for each sample. Three to four hours later, the supernatant was discarded, and the sediment was examined under microscope.
The slides were examined under Olympus CX-4 microscope at ×100 and ×400 magnification using facilities of the Shared Research Center “Complex Fundamental and Applied Studies of the Function of Live System under Far North Conditions” (IB KarRC RAS). All the detected objects were photographed (adaptor U-TV0.5xC-3, camera Levenhuk C510 NG, software Toup View Version ×86, 3.5.434). Identification of parasite eggs was based on available information on their morphology and morphometry (Kozlov 1977; Zajac and Conboy 2012).
Species synonyms for the parasites are given according to the Fauna Europaea database (Gibson et al. 2014).
Results
We examined a total of 93 brown bear fecal samples and identified seven morphologically distinct types of helminth eggs. Because the limitations of the methods in helminth species identification, we give here the names of the genus, family or order of the detected parasites (Table 1).
Trematode eggs were found in three samples (3.2% prevalence), and all of them structurally corresponding to typical representatives of the genus Dicrocoelium sp. (Fig. 2а). Eggs were detected through examination of the sediment: small, dark brown, 41 ± 2 × 25 ± 0.3 μm.
Eggs of cestodes Diphyllobothrium and Anoplocephalidae were found in three and four samples, respectively. The dimensions of Diphyllobothrium sp. eggs were within [67–71] × [41–50] μm. The diameters of Anoplocephalidae eggs and oncospheres (Fig. 2с) were 44 ± 4 [37–51] and 13 ± 1 [11–15] μm.
Nematode eggs could be differentiated into four different morphological types: Capillariidae (1.1% prevalence), Baylisascaris sp. (37.6%), Strongylida 1 (11.8%), and Strongylida 2 (5.3%). Capillariidae eggs (Fig. 2d) were retrieved from a single sample, and their length and width were 60 ± 6 [65–52] × 26 μm. The criterion for differentiating Strongylida in our samples into two groups was the size: Strongylida 1 eggs (Fig. 2f, g) were bigger, [52–90] × [28–58] μm, and Strongylida 2 eggs (Fig. 2h, i) were smaller.
Genetic analysis revealed sufficient genetic material for DNA identification in 21 samples (out of 93), which belonged to 15 individual bears (Fig. 1). Data on parasites from different samples but from the same animal were pooled together (Table 1). The 15 individual brown bears in the survey contained all the egg morphotypes described above. Dicrocoelium, Diphyllobothrium, Anoplocephalidae, Capillariidae, and Strongylida 2 were found in singular individuals, the prevalence of Baylisascaris and Strongylida 1 eggs was 40 and 20%, respectively. Differences in parasite prevalence between samples and between individual animals were insignificant (p > 0.05). Among the three fecal samples from one bear, collected on 01.09.2014 (1 sample) and 11.07.2015 (2 samples), only one (July 2015) contained Baylisascaris sp. eggs.
Discussion
All trematode eggs found in our study seem to belong to Dicrocoelium dendriticum (Rudolphi, 1819), which infects the bile ducts of the liver and gall bladder of its definitive hosts—herbivorous and carnivorous mammals (Kozlov 1977). The life cycle of this species involves two intermediate hosts: terrestrial mollusks and ants. D. dendriticum had previously been found both in brown bear scats (Esaulova et al. 2010, 2012) and in dissected bodies (Ruhlyadev and Ruhlyadeva 1953). When the helminth fauna of U. actors was studied in theVolga-Vyatkaregion by partial helminthological dissection, the intensity of infection with D. dendriticum was 101 to 17,000 specimens (Maslennikova and Kolevatova 2004).
Since the eggs of Diphyllobothrium cestodes are highly variable (Andersen and Halvorsen 1978; Choi et al. 2015), we could not identify them to species relying just on their morphology. The brown bear has so far been reported to host five species of cestodes of the genus Diphyllobothrium: D. cordatum (Leuckart, 1863), D. dendriticum (Nitzsch, 1824), D. latum (Linnaeus, 1758), D. nihonkaiense Yamane et al., 1986, and D. ursi Rausch, 1954 (Rausch 1954; Rogers and Rogers 1976; Kozlov 1977; Arizono et al. 2009; Catalano et al. 2015).
Among the above species, taxonomic catalogs of fish parasites for Karelia and the Murmansk Region report the findings of D. dendriticum and D. latum plerocercoids (Rumyantsev and Ieshko 1997; Mitenev and Schulman 1999). For D. cordatum (pinnipeds as obligate hosts), the White Sea has been mentioned as one of the finding locations (Delamure et al. 1985).
