Abstract
Escape behaviour is the behaviour that birds and other animals display when already caught by a predator. An individual exhibiting higher intensity of such anti-predator behaviour could have greater probabilities of escape from predators. Parasites are known to affect different aspects of host behaviour to increase their own fitness. Vector-transmitted parasites such as malaria parasites should gain by manipulating their hosts to enhance the probability of transmission. Several studies have shown that malaria parasites can manipulate their vectors leading to increased transmission success. However, little is known about whether malaria parasites can manipulate escape behaviour of their avian hosts thereby increasing the spread of the parasite. Here we used an experimental approach to explore if Plasmodium relictum can manipulate the escape behaviour of one of its most common avian hosts, the house sparrow Passer domesticus. We experimentally tested whether malaria parasites manipulate the escape behaviour of their avian host. We showed a decrease in the intensity of biting and tonic immobility after removal of infection with anti-malaria medication compared to pre-experimental behaviour. These outcomes suggest that infected sparrows performed more intense escape behaviour, which would increase the likelihood of individuals escaping from predators, but also benefit the parasite by increasing its transmission opportunities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In any animal population, there are behavioural differences among individuals, where some individuals are bolder, more aggressive or more sociable than others. Although individuals may change their aggressiveness or boldness depending on the ecological situation (e.g. predation risk or parasitism), the behaviour of certain individuals is consistent over time and across situations (Gosling 2001; Sih et al. 2004; Réale et al. 2010). Predation is a major selection pressure that determines the form and the behaviour of animals (Endler 1991; Lima 1998). Therefore, any animal whose behaviour facilitates avoidance of encounters with predators or survival of attacks will increase its fitness (Lind and Cresswell 2005). In birds, escape behaviour is displayed when an individual is already caught by a predator (Møller et al. 2011). An individual exhibiting such anti-predator behaviour increases the probability of being released by biting, struggling, losing feathers, emitting alarm or distress calls or displaying tonic immobility (Högstedt 1983; Edelaar et al. 2012). Hence, individuals with higher intensity of escape behaviour may have enhanced probabilities of escape from predators (Møller et al. 2011).
Parasites exert intense selection on their avian hosts (Loye and Zuk 1991). Therefore, natural selection is expected to favour parasites with mechanisms that enhance their transmission success. Parasites can affect aspects of host behaviour in ways that increase their own fitness (Moore 2002; Schmid-Hempel 2011). The behavioural manipulation hypothesis posits that manipulation of host behaviour by parasites confer fitness benefits to the parasite, usually by increasing transmission success compared to conspecifics that are unable to modify host behaviour (Lefèvre et al. 2008; Poulin 2010).
Avian malaria and related haemosporidians are abundant and diverse parasites infecting several hundred species of birds in almost all continents. Plasmodium species are among the most pathogenic species of avian malaria, being responsible for mass mortality, population declines and even extinctions of many bird species (Van Riper et al. 1986; Valkiūnas 2005). These parasites are transmitted from infected to uninfected hosts by blood-sucking arthropods. Their life cycles are complex, involving sexual stages in the vector and asexual stages in the vertebrate host. Theory predicts that parasites that are dispersed by vectors should gain by manipulating their vector to their own advantage (Schmid-Hempel 2011). Thus, Plasmodium gallinaceum is able to increase the biting rate of its vector, the mosquito Aedes aegypti, leading to an increase in its transmission success (Koella et al. 2002). Moreover, an increase in the attractiveness of infected hosts to the vector should also enhance the probability of transmission from the vertebrate host to the arthropod vector (Hamilton and Hurd 2002). Following this idea, Cornet et al. (2013) experimentally demonstrated that infected birds attract significantly more vectors than uninfected ones, suggesting that malaria parasites manipulate the behaviour of vectors to increase their own transmission. Furthermore, alterations in the behaviour of vertebrate hosts may also benefit parasite transmission. For example, experimental mice carrying Plasmodium gametocytes in their blood showed the weakest mosquito-repellent behaviour, thus allowing mosquitoes to ingest infective forms of the malaria parasites and contributing to transmission of the malaria parasites (Day and Edman 1983).
However, little is known about whether malaria parasites can manipulate escape behaviour of their avian hosts with the aim of promoting the spread of the parasite. So far, only a recent study has explored the association between haemosporidian infection and escape behaviour of avian hosts. Garcia-Longoria et al. (2014) showed a positive relationship between the prevalence of Leucocytozoon and Haemoproteus and the intensity of host escape behaviour in 89 species of birds. Here we explore if Plasmodium relictum, a widespread and highly pathogenic haemosporidian parasite, can manipulate the escape behaviour of one of its most common avian hosts, the house sparrow Passer domesticus (Marzal et al. 2011). With this aim, we conducted an experimental study of this bird–malaria system. We experimentally tested whether malaria parasites can manipulate the escape behaviour of their avian host by breaking up any correlation with potentially confounding variables. If Pl. relictum is able to manipulate anti-predator behaviour in order to increase its probability of transmission, then we should predict a decrease in the intensity of escape behaviour after removal of the infection with anti-malaria medication.
