Abstract
Sarcocystis spp. are protozoan parasites with a heteroxenous life cycle, which produce cysts in the muscle of herbivorous animals. In these animal species, sarcocystosis is frequently asymptomatic, although it may occur with high prevalence. Seven Sarcocystis spp. have been described in red deer (Cervus elephus). The aim of this study was to determine the prevalence of sarcocystosis, and to perform the morphological and molecular characterization of Sarcocystis spp. found in wild red deer of the Nahuel Huapi National Park (NHNP), Patagonia, Argentina. Full necropsies of 62 red deer killed by hunters in the NHNP and neighboring areas were performed. Samples of heart and skeletal muscle were examined histologically and selected samples were also examined by transmission electron microscopy (TEM), PCR and sequencing. Sarcocystis spp. thin walled cysts were detected in 62 % (38/62) of heart, and in 22 % (3/14) of skeletal muscle samples examined histologically. TEM revealed a smooth and thin cyst wall (≤1 μm), with scarce and separated ribbon-like protrusions. A total of three partial and one full 18S ribosomal RNA (rRNA) gene sequences were obtained, and showed the highest identity (≥99 %) with Sarcocystis taeniata, a species described in moose (Alces alces). The morphological and molecular results indicate that red deer in Argentina are frequently infected with S. taeniata, a species for which the definitive host is unknown. The present results also confirm that Sarcocystis spp. using cervids as intermediate host are not host-specific. Further studies are needed to improve the epidemiological knowledge of Sarcocystosis in red deer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Red deer (Cervus elaphus) have been introduced in several countries and have become a serious threat to wildlife and agriculture (Novillo and Ojeda 2008). These ungulates can carry and transmit pathogens of concern to livestock and wildlife species (Haigh et al. 2002; Mackintosh et al. 2002; Gortázar et al. 2006; Böhm et al. 2007). In addition, red deer can damage the local flora. In Argentina, environmental damage by introduced and invasive European red deer has been documented (Novillo and Ojeda 2008). However, information on diseases carried and transmitted by this introduced species is scant.
Sarcocystis spp. are intracellular protozoa with an heteroxenous life cycle, that produce muscle cysts in the intermediate hosts, such as domestic cattle, sheep, pigs, wild cervids, and also humans, and reproduce sexually in the intestine of definitive hosts (DH; several carnivorous or omnivorous animal species) (Dubey et al. 2015). Most Sarcocystis spp. infections (sarcocystosis) are asymptomatic, although disease may occur in intermediate hosts (Dubey et al. 2015; Avapal et al. 2004). Sarcocystis suihominis and Sarcocystis hominis, which infect pigs and cattle, respectively, have public health importance because humans are their definitive hosts in which nausea, abdominal pain and diarrhea have been reported (Tenter 1995; Fayer 2004; Solaymani-Mohammadi and Petri 2006).
Cervid animals are intermediate hosts of several Sarcocystis spp., some of which have unknown definitive hosts and other uses canids or felids as DH (Dubey et al. 2015). In North America and Europe, sarcocystosis has been reported in elk (Cervus canadensis), white-tailed deer (Odocoileus virginianus), mule deer (Odocoileus hemionus), roe deer (Capreolus capreolus), reindeer (Rangifer tarandus tarandus), moose (Alces alces) and red deer (Pond and Speer 1979; Mahrt and Colwell 1980; Crum et al. 1981; Dahlgren and Gjerde 2007; Dahlgren et al. 2008; Gjerde 2013; Dubey et al. 2015). Although no zoonotic Sarcocystis spp. has been reported in cervids the impact of sarcocystosis in these species has not been thoroughly investigated (Pond and Speer 1979; Mahrt and Colwell 1980; Crum et al. 1981; Dubey et al. 2015) and it is not known if Sarcocystis spp. is host-specific for cervid species. Thorough morphological descriptions, cross-infection studies, and/or DNA sequence data are necessary to achieve proper identification of Sarcocystis spp. (Dubey et al. 2015). Several Sarcocystis spp. are reported to affect European red deer. Among these are Sarcocystis cervicanis, Sarcocystis hjorti, Sarcocystis hardangeri, Sarcocystis ovalis, Sarcocystis elongata, Sarcocystis tarandi, and Sarcocystis truncata, most of which have unknown DH (Dahlgren and Gjerde 2010; Gjerde 2014a; Dubey et al. 2015).
