Abstract
A severely underweight alligator snapping turtle Macrochelys temminckii Troost in Harlan, 1835, was found near Tyler, Texas, and taken to the Caldwell Zoo. Blood films were submitted to Texas A&M University, College Station, Texas, for morphological and molecular identification of haemogregarine-like inclusions in the red blood cells. Intraerythrocytic Haemogregarina sp. forms were found on microscopic examination at a parasitemia of <1 %. The morphology and morphometric data for the forms indicate similarity to Haemogregarina macrochelysi n. sp. Telford et al., 2009, previously reported in alligator snapping turtles in Florida and Georgia, but two characteristic stage forms were not shared between H. macrochelysi n. sp. and the parasite found in this report. The haemogregarine 18S ribosomal RNA gene (1555-bp fragment) was amplified and cloned, and five clones sequenced. The sequences were deposited in the NCBI GenBank database. All five showed ∼96 % identity to Haemogregarina balli Paterson and Desser, 1976, Hepatozoon sp., and Hemolivia stellata Petit et al., 1990. A 774-bp segment shared 98-99 % identity with the corresponding Haemogregarina sp. rDNA sequence (KR006985) from Caspian turtles (Mauremys caspica McDowell, 1964) in Iran. A neighbor-joining phylogenetic tree generated from aligned sequences from the clones, 26 hematozoa, Adelina dimidiata Schneider, 1875, and Cryptosporidium serpentis Levine, 1980, revealed the cloned sequences clustered on their own branch within the Haemogregarina spp. clade. No genetic data are available for H. macrochelysi n. sp. at this time, so it remains unclear if this parasite in a Texas alligator snapping turtle is conspecific with H. macrochelysi n. sp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The alligator snapping turtle, Macrochelys temminckii Troost in Harlan, 1835 (Testudines: Chelydridae), is characterized by sharp claws, spiked carapacial ridges, and huge head with strongly hooked beak. It is distributed in North America across the southeastern USA and is endemic to river systems that drain into the Gulf of Mexico from the Suwannee River in Georgia and Florida, and from the Brazos River in Texas (Powell et al. 2016). It is the largest species of freshwater turtle in North America, weighing up to 135 kg. It is highly aquatic although oviposition occurs on land. The alligator snapping turtle is non-migratory and a top-trophic-level predator (Boundy and Kennedy 2006; Ernst and Lovich 2009; Ewert 1976).
Haemogregarines are widely distributed blood parasites among aquatic turtles and have been reported in North America, Europe, Asia, and Australia (Telford 2009). Leeches are thought to be the invertebrate hosts and the transmission vectors for aquatic vertebrate hosts, while ticks are the transmission vectors for parasites of terrestrial reptiles (Telford 2009; Cook et al. 2009; Merino et al. 2009; Siddall and Desser 1991). In the USA, Haemogregarina spp. have been investigated in freshwater turtle populations in Louisiana, Georgia, Tennessee, Kentucky, and Texas (Acholonu 1974; Davis and Sterrett 2011; Edney 1949; Strohlein and Christensen 1984; Wang and Hopkins 1965) and particularly in the alligator snapping turtle M. temminckii in Arkansas, Georgia, and Florida (McAllister et al. 1995; Telford et al. 2009).
Haemogregarines are intraerythrocytic apicomplexans that belong to the suborder Adeleorina of the family Haemogregarinidae. Haemogregarina species have indirect life cycles. They infect a wide variety of vertebrate hosts such as mammals, birds, fishes, snakes, crocodilians, lizards, and turtles in which they undergo asexual cycles of merogony, gametogony, syngamy, and sporogony (Barta et al. 2012; Siddall and Desser 1992). The various types of meronts and merozoites that arise may either initiate further rounds of merogonic replication or differentiate into gamonts (Barta et al. 2012; Smith 1996). The invertebrate transmission vectors in which the sexual stages take place include ticks, mites, other arthropods, and leeches (Telford Jr 2009). Although the complete life cycles for Haemogregarina balli in Nearctic snapping turtles and Haemogregarina stepanowi Danilewsky, 1885, in the European pond turtle have been described, data regarding the complete life cycles for many of these organisms are lacking (Siddall and Desser 1991, 1992; Telford 2009). However, intraerythrocytic stages for a number of Haemogregarina spp. have been described in various hosts from different parts of the world (Barta et al. 2012; Davis and Sterrett 2011).