Materials and methods
Study site and sample collection
The study was carried out in a population of house sparrows in the university campus of Badajoz (38° 52′ N, 6° 58′ W), southwest Spain, during November–December 2012. We captured 70 adult house sparrows with mist nets and recorded their body mass with a Pesola spring balance to the nearest 0.1 g. All birds were individually identified with numbered metal rings. One microcapillary of blood (70 μl) was obtained from the brachial vein of each individual and stored in 500 μl of SET buffer (0.15 M NaCl, 0.05 Tris, 0.001 M EDTA, pH 8.0) until DNA extraction. We also obtained a blood smear from each individual in order to estimate parasite intensity (the number of parasites per individual host). Seven behavioural variables (see below for detailed description) were assessed before the bird was bled and released in the aviaries of the Experimental Garden in the University of Extremadura. After initial molecular screening of haemosporidian infection, we selected 32 haemosporidian-infected sparrows and 38 non-infected individuals for experiments.
Anti-malaria treatment
House sparrows were placed in the aviary where each cage (3.5 × 1.5 × 2.5 m) contained a maximum of eight individuals. Birds were provided with water and food ad libitum and kept for 2 weeks in the aviaries in order to achieve acclimatisation. Due to a possible influence of drugs on the behaviour of sparrows, individuals were randomly assigned to one of two treatments independently of infection status: (1) a control group of 33 individuals (14 infected + 19 non-infected sparrows) that were injected with 0.2 ml phosphate-buffered saline (PBS), or (2) an experimental group of 37 individuals (18 infected + 19 non-infected sparrows) that were subcutaneously injected with 0.02 mg of primaquine + 0.4 mg of chloroquine diluted in 0.2 ml of saline solution (Remple 2004). We also provide a Malarone™ treatment with fixed-dose combination of 250 mg of atovaquone and 100 mg of proguanil hydrochloride to individuals of the treatment group (Palinauskas et al. 2009). One dose contained 0.24 mg of Malarone™ dissolved in 50 μl of drinking water in the water dispensers. We provided the same quantity of water in the dispensers of the control group, but without Malarone. Four infected birds died 2 weeks after inoculation, so the sample size of the control and treatment group was finally reduced to 31 (12 infected + 19 non-infected sparrows) and 35 (16 infected + 19 non-infected sparrows) individuals, respectively. Immediately before treatment, we recorded body mass of all individuals and took a second blood sample for haemosporidian analysis.
Finally, 3 weeks after inoculation, we took a third blood sample to verify the effectiveness of the anti-malaria treatment. In addition, body mass and the seven behavioural variables were recorded again to assess for an effect of haemosporidian infection on body mass and escape behaviour of individuals.
Intensity of blood parasites
Blood samples were fixed in absolute methanol and stained with Giemsa. The intensity of Plasmodium parasites was quantified as the number of parasites per 10,000 erythrocytes under ×1000 magnification with oil immersion (Godfrey et al. 1987).
Molecular detection of blood parasite infections
Haemosporidian parasites (Plasmodium spp. and Haemoproteus spp.) were detected from blood samples using molecular methods (Bensch et al. 2000; Waldenström et al. 2004). DNA from the avian blood samples were extracted in the lab using the standard phenol/chloroform/isoamylalcohol method (Sambrook et al. 2002). Diluted genomic DNA (25 ng/μl) was used as a template in a polymerase chain reaction (PCR) assay for detection of the parasites using nested PCR protocols described by Waldenström et al. (2004). The amplification was evaluated by running 2.5 μl of the final PCR on a 2 % agarose gel. All PCR experiments contained one negative control for every eight samples. In the very few cases of negative controls showing signs of amplification (never more than faint bands in agarose gels), the whole PCR batch was run again to make sure that all positives were true. Positive amplifications were sequenced in order to select the individuals infected by Pl. relictum.
Behavioural variables
Seven aspects of escape behaviour were assessed before a bird was bled. All these variables were recorded in the aviary before and after treatment and were always assessed by the same person (LGL). Several of these variables have been associated with susceptibility to predation by hawks and cats (Møller et al. 2011). It is important to emphasise that the different components of escape behaviour are mostly independent of each other (Møller et al. 2011). The seven behavioural variables were defined as follows:
-
1.
Wriggle score. We scored how much the bird struggled while being held in a hand (a score of 0, no movement; 1, moves rarely; 2, moves regularly, but not always; 3, moves continuously). Individuals that wriggle more may more readily escape from a predator compared to individuals that stay calm.
-
2.