In the United States, Pond and Speer (1979) have reported prevalences of sarcocystosis of 80 % (57/72) for mule deer, 50 % (12/24) for white-tailed deer, 50 % (12/24) for elk, and 13 % (2/15) for bison. In Canada, a health surveillance found a prevalence of the infestation of 100 % (2/2) for elk, 96 % (196/205) for moose, 94 % (17/18) for bison, 75 % (27–36) for mule deer, 75 % (3/4) for bighorn sheep (Ovis canadensis), 73 % (11/15) for mountain goats (Oreamnos americanus), and 49 % (137/277) for white-tailed deer (Mahrt and Colwell 1980). In Argentina, a prevalence of Sarcocystis cruzi cysts of 71.5 % (372/380), and thick-walled Sarcocystis spp. of 23.1 % (88/380) were reported in loin samples from cattle (Moré et al. 2011). Further molecular differentiation of Sarcocystis spp. in the same samples revealed 313, 29, 14, and 2 positive samples for S. cruzi, S. sinensis, S. hirsuta and S. hominis, respectively (Moré et al. 2013). In the same country, Sarcocystis spp. (species not identified) was found in guanacos (Lama guanicoe) with a prevalence of 67 % (8/12) (Beldomenico et al. 2003). However, to the best of our knowledge, the prevalence of sarcocystosis and the identification of the Sarcocystis spp. infecting wild deer has not been reported in Argentina.
The aim of this study was to determine the prevalence of sarcocystosis, and to perform morphological and molecular identification of the Sarcocystis spp. in wild red deer (C. elaphus) in Patagonia, Argentina.
Material and methods
Samples
Field work was conducted during the red deer hunting season in the Nahuel Huapi National Park (NHNP) and in livestock farms located close to this National Park, in the area of San Carlos de Bariloche and Junín de los Andes, Río Negro and Neuquén provinces, respectively, all in Northern Patagonia, Argentina.
Full necropsies were performed on 62 red deer, and samples of heart (n = 62) and skeletal muscle (n = 14) were collected and used for this study.
Histological examination
Sixty two hearts (apical area) and 14 skeletal muscle (gluteal muscles) samples were fixed by immersion in 10 % buffered formalin, pH 7.2, for 24 to 60 h before being embedded in paraffin wax, sectioned at 4 μm, and processed routinely for the production of hematoxylin and eosin stained sections.
Direct microscopic examination
Additional heart and skeletal muscle samples from red deer were collected and frozen at −20 °C. After histologic examination of all the samples was complete, only the heart samples from those animals in which Sarcocystis spp. were detected histologically, were thawed and processed for direct microscopic examination as previously described (Moré et al. 2011). Briefly, 5 to 10 g of each sample were ground in a tissue homogenizer with the addition of 50 ml phosphate buffered saline (PBS), pH 7.2. Two to three milliliter-aliquots of the homogenate were placed in a Petri dish, diluted with 10 ml of PBS and observed in an inverted microscope. Sarcocysts or cysts parts observed were counted, and each sample was categorized according to the presence of low (1–2 cysts/plate), moderate (3–5 cysts/plate), or abundant (≥6 cysts/plate) numbers of cysts.
To collect sarcocysts for further analyzes, five fresh samples of skeletal muscle (gluteal muscles) and the same numbers of samples from heart were refrigerate (4–8 °C), processed as sample of pooled muscles for each animal within 1 week, and then used for direct microscopic examination following the methodology mention above.
Transmission electron microscopy
Ten sarcocysts detected during direct examination of refrigerated sample of pooled muscles homogenates from 3 different animals were collected using micropipettes, fixed in 2 % glutaraldehyde, processed and analyzed by transmission electron microscopy (TEM) as previously described (Moré et al. 2011).
PCR and sequencing
DNA extraction was performed from 10 individual sarcocysts collected during direct microscopic examination of the refrigerated samples, using a commercial kit (Wizard Genomic, Promega, USA) according manufacturer’s instruction. A fragment of the Sarcocystis spp. 18S ribosomal RNA (rRNA) gene was amplified by polymerase chain reaction (PCR) using the primers SarcoFext and SarcoRext previously described (Moré et al. 2013). Amplification products (with an estimated concentration of at least 40 ng/μl) were purified using the QIAQUICK purification kit (QIAGEN, Germany) and submitted for sequencing to the Lightrun service of GATC Biotech with both primers mentioned above. Additionally, two positive samples in the mentioned PCR were amplified with primers ERIB1 and PrimerB in order to amplify the full-length sequence of the 18S rDNA of most Sarcocystis spp. (∼1850 bp). These products were purified, cloned into plasmids and sequenced as previously described (Moré et al. 2013). Sequences obtained were aligned and analyzed using the Geneious software. Consensus sequences obtained were compared with others reported in GenBank by BLAST analysis.