Nearly 300 haemogregarine species are named in previous studies based on morphological and/or biological features, such as the size and the shape of the stages in the host erythrocytes (Levine 1982; Perkins and Keller 2001). However, morphological similarities and the lack of detected life cycle stages, especially the identification of the vector and morphology of the parasite in the vector, have prevented an accurate identification of the different infective forms (Levine 1982). Numerous Haemogregarina spp. have been reported in turtles and tortoises globally, but descriptions of chelonian hematozoa in the USA are limited (Acholonu 1974; McAllister et al. 1995; McAllister 2015; Strohlein and Christensen 1984; Telford et al. 2009; Wang and Hopkins 1965) with some including photomicrographs and selected measurements.
New molecular tools combined with morphological approaches provide acceptable differential diagnoses and taxonomy of Haemogregarina spp. (Lv et al. 2015). To date, the 18S ribosomal RNA (rRNA) gene has been utilized in characterization, identification, and prevalence as well as in taxonomic relationship studies among apicomplexan parasites in reptiles, including freshwater turtles (Dvořáková et al. 2015; Kopečná et al. 2006; Kvičerová et al. 2014; Maia et al. 2014; Rakhshandehroo et al. 2016; Telford et al. 2009).
This study entails the first known Haemogregarina sp. identified in the alligator snapping turtle (M. temminckii) in Texas and includes both morphological and molecular descriptions of this parasite. Moreover, this report provides the first DNA sequence data for a Haemogregarina sp. in the USA.
Materials and methods
An alligator snapping turtle was found in early January 2015 laying by the side of a road near Tyler, Texas, and was taken to the Caldwell Zoo in Tyler. The turtle was drastically underweight. No leeches were present although examination of a stained blood film revealed the presence of haemogregarine-like inclusions in the red blood cells. Unfixed blood films were sent to the Department of Veterinary Pathobiology, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, Texas, for parasite identification.
A blood film was methanol fixed and Giemsa stained (AccuStain, Sigma) for microscopic examination by light microscopy at ×1000 magnification under immersion oil. The parasitemia was estimated based on examination of 1000 erythrocytes. Scanning and morphometric data for the blood stage parasites were obtained, and images were captured using an Olympus IX70 microscope (Olympus America, Inc. Center Valley, PA) equipped with Spot Software 5.2 Macro-Photography© 2016 SPOT Imaging (Diagnostic Instruments, Inc. Avon, MA). The length and width for multiple examples of each developmental stage observed were measured in micrometers and the mean and standard deviation determined. The range in length and width for each stage was recorded. The observed morphology of the blood stage parasites in this study was compared with published morphological data on turtle haemogregarines (McAllister 2015; Telford 2009; Telford et al. 2009).
DNA isolation and amplification
The blood film from one slide was scraped into 100 μl of Dulbecco’s phosphate-buffered saline (PBS) using a sterile scalpel blade. DNA was extracted from the blood following the manufacturer’s protocol (FlexiGene DNA Extraction kit, Qiagen, Redwood City, CA, USA). The extracted DNA concentration was determined (NanoDropND-1000 Spectrophotometer, NanoDrop Technologies, Wilmington, DE, USA) and adjusted to approximately 100 ng/μl.
Forward primer HemoFN (5′-CCGTGGTAATTCTAGAGCTAATACATGAGC-3′) and reverse primer HemoRN (5′-GATAAGGTTTACGAAACTTTCTATATTTA-3′) were designed from an alignment of haemogregarine 18S rRNA gene sequences in the GenBank database to amplify a gene fragment of ∼1550 bp. The amplification reaction was performed according to the manufacturer’s instructions (Phusion® High-Fidelity DNA polymerase, FINNZYMES, New England Biolabs, Inc., Ipswich, MA, USA) in a final volume of 25 μl containing ∼100 ng of DNA. A negative control reaction (no DNA) was included. The amplification profile was initial denaturation at 98 °C for 2 min followed by 35 cycles of denaturation at 98 °C for 30 s, annealing at 53 °C for 30 s, and extension at 72 °C for 2 min with a final extension at 72 °C for 7 min followed by hold at 4 °C (Labnet MultiGene Thermal Cycler, Woodbridge, NJ, USA). All amplicons were electrophoresed through a 1 % agarose gel alongside a 200-bp marker (BioDL200 BioFlux, Bulldog Bio, Portsmout, NH, USA), stained with ethidium bromide, and visualized under ultraviolet transillumination.