Biting. Whether the bird did not bite, when we held our right hand index finger in front of the beak, we gave a score of 0, and if it did a score of 1. We presume that a higher frequency of biting entails an elevated probability of escape from a predator because the predator loses its grip when re-directing its attention towards the biting prey.
-
3.
Feather loss. While the bird was handled, if it lost feathers, we gave a score of 1, or 0 if it did not. Feather loss may result in predators losing their grip of a prey (Møller et al. 2006).
-
4.
Fear scream. While the bird was handled, if it gave a fear scream (a score of 1) or not (a score of 0). Birds giving fear screams attract the attention of secondary predators thereby increasing the probability of escape once captured (Högstedt 1983; Møller and Nielsen 2010).
-
5.
Tonic immobility. At the end of the above procedure, we placed the bird, just before it was released, with our right hand on its back on our flat left hand. When the bird was lying still, we removed the right hand and recorded the time until the bird righted itself and flew away. We allowed tonic immobility up to 30 s, and if the bird had not left yet, we terminated the trial. Tonic immobility is a standard measure of fear in poultry research with both environmental and genetic components (Hoagland 1928; Jones 1986; Boissy 1995; Forkman et al. 2007). Recently, Edelaar et al. (2012) showed that tonic immobility is related to personality and anti-predation behaviour because it is a measure of boldness towards predators. The longer time a bird stays, the higher its level of fear. Tonic immobility has a strongly bimodal distribution, with most individuals having tonic immobility of 0–5 s, but some 10–20 % having 25–30 s (Møller et al. 2011).
-
6.
Alarm call. When the bird departed from our hand whether it gave an alarm call (a score of 1) or not (a score of 0). It has been suggested that the function of this call is to distract the predator or to warn conspecifics (Charnov and Krebs 1975; Platzen and Magrath 2004).
-
7.
Breath rate. Carere and van Oers (2004) showed that breath rate is a signal of acute stress in birds when handled. We recorded the number of inhalations during 30 s while the bird was held in the hand. According to Carere et al. (2001), we should expect a higher number of inhalations with a higher level of fear.
Statistical procedures
Before the trials, escape behaviours were assessed for the same individuals with a 6-day interval, and we calculated the repeatability of these behavioural variables in order to confirm the reliability of our measurements. We used the program R-2.15 (R Development Core Team 2011) with the rptR package to calculate the repeatability of binary variables by using a GLMM with logit-link and multiplicative overdispersion (Nakagawa and Schielzeth 2010). We used an ANOVA to determine differences in quantitative variables (body mass) and chi-square tests to determine differences in qualitative variables (wriggle score, biting, tonic immobility, feather loss and alarm call) between infected and non-infected individuals. Previous studies have criticised the use of Bonferroni correction of multiple statistical tests (Moran 2003; Nakagawa 2004; Garamszegi 2006; Garamszegi et al. 2009) because it could increase the risk of committing type II errors (Nakagawa and Cuthill 2007). It has been suggested that effect sizes and confidence intervals (CIs) reliably reveal the biological importance of results (Nakagawa 2004). To calculate effect sizes, we used the corresponding df to calculate r and standard errors (based on r) to approximate the corresponding CIs (Nakagawa and Cuthill 2007). 95 % CIs are presented as lower/upper limits. We also present significance levels for illustrative purposes. In behavioural studies, the following benchmark is used for interpretations: r ≈ 0.1 is a small effect, r ≈ 0.3 an intermediate effect and r ≈ 0.5 a strong effect (Cohen 1988; Møller and Jennions 2002). The magnitude of effects in biological studies is typically intermediate accounting for 5–7 % of the variance, thus being equivalent to effect sizes of 0.22–0.26 (Møller and Jennions 2002).
With the aim to test the hypothesis that malaria infection could modify the escape behaviour of house sparrows, we analysed the variation in escape behaviour before and after the administration of anti-malaria medication or placebo in malaria-infected sparrows (N = 56 observations taken from 28 individuals). We also tested this hypothesis separately in non-infected sparrows that received anti-malaria medication or placebo (N = 76 observations from 38 individuals). Therefore, the second group was used as a second control to ensure that the application of the medication was not the cause of possible changes in escape behaviour. We used a linear mixed model approach to analyse these two subsets of data. The dependent variables were the behavioural variables modelled with the appropriate error structure and link functions (i.e. binomial errors for biting, feather loss, alarm call, and distress, and Poisson errors for wriggle and tonic immobility). The input variables introduced in the models were body mass, tarsus length, sex, time (included as a factor with two levels, i.e. before–after the application of the treatment), treatment (PBS medication), and the interaction term between time and treatment. Body mass and tarsus length were included to control for difference among individuals in body condition that could influence escape behaviour and, therefore, they were treated as confounding variables. We predicted that if the treatment have an effect on behaviour, then the interaction term between treatment and time should be statistical significant. The significance of this interaction term was tested comparing with a likelihood ratio test (LRT) the global model with a reduced model on which the interaction term was previously removed leaving all other input variables in the reduced model. In these models, we also included the aviary and subject identification as random terms to control for these source of variation and to control for pseudo-replication caused because we have two observations of escape behaviour (i.e. before and after the application of the treatment) taken from the same individual. The models concerning the dependent variables “alarm call” and “feather loss” did not converge because the data was unbalanced with very few experimental or control individuals giving an alarm call or losing a feather. Therefore, we excluded these escape behavioural variables in the analyses concerning our experimental approach.