Results
Histology and direct microscopic examination
Sarcocystis spp. cysts were observed in HE sections of 38/62 (62 %) of heart (Fig. 1) and in 3/14 (22 %) of skeletal muscle samples. The sarcocysts were always seen within skeletal or cardiac muscle fibers which in most cases showed no muscle fiber degeneration, although segmental hyaline change was occasionally seen in infected muscular fibers. All cysts contained numerous mature banana-shape bradyzoites. Three out of 38 heart samples containing sarcocysts showed mild, interstitial, focal, lymphoplasmacytic myocarditis, while one of those 38 samples showed steatosis. Hyalin degeneration of fibers was observed in the three skeletal muscle samples containing sarcocysts but not in any of the infected myocardial fibers. Very few lymphocytes were occasionally present surrounding the infected muscle cells.
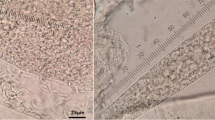
By direct microscopic examination of 34 heart samples (four samples were not analyzed due to insufficient amount of tissue), Sarcocystis spp. infection was categorized as low in 44 % (15/34), moderate in 27 % (9/34), and abundant in 29 % (10/34). All five sample of pooled muscles evidenced microscopical sarcocysts. All sarcocysts observed were fusiform, clearly divided by septa and measured up to 700 μm long and 40–90 μm wide. The sarcocyst wall was thin (≤1 μm) and had scarce and hair-like villar protrusions (Fig. 2).
All the cysts processed by TEM had a smooth and thin wall (≤1 μm), with scarce and separated ribbon-like protrusions (vp). The vp were 0.5–1 μm long and 0.1–0.2 μm thick (Fig. 3). The ground substance layer measured 0.5 μm and lacked microtubules. Bradyzoites contained numerous micronemes located in the anterior half and amylopectin granules located in the posterior half (Fig. 3).
Transmission electron microscopical (TEM) examination of the cyst wall from a microscopical sarcocyst in reed deer (C. elaphus). Note the scarce ribbon-like villar protrusions (vp), a thin ground substance layer (gs), and sectioned bradyzoites (br) containing several electron lucid amylopectin granules (a)
PCR and sequencing
A total of three consensus sequences (857, 845 and 765 bp) were obtained from different samples amplified with SarcoFext/SarcoRext and deposited in the GenBank (accession numbers KT626599, KT626600, and KT626601). The three sequences showed the highest identity (≥99 %) with Sarcocystis taeniata sequences (KF831279, KF831292, among others) and also 98 % with a Sarcocystis grueneri sequence (KC556825). Two cloned amplification products were obtained and identical consensus sequences of 1886 bp were obtained; BLASTn comparison revealed a 99 % sequence identity with S. taeniata sequences (KF8312895, KF8312896, KF831289, among others). The full-length 18S rRNA gene sequence obtained in the present study was deposited in the GenBank with the accession number KT626602.
Discussion
The results of morphological and molecular studies conducted on sarcocysts obtained in the present study lead to the identification of S. taeniata, a species previously described in moose (A. alces) from Canada (Gjerde 2014b), but none of the Sarcocystis spp. previously described in red deer was identified. S. taeniata was recently described in moose, cyst wall morphology was based only in scanning electron microscopy (SEM), which did not allow to differentiate it from S. grueneri (Gjerde 2014b). Differentiation between these two species was achieved by 18S rRNA and cox1 gene sequence comparison (Gjerde 2014b). In the present study, the scarce and thin ribbon-like villar protrusions detected in the TEM observation are in agreement with the previous S. taeniata SEM images from Gjerde in 2014. Additionally, the 18S rRNA sequences obtained in the present study showed the highest identity (≥99 %) with S. taeniata sequences in GenBank. Phylogenetic analysis of the mentioned sequences evidenced a close relation with S. grueneri sequences from reindeer (R. t. tarandus) (Gjerde 2014b). This species of Sarcocystis uses reindeer as intermediate host, and fox (Vulpes vulpes and Vulpes lagopus), raccoon-dog (Nyctereutes procyonides) and dog as DH (Dubey et al. 2015). The DH of S. taeniata remains unknown. Based on these similarities, it is possible to suggest that S. taeniata uses canids as DH. Experimental infection and molecular studies are required to confirm/rule out this hypothesis.