Cloning and phylogenetic analyses
Appropriately sized amplicons of ∼1550 bp were directly ligated within 24 h of PCR into the pJET1.2/blunt plasmid vector (CloneJET PCR Cloning Kit, Fermentas, Inc., Burlington, Ontario, Canada) and chemically competent Escherichia coli cells (TOP 10 One Shot®INVαF; Invitrogen, Grand Island, NY, USA) transformed following manufacturers’ instructions. Twenty transformed colonies were randomly chosen for colony PCR to verify the insert, and five verified colonies were expanded in overnight cultures. Plasmid DNA was extracted from the cultures (EZ-10 Spin Column Plasmid DNA Minipreps Kit, Bio Basic Inc., Amherst, NY, USA), quantified as above and adjusted to a concentration of approximately 150 ng/μl for automated bi-directional sequencing (Eton Bioscience Inc, San Diego, CA, USA) using pJET1.2 sequencing primers (CloneJET PCR Cloning Kit).
The resulting sequences were analyzed using Sequencher Version 5.1 and manually trimmed to remove ambiguous or unreadable data. Chromatogram-based contiguous sequences were generated for each of the five clones, and similarities to 18S rRNA gene sequences in the GenBank database were determined using the NCBI Basic Local Alignment Tool (BLAST) (Altschul et al. 1990). The obtained sequences were deposited in the NCBI GenBank database.
The sequences were aligned using Muscle (http://www.ebi.ac.uk/Tools/msa/muscle/) with additional sequences of Haemogregarina species and those of closely related genera, and Cryptosporidium serpentis as the outgroup, selected from the NCBI GenBank database (Table 1). The sequences in the Muscle alignment were trimmed to equivalent lengths BioEdit (Hall 1999), and the final alignment was used to construct a maximum likelihood tree in DIVEIN (https://indra.mullins.microbiol.washington.edu/DIVEIN/diver.html) under the default settings. The resulting tree was viewed and appropriately labelled using Mega 6.0 (Tamura et al. 2013).
Results
Host
The alligator snapping turtle was not treated. It is currently in good health (Fig. 1) and remains in residence at the Caldwell Zoo, Tyler, Texas.
Morphology
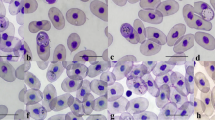
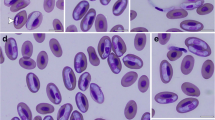
Intraerythrocytic Haemogregarina sp. forms with morphology typical of different blood stages were found on microscopic examination of a Giemsa-stained blood film (Fig. 2). The parasitemia was <1 %. No extracellular organisms were identified, nor were organisms found within white blood cells.