Ethical note
All the experiments comply with the current laws of Spain, where the experiments were performed.
Results
Repeatability of behavioural variables
Repeatability based on multiple measures for each behavioural variable within individuals is reported in Table 1. Six out of seven measures of escape behaviour of individuals at repeated captures were consistent (repeatabilities ranged from 0.53 to 0.93). Only breath rate did not have a high repeatability. Hence, we excluded breath rate from further analyses in order to avoid biased results.
Relationship between malaria parasites and escape behaviour
Effects of medication on prevalence and intensity of blood parasite infections
As expected from the anti-malarial treatment, there was a decrease in prevalence of Plasmodium infection in all the medicated sparrows with an intermediate effect (McNemar N = 16; χ 2 15 = 14.06, P < 0.001, r = 0.741, 95 % CI = 0.389/0.904), while for controls, there was no change in prevalence of infection (McNemar N = 12; χ 2 11 = 0.001, P = 1.00, r = 0.000, 95 % CI = −0.442/0.442). Likewise, parasite load decreased in infected individuals from the experimental group (change in parasite load (standard error, SE) = 12.11 (22.78); Wilcoxon matched-pairs signed-ranks test, N = 16; z = −3.73, P = 0.000, r = 0.678, 95 % CI = 0.337/0.862), while it remained similar in infected individuals from the control group (change in parasite load (SE) = 15.00 (28.20); Wilcoxon matched-pairs signed-ranks test, N = 12; z = −1.28, P = 0.201, r = 0.231, 95 % CI = −0.165/0.654).
Effects of the malaria infection in escape behaviour
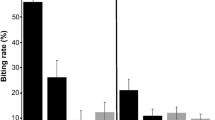
We found that two out of the seven variables related to escape behaviour changed in infected house sparrows treated with anti-malaria medication. On the contrary, the escape behaviour of non-infected house sparrows did not change after the medication. Specifically, we found that the probability of biting significantly decreased in malaria infected birds after the experimental reduction of the prevalence and intensity of Pl. relictum (Fig. 1). This was supported because the interaction term between time and treatment was statistically significant (LRT = 4.59, df = 1, P = 0.032) (Table 2). Likewise, we found that the tonic immobility significantly increased in infected sparrows that received an anti-malaria treatment (Fig. 2). This was supported because the interaction term between time and treatment was statistically significant (LRT = 47.7, df = 1, P < 0.001) (Table 2). These results were not confounded by either body condition of the birds before treatment or the aviaries where the experiment was performed, because we controlled for this source of variation in our mixed model approach (Table 2).
On the contrary, we found no effect of the anti-malaria medication per se on the escape behaviour of sparrows. In this sense, any of the variables related to escape behaviour significantly varied among the non-infected sparrows after the administration of anti-malaria medication or PBS (all P > 0.1) (Table 3). These results were not confounded by either body condition of the birds before treatment or the aviaries where the experiment was performed, because we controlled for this source of variation in our mixed model approach (Table 3).
Discussion
The behavioural manipulation hypothesis posits that parasites can change the behaviour of their hosts to their own selective advantage, usually by increasing their reproductive fitness (Thomas et al. 2005; Lefèvre et al. 2008). Here we have tested this hypothesis using an experimental approach in a bird host–malaria system. We clearly showed that biting and tonic immobility changed after clearance of the infection. Moreover, we showed that the anti-malaria drug on its own did not provoke a change in behaviour of the host.
Individual measurements of escape behaviour in birds when captured by a human were consistent across repeated measurements in a population of wild-caught house sparrows. The repeatability of escape behaviour was high for behavioural traits, which confirms the validity of our field estimates, as did previous studies of repeatability of animal behaviour (Dingemanse 2002; Biro 2012).