Cestodes D. dendriticum and D. latum are widespread distributed in the Holarctic region. The second intermediate host for D. dendriticum, which has been reported from more northern locations than D. latum, is land-locked and sea-going salmonids, whereas for D. latum the main intermediate hosts are perch, pike, and burbot (Delamure et al. 1985; Scholz et al. 2009). Considering the region and sampling sites, one can surmise the distribution of D. dendriticum in the area.
Cestodes of the family Anoplocephalidae are common parasites of herbivorous mammals. There is no information on the prevalence of these cestodes in bears (either from feces or from necropsies). The detection of Anoplocephalidae eggs in carnivores’ scats is usually classified as artifacts and attributed to consumption of infected prey (Elmore et al. 2013). The presence of Anoplocephalidae eggs in bear fecal samples can be the result of preying on some herbivorous mammals. Neither can one exclude accidental contamination of the samples with feces of rodents, which were very abundant in the study area during the period in question and were infected with cestodes of the family Anoplocephalidae (Anoplocephaloides dentata and Paranoplocephala sp.) at a rate of around 30%.
The eggs of Baylisascaris sp. nematodes detected in the samples probably belong to the widespread ursine specialist parasite Baylisascaris transfuga (Rudolphi, 1819). This parasite with a direct life cycle infests animals as they swallow invasive eggs. Baylisascaris transfuga has been reported from representatives of the genus Ursus in Europe, Asia and North America (Kozlov 1977; Rogers and Rogers 1976; Worley et al. 1976; De Ambrogi et al. 2011; Aghazadeh et al. 2015; Catalano et al. 2015), both from wild populations and captive animals (Pasechnik 2010). The prevalence of Baylisascaris eggs in U. arctos fecal samples ranges from 5 to 50% (Esaulova et al. 2012; Aghazadeh et al. 2015; Vavilova et al. 2015) and demonstrates a clear seasonal pattern with peaks in the summer season (Burdelev 1953; Gau et al. 1999). Parasitological dissections of bears sometimes yielded the prevalence of B. transfuga infection at more than 75% and intensity of up to 480 parasites (Worley et al. 1976; Maslennikova and Kolevatova 2004).
Only one sample in our study contained eggs of the Capillariidae type (Fig. 2d). The eggs differed in size from the eggs of Eucoleus aerophilus (Creplin, 1839) and Pearsonema plica (Rudolphi, 1819), the species previously discovered in U. arctos at dissection (Ruhlyadev and Ruhlyadeva 1953; Tranbenkova 2006). According to Esaulova et al. (2010, 2012) fecal samples of brown bear inhabiting in the Far East contained the eggs of Aonchotheca putorii (Rudolphi, 1819), a widespread parasite of predaceous mammals (Kozlov 1977), including U. americanus (Pence et al. 1983; Foster et al. 2004). Morphologically, the eggs in our samples were similar to A. putorii eggs.
We subdivided Strongylida eggs into two groups, based exclusively on their size. Larger eggs (Strongylida 1) may belong to nematodes Uncinaria sp. (Strongylida: Ancylostomatidae) parasitic on carnivores. Data is available on the prevalence of four species of this nematode genus in brown bear intestines: Uncinaria rauschi Olsen, 1968 and U. yukonensis (Wolfgang, 1956) are ursine specialists (the former reported only from the Nearctic, and the latter from the Holarctic realm), U. stenocephala (Railliet, 1884) is a Holarctic generalist parasite; U. skrjabini Matschulsky, 1949 is a Palearctic species parasitic in mustelids (Ruhlyadev and Ruhlyadeva 1953; Gubanov 1964; Rausch et al. 1979; Catalano et al. 2015). Strongylid eggs are frequently identified in U. arctos scats at a prevalence rate of nearly 10% (Gau et al. 1999; Esaulova et al. 2012; Aghazadeh et al. 2015; Vavilova et al. 2015).