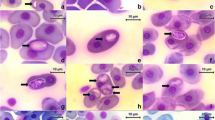
Among the intraerythrocytic stages observed were trophozoites, premeronts, meronts, and gamonts (Figs. 2 and 3). Trophozoites were the smallest forms and occurred individually in the erythrocyte with length × width dimensions of 8.75 ± 0.5 × 3.75 ± 1.4 μm (range, 8–9 × 3–5; n = 4) (Fig. 2a–c). Trophozoites possessed a prominent eccentric nucleus and vacuolated cytoplasm. Premeronts were the most common form seen in the blood film (Fig. 2d–f). The parasites were elongated with a central or slightly eccentric nucleus located toward the side of the parasite. Premeronts measured 13.42 ± 0.80 × 5.95 ± 0.41 μm (range, 12–15 × 5–7; n = 25). Meronts were elongated and slightly curved, measuring 11.1 ± 1.7 × 4.9 ± 0.7 μm (range, 10–14 × 4–6, n = 10), and contained a single nucleus (Fig. 3a–b) or multiple irregular nuclei (Fig. 3c–f). The immature gamont possessed a nucleus located toward or at one end (Fig. 3g–i) and were 11.6 ± 0.8 μm × 5.1 ± 0.4 μm (range 11–13 × 5–6, n = 6). Gamonts were slender and recurved with twin folded approximately equal limbs. The gamont total length was 33 ± 7.02 × 3.3 ± 1.4 μm (range, 26-40 × 3-4, n = 4) with LW 133.75 ± 39.4 μm2 (78–160) and L/W 9.6 ± 0.9 (8.6–10.8). The length of the gamont folded limbs ranged from 14 to 15 μm with a width across both limbs of 6-8 μm (Fig. 3j, k). The nucleus was located at one end of the structure in the bend of the limbs. Encapsulated gamonts were rare and appeared as non-staining bodies (Fig. 3l).
A voucher Giemsa-stained specimen slide has been deposited in the Systematics Research Collections, University of Nebraska State Museum, Lincoln, NE, under Accesson Number P-2016-057.
Molecular characterization
Genomic DNA from the alligator snapping turtle blood film was obtained with a concentration of 696 ng/μl. The primary PCR successfully amplified the 18S rRNA gene region with the designed primers, yielding amplicons of the expected size of ∼1550 bp. Twenty transformed colonies were verified to contain the proper insert by PCR and five (clones 2, 4, 6, 7, and 8) were selected for sequence analysis. The cloned sequences were 1555 bp in length and varied in sequence from one another, ranging from 99.3 to 99.9 % identity (Table 2). Clone 6 was the most variable, differing from the other clones in 8 to 10 nucleotide positions (99.3–99.5 % identity).
The 18S rDNA 1555-bp insert sequence of the five clones all showed ∼96 % identity to H. balli Paterson and Desser, 1976, Hepatozoon sp., and Hemolivia stellata Petit et al., 1990 (GenBank accession nos. HQ224959, FJ719813, and KP881349, respectively) by BLAST analysis. Higher identity was found between Haemogregarina sp. (KR006985) from Iran (99 % identity with clones 2, 6, and 8; 98 % identity with clones 4 and 7) by BLAST analysis of a 774-bp segment corresponding to that deposited for KR006985. The obtained sequences were deposited in the NCBI Genbank database under accession numbers KX507246, KX507247, KX507248, KX507249, and KX507250 for Haemogregarina sp. clones 2, 4, 6, 7, and 8, respectively.
The five cloned sequences were aligned with 26 18S rRNA gene sequences from closely related taxa, and from Adelina dimidiata and C. serpentis (Table 1). The final aligned length was 1382 bp after trimming sequences as needed to uniform corresponding fragments. The maximum likelihood phylogenetic tree generated from the final alignment shows three well-supported clades of the adeleorinid parasites infecting a variety of hosts (amphibians, reptiles, and mammals) (Fig. 4). The first clade contains two isolates of Dactylosoma ranarum (Kruse, 1890), the second contains Haemogregarina spp., and the third clade includes both Hemolivia spp. and Hepatozoon spp. but branches into two separate clusters, one holding the seven Hemolivia spp. isolates and one holding the five Hepatozoon spp. isolates. The tree topology shows the Haemogregarina sp. cloned sequences from this study within the Haemogregarina spp. clade, but in a separate branch clustered together, distinct from the previously reported sequences (Fig. 4).
Discussion
The alligator snapping turtle in this report was found in the winter in an emaciated state. The finding of intraerythrocytic parasites caused speculation regarding the possible impact of the infection on the turtle’s health. However, generally, infections by haemogregarine blood parasites are considered benign, despite being persistent (Davis and Sterrett 2011). Davis and Sterrett (2011) suggest that the high incidence of haemogregarine infections in aquatic turtles, averaging 70 % in North American published reports, is evidence of the benign effect of these parasites on these hosts.