Avian malaria parasites were considered for many years to have low pathogenicity because many studies failed to show a correlation between haemosporidian infections and fitness components of their hosts (Fallis and Desser 1997; Dufva and Allander 1995; Dawson and Bortolotti 2000). However, the demonstration of effects of parasites requires an experimental approach (Keymer and Read 1991; Marzal et al. 2005; Knowles et al. 2010). Interestingly, we found that two components of anti-predator escape behaviour changed with the reduction in malaria infection. Specifically, medicated sparrows showed a lower frequency of biting behaviour and spent more time in tonic immobility before flying away. We also found that body mass of infected birds increased after treatment with an anti-malaria drug. However, non-infected individuals also increased their body mass, independently to the treatment. Therefore, the change in behaviour we found was independent of change in body mass since also non-infected individuals change their body mass during the course of the study. Additionally, we did not find any change in behaviour in non-infected individuals treated either with anti-malaria medication or PBS, as expected. The direction of the change in behaviour in infected individuals treated with anti-malaria medication shows a trend towards parasite-free individuals being more scared or shy. Numerous studies have shown that parasites can modify specific anti-predator behaviours to increase their own fitness (see reviews in Moore 2002; Lafferty and Shaw 2013). These modifications include examples in which parasites alter the behaviour of their intermediate hosts in ways that favour predation of infected hosts, thus enhancing trophic transmission. For example, a rodent infected with Toxoplasma gondii is known to lose fear against predators (definitive host), thus increasing the transmission of the parasite to its final host (Berdoy et al. 2000; Vyas et al. 2007; Webster and McConkey 2010). In the case of malaria parasites, the death of an infected host by a predator entails the end of transmission of the parasite. Here, we showed that sparrows were more aggressive against the predator and performed more intense escape behaviour when infected with malaria parasites. Theoretically, these changes in host behaviour should benefit the parasite. In this sense, intense escape behaviour that allows a bird to escape from a predator attack would increase the likelihood of survival (Møller et al. 2011). For example, we can assume that a bird biting more frequently or harder would enjoy an elevated probability of escape. In addition, death-feigning birds as in tonic immobility appear alert and may take advantage of escape opportunities when attacking by a predator. It has also been shown that individuals with the shortest duration of tonic immobility, and thus the lowest level of fear (Edelaar et al. 2012), could be more likely to adopt a more active behavioural strategy (Erhard et al. 1999) that may facilitate escape. Indirectly, this would also benefit the parasite by increasing their transmission opportunities, the key factor determining parasite fitness. To the best of our knowledge, this is the first study experimentally showing a modification in host escape behaviour provoked by a malaria parasite.
The manipulation behaviour hypothesis posits that parasites may induce behavioural changes in their host in ways to benefit their transmission to other hosts and hence increase their own fitness (Lefèvre et al. 2008; Poulin 2010). However, hosts usually do not obtain any benefit from these parasite manipulations (Levri 1995; Vyas et al. 2007). For example, infection with malaria parasites has been shown to alter the behaviour of mosquitoes by increasing biting rates, thus enhancing parasite transmission (Cator et al. 2014), which could potentially lead to a decrease in avian host fitness because of blood loss and infection with pathogens (Lehane 2005). However, these behavioural alterations in the mosquitoes may also increase mortality of infected mosquitoes (Anderson et al. 2000) and hence decrease the negative effects on vertebrate hosts. Our findings revealed that sparrows could also benefit from Plasmodium manipulation, showing the complexity of the relationships in the scenario avian host−mosquito vector−Plasmodium parasite. Furthermore, because haemotozoa-infected birds are more prone to be depredated, thereby reducing the transmission success of parasites (Møller and Nielsen 2007), an increase in escape behaviour in infected birds would facilitate escape from predators and enhance parasite transmission. In this sense, we showed that malaria-infected sparrows performed more intense escape behaviour, which would indeed increase their likelihood of escape from predators and survival. If the performance of intense escape behaviour may clearly benefit an individual host, the question raised from here is why sparrows do not always exhibit a maximum in this behaviour, regardless to their infection status. One explanation could be that this anti-predatory behaviour should have some associated costs, and hence there could be an optimisation of benefits associated with escape weighed against costs of the performance of the behaviour. In this sense, it has been shown that aggressive behaviour in birds can incur short-term costs in terms of energy and risk of injuries (Brown 1997; Viera et al. 2011), which may result in long-term fitness costs (Hagelin 2002). In addition, it has been shown that high levels of steroid hormones may facilitate energy-intensive escape responses in birds (Hau et al. 2010), but it can also impose immuno-suppression leading to diseases and parasite infections (Saino et al. 1995; Deviche and Parris 2006). Alternatively, escape behaviour may show behavioural plasticity, where the expression of some behavioural traits (e.g. shyness and boldness) may vary within and among individuals across different environmental conditions (Réale et al. 2007; Stamps and Groothuis 2010; Poulin 2013). Future studies examining the costs and benefits of escape behaviour in parasitised birds are desirable for understanding these variations.