We could not identify the exact species of the eggs denoted here as Strongylida 2. Since bears are omnivorous, their helminth fauna often comprises parasites typical of other animal groups (Rausch et al. 1979; Gau et al. 1999; Tranbenkova 2006; Aghazadeh et al. 2015). One should also bear in mind that once on the ground, feces soon get colonized by bacteria and fungi, and then by nematodes with close trophic links to soil microorganisms. Fecal samples of bears from the Murmansk Region were found to contain free-living soil-dwelling nematodes of families Diplogasteridae, Rhabditidae, Bunonematidae, which show preference for saprobiotic detritus and bacterial flora, as well as mycotrophic nematodes of the genus Aphelenchoides. Many species of non-parasitic nematodes feature high rates of individual development, wherefore larvae and eggs of nematodes of these groups can be present on the mounts in addition to adult stages. Admittedly, Strongylida 2 eggs can belong to either parasitic or free-living nematodes.
When the study is limited to fecal examination (without genetic fingerprinting of each fecal sample), not only species identification by the morphotype of the eggs is problematic, but also estimation of the parasite’s prevalence in the host population is rather approximate, since it is nearly impossible to reliably determine which individual host (or even host species) the sample came from. The rare exception here is the samples collected directly from the rectum of anesthetized bears (Gau et al. 1999).
In our study, we employed genetic analysis to deal with the problem of estimating prevalence. Unfortunately, the methods of DNA extraction from animal feces are not faultless. The brown bear population of the Tersky District is around 250 animals (Tirronen et al. 2015). We managed to identify DNA fragments in 21 samples coming from 15 individual bears. Per sample and per animal estimates of the prevalence of different morphotypes of parasites were similar. There usually was only one scat sample per bear. On the other hand, among the three examined samples coming from one bear, Baylisascaris eggs were detected in only one sample, although the “positive” and the “negative” scats were collected on the same day.
Underestimated prevalence levels yielded by coprological studies may be the consequence of the low “sensitivity” of flotation techniques. In the raccoon (Procyon lotor), for instance, Baylisascaris procyonis eggs were found in 66% of fecal samples taken from infected hosts (Page et al. 2005). B. transfuga eggs were not detected in the scats of bears in which the nematode infection was confirmed both by positive PCR tests and dissection of the intestine (Aghazadeh et al. 2015). Another possible factor for the absence of eggs from the scats of infected hosts is the periodicity of egg shedding rates in Baylisascaris nematodes (Reed et al. 2012). We can therefore surmise that the actual prevalence of B. transfuga in the brown bear population of the Kola Peninsula is similar to known maximums (over 70%). The parasite’s reinvasion success and steadily high abundance are probably promoted by the high local density of the brown bear population in White Sea coastal habitats, where the relative abundance of the animals during the study period was up to 7 bears per 10 km2 (Tirronen et al. 2015).
Thus, in our study, brown bears were found to be infected with helminth eggs of Dicrocoelium (3%), Diphyllobothrium (3%), Anoplocephalidae (4%), Capillariidae (1%), Bayliscaris (38%), Strongylida 1 (12%), and Strongylida 2 (5%), which may belong to 7–10 different species. The main specific feature of the helminth fauna is the high rate of infection with the nematode Baylisascaris transfuga. The above list is certainly incomplete, including only the most common species detectable by the techniques used in the study. Both extra intestinal (Trichinella, Dirofilaria) and intestinal (trematodes, Taenia cestodes) parasites of the brown bear may have remained unaccounted.
References
Aghazadeh M, Elson-Riggins J, Reljić S, De Ambrogi M, Huber D, Majnarić D, Hermosilla C (2015) Gastrointestinal parasites and the first report of Giardia spp. in a wild population of European brown bears (Ursus arctos) in Croatia. Veterinarski Arhiv 85:201–210