In the UK, an increase in the prevalence of Sarcocystis spp. was reported in free-range deer (Böhm et al. 2006) over a 7-year period. It has been suggested that this condition may reflect the high animal concentration and frequent contact of affected animals with the DH. In Patagonia, although the evolution of the prevalence of sarcocystosis is not known, the high prevalence of Sarcocystis spp. infection in heart (62 %) identified in red deer in this study, suggests that there is also a frequent contact between the DH and deer carcasses. Additionally, the selection of hearth tissues sampled in the present study could have an influence in the outcome of S. taeniata, which may have a “predilection” of myocardium in red deers.
It is known that a major cause of death of European red deer in Patagonia is hunting and predation by pumas (Felis concolor) (Flueck et al. 2005; Novillo and Ojeda 2008). It is therefore possible that pumas play a role as definitive host of Sarcocystis spp., and also that hunting activity (which usually involves abandoning significant parts of the deer carcasses in the field) could increase access to those carcasses by other potential DH, such as feral dogs (Canis lupus familiaris), Andean fox (Lycalopex culpaeus), Patagonian weasel (Lyncodon patagonicus), or scavenger birds such as the Andean condor (Vultur gryphus) and Southern crested caracara (Caracara plancus). More studies are needed in order to identify properly other Sarcocystis spp. affecting wild red deers in Argentina.
Most samples analyzed in the present study showed cysts in the muscle which were not associated with gross or microscopic lesions; however, a few samples showed focal lymphoplasmacytic myocarditis and hyaline degeneration of skeletal muscle. Similar lesions have been reported in experimental infections with S. cruzi in calves and with Sarcocystis tenella in lambs (Dubey et al. 2015)as well as with Sarcocystis spp. in rocky montain elk calves (Foreyt et al. 1995) .
The result of the present study indicates that wild European reed deer in Argentina are frequently infected with S. taeniata, a species which affects also moose. The present study represents the first report of S. taeniata in the Southern Hemisphere and in a different cervid species than moose. Moreover, the present results support the hypothesis that Sarcocystis spp. from wild cervids could affect more than one cervid animal species as intermediate host, as S. taeniata, which was thought to infect only moose, has now been demonstrated to infect also red deer.
The potential for Sarcocystis taeniata of deer to be transmitted to native wildlife and livestock, as well as its potential zoonotic potential requires further investigation.
References
Avapal RS, Sharma JK, Juyal PD (2004) Pathological changes in Sarcocystis infection in domestic pigs (Sus scrofa). Vet J 168(3):358–61. doi:10.1016/j.tvjl.2003.11.006
Beldomenico PM, Uhart M, Bono MF, Marull C, Baldi R, Peralta JL (2003) Internal parasites of free-ranging guanacos from Patagonia. Vet Parasitol 118:71–7. doi:10.1016/j.vetpar.2003.09.008
Böhm M, White PC, Daniels MJ, Allcroft DJ, Munro R, Hutchings MR (2006) The health of wild red and sika deer in Scotland: an analysis of key endoparasites and recommendations for monitoring disease. Vet J 171(2):287–94. doi:10.1016/j.tvjl.2004.10.020
Böhm M, White PCL, Chambers J, Smith L, Hutchings MR (2007) Wild deer as a source of infection for livestock and humans in the UK. Vet J 174:260–276. doi:10.1016/j.tvjl.2006.11.003
Crum JM, Fayer R, Prestwood AK (1981) Sarcocystis spp. in white tail deer I. Definitive and intermediate host spectrum with a description of Sarcocystis odocoileo canis n.sp. J Wildl Dis 17(4):567–79
Dahlgren SS, Gjerde B (2007) Genetic characterisation of six Sarcocystis species from reindeer (Rangifer tarandus tarandus) in Norway based on the small subunit rRNA gene. Vet Parasitol 146(3–4):204–13. doi:10.1016/j.vetpar.2007.02.023
Dahlgren SS, Gjerde B (2010) Molecular characterization of five Sarcocystis species in red deer (Cervus elaphus), including Sarcocystis hjorti n. sp., reveals that these species are not intermediate host specific. Parasitol 137(5):815–40. doi:10.1017/S0031182009991569