Another remaining question concerns the identification of the mechanisms that Plasmodium may use to enhance the escape behaviour of house sparrows. Although the molecular mechanisms are still poorly understood, three main mechanisms have been proposed to be used by parasites to alter host behaviour following infection (Adamo and Webster 2013; Poulin 2010). First, some parasites secrete substances capable of altering neuronal activity of the host provoking a change in host behaviour. For instance, Schistosoma mansoni, a trematode, secretes opioid peptides altering neuronal functions in its host (Kavaliers et al. 1999). Second, parasites can also modify the behaviour by interfering with physiological and biochemical pathways, inducing an indirect change in the behaviour of the infected host. For example, larval stages of helminths can promote changes in concentrations of serotonin, dopamine, and other neurotransmitters in the brain of hosts, thereby modifying their behaviour (Poulin et al. 2003). Third, parasites may use genomics and proteomics to induce changes in the brain of the host. For example, T. gondii, an obligate parasitic protozoan, secretes a protein kinase in cells of an infected host thereby altering the expression of genes involved in immune function and neural signalling of the host (Hakimi and Cannella 2011). We do not know the mechanism used by Plasmodium to modify the behaviour of its vertebrate hosts. Plasmodium parasites are known to alter the behaviour of their intermediate hosts. For example, it has been experimentally shown that Pl. gallinaceum affects the host-seeking behaviour of its mosquito vector A. aegypti to increase its transmission success (Koella et al. 2002). More recently, malaria-infected mosquitoes have been shown to express enhanced attraction to human odour (Smallegange et al. 2013). Such modifications could be produced by alteration of an enzyme in the salivary glands of mosquitoes that hamper the blood meal process (Rossignol et al. 1984) and alterations in proteins in the head of sporozoite-infected mosquitoes indicating a possible dysfunction in the central neural system (Lefevre et al. 2007).
In conclusion, this is the first study to experimentally show a modification in host escape behaviour provoked by a malaria parasite, as expected for parasite manipulation of host behaviour. Further studies of mechanisms involved in parasite-induced changes in escape behaviour may provide powerful tools for understanding bird–malaria interactions.
References
Adamo SA, Webster JP (2013) Neural parasitology: how parasites manipulate host behaviour. Exp Biol 216:1–2
Anderson RA, Knols BGJ, Koella JC (2000) Plasmodium falciparum sporozoites increase feeding-associated mortality of their mosquito hosts Anopheles gambiae. Parasitology 4:329–333
Bensch S, Stjernman M, Hasselquist D, Ostman O, Hansson B, Westerdahl H, Pinheiro RT (2000) Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc Biol Sci 267:1583–1589
Berdoy M, Webster JP, Macdonald DW (2000) Fatal attraction in rats infected with Toxoplasma gondii. Proc Biol Sci 267:1591–1594
Biro PA (2012) Do rapid assays predict repeatability in labile (behavioural) traits? Anim Behav 83:1295–1300
Boissy A (1995) Fear and fearfulness in animals. Q Rev Biol 70:165–191
Brown CR (1997) Purple Martin (Progne subis). In: Pool A, Giil E (eds) The birds of North America. Philadelphia, and the American Ornithologists Union, Washington DC, USA.
Carere C, Van Oers K (2004) Shy and bold great tits (Parus major): body temperature and breath rate in response to handling stress. Physiol Behav 82:905–912
Carere C, Welink D, Drent PJ, Koolhaas JM, Groothuis TG (2001) Effect of social defeat in a territorial bird (Parus major) selected for different coping styles. Physiol Behav 73:427–433
Cator LJ, Lynch PA, Thomas MB, Read AF (2014) Alterations in mosquito behaviour by malaria parasites: potential impact on force of infection. Malar J 13:164
Charnov LE, Krebs RJ (1975) The evolution of alarm calls: altruism or manipulation? Am Nat 109:107–112
Cohen J (1988) Statistical power analysis for the behavioral science. Lawrence Erlbaum Associates, Hillsdale
Cornet S, Nicot A, Rivero A, Gandon S (2013) Malaria infection increases bird attractiveness to uninfected mosquitoes. Ecol Lett 16:323–329
Dawson RD, Bortolotti GR (2000) Effects of hematozoan parasites on condition and return rates of American kestrels. Auk 117:373–380
Day JF, Edman JD (1983) Malaria renders mice susceptible to mosquito feeding when gametocytes are most infective. J Parasitol 69:163–170
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation Statistical Computing