Andersen K, Halvorsen O (1978) Egg size and form as taxonomic criteria in Diphyllobothrium (Cestoda, Pseudophyllidea). Parasitology 76:229–240
Andreassen R, Schregel J, Kopatz A, Tobiassen C, Knappskog PM, Hagen SB, Kleven O, Schneider M, Kojola I, Aspi J, Rykov A, Tirronen KF, Danilov PI, Eiken HG (2012) A forensic DNA profiling system for Northern European brown bears (Ursus arctos)//Forensic Science International. Genetics 6:798–809. doi:10.1016/j.fsigen.2012.03.002
Arizono N, Shedko M, Yamada M, Uchikawa R, Tegoshi T, Takeda K, Hashimoto K (2009) Mitochondrial DNA divergence in populations of the tapeworm Diphyllobothrium nihonkaiense and its phylogenetic relationship with Diphyllobothrium klebanovskii. Parasitol Int 58:22–28. doi:10.1016/j.parint.2008.09.001
Burdelev TE (1953) Studies of the seasonal dynamics of major helminth infections in carnivores in the Moscow Zoo. In: Collected papers on Helminthology, in Honor of Academician K.I. Skryabin’s 75th Anniversary. Moscow, pp 93–98 (in Russian)
Catalano S, Lejeune M, Tizzani P, Verocai GG, Schwantje H, Nelson C, Duignan PJ (2015) Helminths of grizzly and black bears in Alberta and British Columbia, Canada. Can J Zool 93:765–772. doi:10.1139/cjz-2015-0063
Choi S, Cho J, Jung B-K, Kim D-G, Jeon SJ, Jeon H-K, Eom KS, Chai J-Y (2015) Diphyllobothrium nihonkaiense: wide egg size variation in 32 molecularly confirmed adult specimens from Korea. Parasitol Res 114:2129–2134. doi:10.1007/s00436-015-4401-7
De Ambrogi M, Aghazadeh M, Hermosilla C, Huber D, Majnaric D, Reljic S, Elson-Riggins J (2011) Occurrence of Baylisascaris transfuga in wild populations of European brown bears (Ursus arctos) as identified by a new PCR method. Vet Parasitol 179:272–276. doi:10.1016/j.vetpar.2011.02.025
Delamure SL, Skryabin AS, Serdiukov AM (1985) Diphyllobothriata—flatworm parasites of man, mammals and birds. Nauka, Moscow (in Russian)
Elmore SA, Lalonde LF, Samelius G, Alisauskas RT, Gajadhar AA, Jenkins EJ (2013) Endoparasites in the feces of arctic foxes in a terrestrial ecosystem in Canada. International Journal for Parasitology: Parasites and Wildlife 2:90–96. doi:10.1016/j.ijppaw.2013.02.005
Esaulova NV, Naidenko SV, Lukarevskii VS, Hernandez-Blanco JА, Sorokin PА, Litvinov МN, Kotlyar АК, Rozhnov VV (2010) The parasite fauna of carnivores in Ussuriisky reserve. Russian Journal of Parasitology (4):22–28 (in Russian)
Esaulova NV, Seryodkin IV, Konyaev SV, Malkina AV, Borisov MY (2012) Fauna of Bear’s helminthes from Sakhalin island and south of Russian Far East. Russian Journal of Veterinary Small pets and wild animals (4):16–19 (in Russian)
Foster GW, Cunningham MW, Kinsella JM, Forrester DJ (2004) Parasitic helminths of black bear cubs (Ursus americanus) from Florida. J Parasitol 90:173–175. doi:10.1645/GE-127R
Gau RJ, Kutz SJ, Elkin BT (1999) Parasites in grizzly bears from the Central Canadian Arctic. J Wildl Dis 35:618–621. doi:10.7589/0090-3558-35.3.618
Gibson D, Bray R, Hunt D, Georgiev B, Scholz T, Harris P, Bakke T, Pojmanska T, Niewiadomska K, Kostadinova A, Tkach V, Bain O, Durette-Desset M, Gibbons L, Moravec F, Petter A, Dimitrova Z, Buchmann K, Valtonen E, de Jong Y (2014) Fauna Europaea: helminths (animal parasitic). Biodiversity Data Journal 2:e1060. doi:10.3897/BDJ.2.e1060
Gubanov NM (1964) Fauna of helminthes in Yakutia game mammals. Nauka, Moscow (in Russian)
Ivashkin VM, Kontrimavichius VL, Nazarova NS (1971) Methods for collection and study of helminths in terrestrial mammals. Nauka, Moscow (in Russian)
Kozlov DP (1977) Analysis of the helminthes of predatory mammals of the USSR. Nauka, Moscow (in Russian)
Linacre A, Gusmão L, Hecht W, Hellmann AP, Mayr WR, Parson W, Prinz M, Schneider PM, Morling N (2011) ISFG: recommendations regarding the use of non-human (animal) DNA in forensic genetic investigations. Forensic Science International-Genetics 5:501–505. doi:10.1016/j.fsigen.2010.10.017
Maslennikova OV, Kolevatova AI (2004) Helminth fauna of carnivores (Canidae, Ursidae, Felidae) in Kirov region. Proc Institute of Gelmintology 40:190–199 (in Russian)