Intracellular haematozoa (Order Eucoccidioida, suborder Adeleorina) were found at a low parasitemia in erythrocytes from the alligator snapping turtle in this study. The morphology and morphometric data for the parasite forms indicate conformity to Hemogregarina spp. previously reported in freshwater turtles in the USA (Davis and Sterrett 2011; Telford et al. 2009). The intraerythrocytic premeront and immature gamont in the current report are similar to the haemogregarine small and large forms, respectively, described in M. temminckii in Arkansas (McAllister et al. 1995). However, the medium form described as resembling a microgamont by McAllister and others (1995), was not seen in the blood film from the Texas alligator snapping turtle in this study.
The various trophozoites, premeronts, meronts, and gamonts observed in the current study generally resemble those seen in Haemogregarina macrochelysi n. sp. (Apicomplexa: Haemogregarinidae) from alligator snapping turtles in the states of Georgia and Florida (Telford et al. 2009). However, Telford et al. (2009) described H. macrochelysi n. sp. as set apart from all other described species by macromeronts that contained 150 or more nuclei in circulating blood. These were not seen in the Haemogregarina sp. in this current study; no more than 4 nuclei within a meront was observed. Another distinguishing feature between the two haemogregarines from this host is the appearance of the meront nuclei. Multiple nuclei within a meront were uniform in shape and size in H. macrochelysi n. sp., whereas they were irregular in the Haemogregarina sp. In the current study, encapsulated gamonts were observed, which were not reported for H. macrochelysi n. sp. (Telford et al. 2009). We were unable to determine the invertebrate vector for the Haemogregarina sp. in the alligator snapping turtle in this study. Whether the haemogregarine in the alligator snapping turtle in this current study is conspecific with H. macrochelysi n. sp. or with the haemogregarine previously described in the alligator snapping turtle in Arkansas remains to be definitively determined. While molecular comparisons would undoubtedly resolve this question, no genetic data are available for either the Arkansas isolate or H. macrochelysi n. sp. at this time.
Molecular characterization of the Haemogregarina sp. in the current study was based on 18S rRNA gene sequence analysis of five clones. As previously reported in cloned haemogregarine 18S rRNA genes (Perkins and Keller 2011), sequence variation was found among the five Haemogregarina sp. clones and none was identical to each other. Nonetheless, the clones were more like one another than to other reported 18S rDNA sequences for haemogregarines. Interestingly, the highest sequence identity (98-99 %) was to an unnamed Haemogregarina sp. found in the Caspian freshwater turtle Mauremys caspica in Fars Province in southern Iran (Rakhshandehroo et al. 2016). Only a short 18S rDNA sequence (774 bp) is available for this parasite (GenBank accession no. KR006985), precluding its incorporation in the phylogenetic analysis. H. balli found in the common snapping turtle Chelydra serpentina serpentina Linnaeus, 1758, might be expected to be more closely related to a Haemogregarina sp. of an alligator snapping turtle, but a comparison of this corresponding 18S rRNA gene fragment shows only 96.3 % identity between the two. Unfortunately, there are no genetic data from US haemogregarines available for comparison to those obtained in this study for the Haemogregarina sp. in the alligator snapping turtle.
This study entails morphological and molecular characterization of a Haemogregarina sp. in an alligator snapping turtle in Texas. The findings of this study compare with available morphological data of haemogregarine developmental stages previously described and provide the first genetic data for a Haemogregarina sp. in this hemisphere.