Deviche P, Parris J (2006) Testosterone treatment to free-ranging male dark-eyed juncos (Junco hyemalis) exacerbates hemoparasitic infection. Auk 123:548–562
Dingemanse N (2002) Repeatability and heritability of exploratory behaviour in great tits from the wild. Anim Behav 64:929–938
Dufva R, Allander K (1995) Intraspecific variation in plumage coloration reflects immune response in Great tit (Parus major) males. Funct Ecol 9:785–789
Edelaar P, Serrano D, Carrete M, Blas J, Potti J, Tella JL (2012) Tonic immobility is a measure of boldness toward predators : an application of Bayesian structural equation modeling. Behav Ecol 23:619–626
Endler JA (1991) Interactions between predators and prey. In: Krebs JR, Davies NB (eds) Behavioural ecology an evolutionary approach. Blackwell, Oxford, pp 169–196
Erhard HW, Mendl M, Christiansen SB (1999) Individual differences in tonic immobility may reflect behavioural strategies. Appl Anim Behav Sci 64:31–46
Fallis A, Desser S (1997) On species of Leucocytozoon, Haemoproteus and Hepatocystis. Parasit Protozoa 3:239–266
Forkman B, Boissy A, Meunier-Salaün MC, Canali E, Jones RB (2007) A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol Behav 92:340–374
Garamszegi LZ (2006) Comparing effect sizes across variables: generalization without the need for Bonferroni correction. Behav Ecol 17:682–687
Garamszegi LZ, Calhim S, Dochtermann N, Hegyi G, Hurd PL, Jorgensen C, Kutsukake N, Lajeunesse MJ, Pollard KA, Schielzeth H, Symonds MRE, Nakagawa S (2009) Changing philosophies and tools for statistical inferences in behavioral ecology. Behav Ecol 20:1363–1375
Garcia-Longoria L, Garamszegi LZ, Møller AP (2014) Host escape behaviour and blood parasite infections in birds. Behav Ecol 25:890–900
Godfrey RD, Fedynich AM, Pence DB (1987) Quantification of hematozoa in blood smears. J Wildl Dis 23:558–565
Gosling SD (2001) From mice to men: what can we learn about personality from animal research? Psychol Bull 127:45–86
Hagelin JC (2002) The kinds of traits involved in male-male competition: a comparison of plumage, behaviour, and body size in quail. Behav Ecol 1:32–41
Hakimi M, Cannella D (2011) Apicomplexan parasites and subversion of the host cell microRNA pathway. Trends Parasitol 27:481–486
Hamilton J, Hurd H (2002) The behavioural ecology of parasites. CABI Publi, Wallingford, UK
Hau M, Ricklefs RE, Wikelski M, Lee KA, Brawn JD (2010) Corticosterone, testosterone and life-history strategies of birds. Proc R Soc B 277:3203–3212
Hoagland H (1928) On the mechanism of tonic immobility in vertebrates. J Gen Physiol 11:715–741
Högstedt G (1983) Adaptation unto death : function of fear screams. Am Nat 121:562–574
Jones RB (1986) The tonic immobility reaction of the domestic fowl: a review. World Poult Sci J 42:82–96
Kavaliers M, Colwell DD, Choleris E, Ossenkopp KP (1999) Learning to cope with biting flies: rapid NMDA-mediated acquisition of conditioned analgesia. Behav Neurosci 113:126–135
Keymer AR, Read AF (1991) Behavioural ecology: the impact of parasitism. In: Toft CA, Aeschlimann A, Bolis L (eds) Parasite-host associations, coexistence or conflict? Oxford University Press, Oxford, pp 37–61
Knowles SCL, Palinauskas V, Sheldon BC (2010) Chronic malaria infections increase family inequalities and reduce parental fitness: experimental evidence from a wild bird population. J Evol Biol 23:557–569
Koella JC, Rieu L, Paul REL (2002) Stage-specific manipulation of a mosquito’s host-seeking behavior by the malaria parasite Plasmodium gallinaceum. Behav Ecol 13:816–820
Lafferty KD, Shaw JC (2013) Comparing mechanisms of host manipulation across host and parasite taxa. J Exp Biol 216:56–66
Lefevre T, Thomas F, Schwartz A, Levashina E, Blandin S, Brizard JP, Le Bourligu L, Demettre E, Renaud F, Biron D (2007) Malaria Plasmodium agent induces alteration in the head proteome of their Anopheles mosquito host. Proteomics 7:1908–1915
Lefèvre T, Roche B, Poulin R, Hurd H, Renaud F, Thomas F (2008) Exploiting host compensatory responses: the “must” of manipulation? Trends Parasitol 24:435–439
Lehane MJ (2005) The biology of blood-sucking in insects. Cambridge University Press, Cambridge, UK
Levri EP (1995) Parasite-induced change in host behavior of a freshwater snail : parasitic manipulation or byproduct of infection ? Behav Ecol 10:234–241
Lima S (1998) Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Adv Study Behav 27:215–290
Lind J, Cresswell W (2005) Determining the fitness consequences of antipredation behavior. Behav Ecol 16:945–956