Dahlgren SS, Gouveia-Oliveira R, Gjerde B (2008) Phylogenetic relationships between Sarcocystis species from reindeer and other Sarcocystidae deduced from ssu rRNA gene sequences. Vet Parasitol 151(1):27–35. doi:10.1016/j.vetpar.2007.09.029
Dubey JP, Calero-Bernal R, Rosenthal BM, Speer CA, Fayer R (2015) Sarcocystosis of animals and humans. CRC Press, Boca Raton FL, USA
Fayer R (2004) Sarcocystis spp. in human infections. Clin Microbiol Rev 17:894–902. doi:10.1128/CMR.17.4.894-902.2004
Flueck WT, Smith-Flueck JM, Bonino N (2005) A preliminary analysis of cause specific and capture-related mortality and survival of adult red deer in northwestern Patagonia. Ecol Austral 15:23–30
Foreyt WJ, Baldwin TJ, Lagerquist JE (1995) Experimental infections of Sarcocystis spp. in Rocky Mountain elk (Cervus elaphus) calves. J Wildl Dis 31(4):462–6
Gjerde B (2013) Phylogenetic relationships among Sarcocystis species in cervids, cattle and sheep inferred from the mitochondrial cytochrome c oxidase subunit I gene. Int J Parasitology 43(7):579–91. doi:10.1016/j.ijpara.2013.02.004
Gjerde B (2014a) Sarcocystis species in red deer revisited: with a re-description of two known species as Sarcocystis elongata n. sp. and Sarcocystis truncata n. sp. based on mitochondrial cox1 sequences. Parasitol 141(3): 441–52. doi: 10.1017/S0031182013001819
Gjerde B (2014b) Morphological and molecular characteristics of four Sarcocystis spp. in Canadian moose (Alces alces), including Sarcocystis taeniata n. sp. Parasitol Res 113(4):1591–604. doi:10.1007/s00436-014-3806-z
Gortázar C, Acevedo P, Ruiz-Fons F, Vicente J (2006) Disease risk and overabundance of game species. Eur J Wild Res 52:81–87. doi:10.1007/s10344-005-0022-2
Haigh JC, Mackintosh CG, Griffin F (2002) Viral, parasitic, and prion diseases of farmed deer and bison. Rev Sci Tech Off Int Epiz 21(2):219–48
Mackintosh CG, Haigh JC, Griffin F (2002) Bacterial diseases of farmed deer and bison. Rev Sci Tech Off Int Epiz 21(2):249–63
Mahrt JL, Colwell DD (1980) Sarcocystis in wild ungulates in Alberta. J Wildl Dis 16(4):571–6
Moré G, Abrahamovich P, Jurado S, Bacigalupe D, Marin JC, Rambeaud M, Venturini L, Venturini MC (2011) Prevalence of Sarcocystis spp. in Argentinean cattle. Vet Parasitol 177(1–2):162–5. doi:10.1016/j.vetpar.2010.11.036
Moré G, Schares S, Maksimov A, Conraths FJ, Venturini MC, Schares G (2013) Development of a multiplex real time PCR to differentiate Sarcocystis spp. affecting cattle. Vet Parasitol 197:85–94. doi:10.1016/j.vetpar.2013.04.024
Novillo A, Ojeda RA (2008) The exotic mammals of Argentina. Biol Invasions 10:1333–44. doi:10.1007/s10530-007-9208-8
Pond DB, Speer CA (1979) Sarcocystis in free-ranging herbivores on the National Bison Range. J Wildl Dis 15(1):51–3
Solaymani-Mohammadi S, Petri WA Jr (2006) Zoonotic implications of the swine-transmitted protozoal infections. Vet Parasitol 140(3–4):189–203. doi:10.1016/j.vetpar.2006.05.012
Tenter AM (1995) Current research on Sarcocystis species of domestic animals. Int J Parasitol 25(11):1311–30
Acknowledgments
We would like to thank Isidoro Ercoli for excellent technical assistance. The authors also thank hunters, hunting guides, park rangers, and farmers from the Nahuel Huapi National Park for providing assistance during field campaigns; the Weiss Smoking Plant at San Carlos de Bariloche city, for allowing access to red deer carcass; and the Argentinean National Park Administration (APN) for research permission (no. 721, no. 1339).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Funding
This work was financially supported by the Wildlife Health Fund, Wildlife Conservation Society, USA; the Rufford Small Grant for Nature Conservation, Rufford Foundation (RSG 3802–07, 5738–1, 15445-B), UK; the Laboratory of Immunoparasitology, Faculty of Veterinary Sciences, National University of La Plata, Argentina; the California Animal Health and Food Safety Laboratory, University of California, San Bernardino, Davis, CA.
Rights and permissions
About this article
Cite this article
Reissig, E.C., Moré, G., Massone, A. et al. Sarcocystosis in wild red deer (Cervus elaphus) in Patagonia, Argentina. Parasitol Res 115, 1773–1778 (2016). https://doi.org/10.1007/s00436-016-4915-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-4915-7