References
Acholonu A (1974) Haemogregarina pseudemydisn. sp. (Apicomplexa: Haemogregarinidae) and Pirhemocyton chelonarumn n. sp. in turtles from Louisiana. J Protozool 21(5):659–664
Altschul SF, Gish W, Miller EW, Myers DJ, Lipman (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410
Barta JR, Ogedengbe JD, Martin DS, Smith TG (2012) Phylogenetic position of the adeleorinid coccidia (Myzozoa, Apicomplexa, Coccidia, Eucoccidiorida, Adeleorina) inferred using 18S rDNA sequences. J Eukaryot Microbiol 59(2):171–180
Boundy J, Kennedy C (2006) Trapping survey results for the alligator snapping turtle (Macrochelys temminckii) in southeastern Louisiana, with comments on exploitation. Chelonian Conserv Biol 5(1):3–9
Cook CA, Smit NJ, Davies AJ (2009) A redescription of Haemogregarina fitzsimonsi Dias, 1953 and some comments on Haemogregarina parvula Dias, 1953 (Adeleorina: Haemogregarinidae) from southern African tortoises (Cryptodira: Testudinidae), with new host data and distribution records. Folia Parasitol 56(3):173–179
Criado-Fornelio A, Ruas JL, Casado N, Farias NAR, Soares MP, Müller G, Brum JGW, Berne MEA, Buling-Saraña A, Barba-Carretero JC (2006) New molecular data on mammalian Hepatozoon species (Apicomplexa: Adeleorina) from Brazil and Spain. J Parasitol 92(1):93–99
Davis A, Sterrett S (2011) Prevalence of haemogregarine parasites in three freshwater turtle species in a population in northeast Georgia, USA. Int J Zool Res 7(2):156–163
Dvořáková N, Kvičerová J, Hostovský M, Široký P (2015) Haemogregarines of freshwater turtles from Southeast Asia with a description of Haemogregarina sacaliae n. sp. and a redescription of Haemogregarina pellegrini Laveran and Pettit, 1910. Parasitology 142(06):816–826
Dvořáková N, Kvičerová J, Papoušek I, Javanbakht H, Tiar G, Kami H, Široký P (2014) Haemogregarines from western Palaearctic freshwater turtles (genera Emys, Mauremys) are conspecific with Haemogregarina stepanowi Danilewsky, 1885. Parasitology 141(04):522–530
Edney JM (1949) Haemogregarina stepanowi Danilewsky (1885) in middle Tennessee turtles. J Tenn Acad Sci 24(3):220–223
Ernst CH, Lovich JE (2009) Turtles of the United States and Canada. JHU Press, Baltimore
Ewert MA (1976) Nests, nesting and aerial basking of Macroclemys under natural conditions, and comparisons with Chelydra (Testudines: Chelydridae). Herpetologica 32:150–156
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Kopečná J, Jirků M, Oborník M, Tokarev YS, Lukeš J, Modrý D (2006) Phylogenetic analysis of coccidian parasites from invertebrates: search for missing links. Protist 157(2):173–183
Kvičerová J et al (2014) Hemolivia and Hepatozoon: haemogregarines with tangled evolutionary relationships. Protist 165(5):688–700
Levine ND (1982) Some corrections in haemogregarine (Apicomplexa: Protozoa) nomenclature. J Protozool 29(4):601–603
Lv Z, Wu Z, Zhang L, Ji P, Cai Y, Luo S, Wang H, Li H (2015) Genome mining offers a new starting point for parasitology research. Parasitol Res 114(2):399–409
Maia JP, Harris DJ, Carranza S, Gómez-Díaz E (2014) A comparison of multiple methods for estimating parasitemia of hemogregarine hemoparasites (Apicomplexa: Adeleorina) and its application for studying infection in natural populations. PLoS ONE 9(4):e95010. doi:10.1371/journal.pone.0095010
McAllister CT (2015) Hematozoa (Apicomplexa: Haemogregarinidae, Hepatozoidae) from two turtles (Testudines: Chelydridae, Emydidae) and two snakes (Ophidia: Colubridae, Viperidae) in southeastern Oklahoma. Proc Okla Acad Sci 95:119–124
McAllister CT, Upton SJ, Trauth SE (1995) Hemogregarines (Apicomplexa) and Falcaustra chelydrae (Nematoda) in an alligator snapping turtle, Macroclemys temminckii (Reptilia: Testudines), from Arkansas. J Helminthol Soc Wash 62(1):74–77
Merino S, Vásquez RA, Martínez J, Celis-Diez JL, Gutiérrez-Jiménez L, Ippi S, Sánchez-Monsalvez I, Martínez-De La Puente J (2009) Molecular characterization of an ancient Hepatozoon species parasitizing the ‘living fossil’marsupial ‘Monito del Monte’Dromiciops gliroides from Chile. Biol J Linnean Soc 98(3):568–576