Mitenev VK, Schulman BS (1999) Parasites of fish in the Murmansk region. Systematic catalog, PINRO, Murmansk (in Russian)
Page LK, Gehrt SD, Titcombe KK, Robinson NP (2005) Measuring prevalence of raccoon roundworm (Baylisascaris procyonis): a comparison of common techniques. Wild life Society Bulletin 33:1406–1412. doi:10.2193/0091-7648(2005)33[1406:MPORRB]2.0.CO;2
Pasechnik VE (2010) Distribution and specific structure of helminths and coccidia at brown bears in the Russian Federation. Russian Journal of Parasitology (1):15–21 (in Russian)
Pence DB, Crum JM, Conti J (1983) Ecological analyses of helminth populations in the black bear, Ursus americanus, from North America. J Parasitol 69:933–950
Rausch RL (1954) Studies on the helminth fauna of Alaska. XXI. Taxonomy, morphological variation, and ecology of Diphyllobothrium ursi n. sp. Provis on Kodiak Island. J Parasitol 40:540–563
Rausch RL, Krechmar AV, Rausch VR (1979) New records of helminths from the brown bear, Ursus arctos L., in the Soviet Far East. Can J Zool 57:1238–1243
Reed C, Henke SE, Kresta AE (2012) Frequency of deposition and location of Baylisascaris procyonis eggs in raccoon feces. Journal of Wildlife Diseases 48:190–94. doi: http://dx.doi.org/10.7589/0090–3558-48.1.190
Rogers LL, Rogers SM (1976) Parasites of bears: a review. In: Pelton MR, Lentfer JW, Folk GE (eds) Bears—their biology and management. Papers of the Third International Conference on Bear Research and Management, Paper, vol 42. IUCN, Morges, pp 411–430
Ruhlyadev DN, Ruhlyadeva MP (1953) To study fauna of helminthes in brown bear. In: Collected papers on helminthology, in honor of academician K.I. Skryabin’s 75th Anniversary. Moscow, pp 598-602 (in Russian)
Rumyantsev EA, Ieshko EP (1997) Parasites of fishes in water bodies of the Karelia. KarRC RAS, Petrozavodsk (in Russian)
Scholz T, Garcia HH, Kuchta R, Wicht B (2009) Update on the human broad tapeworm (genus Diphyllobothrium), including clinical relevance. Clin Microbiol Rev 22:146–160. doi:10.1128/CMR.00033-08
Taberlet P, Cammara J, Griffin S, Uhrès E, Hanotte O, Waits L, Dubois-Paganon C, Burke T, Bouvet J (1997) Noninvasive genetic tracking of the endangered Pyrenean brown bear population. Mol Ecol 6:869–876. doi:10.1046/j.1365-294X.1997.00251.x
Tirronen KF, Panchenko DV, Kuznetsova AS (2015) Brown bear (Ursus arctos L.) of the White sea of the Kola Peninsula. Herald of game management 12:125–136 (in Russian)
Tranbenkova NA (2006) Helminth infestations in the Brown bear of Kamchatka. In: Seryodkin IV, Paczkowski J, Shuntov VP, Raygorodetsky GR (eds) Kamchatka brown bear: ecology, conservation, and sustainable use. Dal’nauka, Vladivostok, pp 137–141
Vavilova OV, Korablev NP, Volkov NO, Ogurtsov SS (2015) Helminths of large predators in central forest state natural biosphere reserve. Gerald of Tver State University. Series: Biology and Ecology (4):40–47 (in Russian)
Worley DE, Fox JC, Winters JB, Jacobson RH, Greer KR (1976) Helminth and arthropod parasites of grizzly and black bears in Montana and adjacent areas. In: Pelton MR, Lentfer JW, Folk GE (eds) Bears—their biology and management. Papers of the Third International Conference on Bear Research and Management. Paper 45. IUCN, Morges, pp 455–464
Zajac AM, Conboy GA (2012) Veterinary clinical parasitology, 8rd edn. Wiley-Blackwell, Chichester
Acknowledgements
The authors are grateful to A.A. Sushchuk, PhD, Researcher at the Laboratory of Animal and Plant Parasitology (Institute of Biology KarRC RAS) for determinations of free-living soil-dwelling nematodes, and P.E. Dekola (KarRC RAS) for assistance during the field season. The research was funded by federal budgetary allocations for implementation of state assignment (0221-2014-0030; 0221-2014-0037); and Russian Foundation for Basic Research grant (№ 14-04-31796).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Bugmyrin, S.V., Tirronen, K.F., Panchenko, D.V. et al. Helminths of brown bears (Ursus arctos) in the Kola Peninsula. Parasitol Res 116, 1755–1760 (2017). https://doi.org/10.1007/s00436-017-5456-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5456-4