Paterson WB, Desser SS (1976) Observations on Haemogregarina balli sp. n. from the common snapping turtle, Chelydra serpentina. J Protozool 23(2):294–301. doi:10.1111/j.1550-7408.1976.tb03775.x
Perkins SL, Keller AK (2001) Phylogeny of nuclear small subunit rRNA genes of hemogregarines amplified with specific primers. J Parasitol 87(4):870–876
Petit G, Landau I, Baccam D, Lainson R (1990) Description et cycle biologique d’ Hemolivia stellata n.g., n.sp., hémogrégarinede crapauds Brésiliens. Ann Parasitol Hum Comp 65:3–15
Powell R, Conant R, Collins JT (2016) Peterson field guide to reptiles and amphibians: eastern and central North America, 4th edn. Houghton Mifflin Harcourt, Boston
Rakhshandehroo E, Sharifiyazdi H, Ahmadi A (2016) Morphological and molecular characterisation of Haemogregarina sp. (Apicomplexa: Adeleina: Haemogregarinidae) from the blood of the Caspian freshwater turtle Mauremys caspica (Gmelin) (Geoemydidae) in Iran. Syst Parasitol 93(5):517–524
Siddall ME, Desser SS (1991) Merogonic development of Haemogregarina balli (Apicomplexa: Adeleina: Haemogregarinidae) in the leech Placobdella ornata (Glossiphoniidae), its transmission to a chelonian intermediate host and phylogenetic implications. J Parasitol 77(3):426–436. doi:10.2307/3283131
Siddall ME, Desser SS (1992) Prevalence and intensity of Haemogregarina balli (Apicomplexa: Adeleina: Haemogregarinidae) in three turtle species from Ontario, with observations on intraerythrocytic development. Can J Surg 70(1):123–128. doi:10.1139/z92-018
Sloboda M, Kamler M, Bulantová J, Votýpka J, Modrý D (2007) A new species of Hepatozoon (Apicomplexa: Adeleorina) from Python regius (Serpentes: Pythonidae) and its experimental transmission by a mosquito vector. J Parasitol 93(5):1189–1198
Smith TG (1996) The genus Hepatozoon (Apicomplexa: Adeleina). J Parasitol 82:565–585
Strohlein DA, Christensen BM (1984) Haemogregarina sp. (Apicomplexa: Sporozoea) in aquatic turtles from Murphy’s Pond, Kentucky. T Am Microsc Soc 103(1):98–101. doi:10.2307/3226539
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis, Version 6.0. Mol Biol Evol 30(12):2725–2729
Telford SR Jr (2009) Hemoparasites of the reptilia: color atlas and text. CRC Press, Boca Raton
Telford SR Jr, Norton TM, Moler PE, Jensen JB (2009) A new Haemogregarina species of the alligator snapping turtle, Macrochelys temminckii (Testudines: Chelydridae), in Georgia and Florida that produces macromeronts in circulating erythrocytes. J Parasitol 95(1):208–214
Troost G (1835) In: Harlan R (ed) Medical and physical researches; or, Original memoires in medicine, surgery, physiology, geology, zoology, and comparative anatomy. L.R. Bailey, Philadelphia
Wang CC, Hopkins SH (1965) Haemogregarina and Haemoproteus (Protozoa, Sporozoa) in blood of Texas freshwater turtles. J Parasitol 51:682–683
Xiao L, Escalante L, Yang C, Sulaiman I, Escalante AA, Montali RJ, Fayer R, Lal AA (1999) Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl Environ Microbiol 65(4):1578–1583
Acknowledgments
The authors thank the Caldwell Zoo in Tyler, Texas, for providing this sample for characterization. We thank Ann Buchanan, DVM, and Casey Plummer at the Caldwell Zoo in particular for communicating with us regarding this case. We thank Dr. Thomas M. Craig in the Department of Veterinary Pathobiology, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, for the helpful contributions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alhaboubi, A.R., Pollard, D.A. & Holman, P.J. Molecular and morphological characterization of a haemogregarine in the alligator snapping turtle, Macrochelys temminckii (Testudines: Chelydridae). Parasitol Res 116, 207–215 (2017). https://doi.org/10.1007/s00436-016-5280-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5280-2