Loye JE, Zuk M (1991) Bird-parasite interactions: ecology, evolution and behaviour. Oxford University Press, Oxford
Marzal A, de Lope F, Navarro C, Møller AP (2005) Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia 142:541–545
Marzal A, Ricklefs RE, Valkiūnas G, Albayrak T, Arriero E, Bonneaud C, Czirják GA, Ewen J, Hellgren O, Hořáková D et al (2011) Diversity, loss, and gain of malaria parasites in a globally invasive bird. PLoS ONE 6:8
Møller AP, Jennions M (2002) How much variance can be explained by ecologists and evolutionary biologists? Oecologia 132:492–500
Møller AP, Nielsen JT (2007) Malaria and risk of predation: a comparative study of birds. Ecology 88:871–881
Møller AP, Nielsen JT (2010) Fear screams and adaptation to avoid imminent death: effects of genetic variation and predation. Ethol Ecol Evol 22:183–202
Møller AP, Nielsen JT, Erritzøe J (2006) Losing the last feather: feather loss as an antipredator adaptation in birds. Behav Ecol 17:1046–1056
Møller AP, Christiansen S, Mousseau T (2011) Sexual signals, risk of predation and escape behavior. Behav Ecol 22:800–807
Moore J (2002) Parasites and the behavior of animals. Oxford University Press, Oxford
Moran MD (2003) Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 102:403–405
Nakagawa S (2004) A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav Ecol 15:1044–1045
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc 82:591–605
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev Camb Philos Soc 85:935–956
Palinauskas V, Valkiūnas G, Krizanauskiene A, Bensch S, Bolshakov CV (2009) Plasmodium relictum (lineage P-SGS1): Further observation of effects on experimentally infected passeriform birds, with remarks on treatment with Malarone. Exp Parasitol 123:134–139
Platzen D, Magrath RD (2004) Parental alarm calls suppress nestling vocalization. Proc Biol Sci 271:1271–1276
Poulin R (2010) Parasite manipulation of host behavior: an update and frequently asked questions. In: Brockmann J (ed) Advances in the study of behavior. Academic Press Burlington, New Jersey, pp 151–186
Poulin R (2013) Parasite manipulation of host personality and behavioural syndromes. J Exp Biol 216:18–26
Poulin R, Nichol K, Latham ADM (2003) Host sharing and host manipulation by larval helminths in shore crabs: cooperation or conflict? Int J Parasitol 33:425–433
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biol Rev Camb Philos Soc 82:291–318
Réale D, Dingemanse NJ, Kazem AJN, Wright J (2010) Evolutionary and ecological approaches to the study of personality. Philos Trans R Soc Lond B Biol Sci 365:3937–3946
Remple DJ (2004) Intracellular hematozoa of raptors: a review and update. J Avian Med Surg 18:75–88
Rossignol PA, Ribeiro JM, Spielman A (1984) Increased intradermal probing time in sporozoite-infected mosquitoes. Am J Trop Med Hyg 33:17–20
Saino N, Møller AP, Bolzernaa AM (1995) Testosterone effects on the immune system and parasite infestations in the barn swallow (Hirundo rustica): an experimental test of the immunocompetence hypothesis. Behav Ecol 6:397–404
Sambrook J, Fritsch EF, Maniatis T (2002) Molecular cloning: a laboratory manual. Cold Sprin, New York, USA
Schmid-Hempel P (2011) Evolutionary parasitology: the integrated study of infections, immunology, ecology and genetics. Oxford University Press, Oxford, UK
Sih A, Bell AM, Johnson JC, Ziemba RE (2004) Behavioral syndrome: an integrative overview. Q Rev Biol 79:241–277
Smallegange RC, van Gemert GJ, van de Vegte-Bolmer M, Gezan S, Takken W, Sauerwein RW, Logan JG (2013) Malaria infected mosquitoes express enhanced attraction to human odor. PLoS ONE 8:5
Stamps JA, Groothuis TGG (2010) Developmental perspectives on personality: implications for ecological and evolutionary studies of individual differences. Philos Trans R Soc Lond B Biol Sci 365:4029–4041
Thomas F, Renaud F, Guégan JF (2005) Parasitism and ecosystems. Oxford Uiversity Press, Oxford, UK
Valkiūnas G (2005) Avian malaria parasites and other Haemosporidia. CRC Press, Boca Raton, FL, USA
Van Riper C III, Van Riper SG, Goff ML, Laird M (1986) The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol Monogr 56:327–344
Viera VM, Viblanc VA, Filippi-Codaccioni O, Côté SD, Groscolas R (2011) Active territory defence at a low energy cost in a colonial seabird. Anim Behav 82:69–76
Vyas A, Kim SK, Giacomini N, Boothroyd JC, Sapolsky RM (2007) Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci U S A 104:6442–6447
Waldenström J, Bensch S, Hasselquist D, Ostman O (2004) A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. J Parasitol 90:191–194
Webster JP, McConkey GA (2010) Toxoplasma gondii-altered host behaviour: clues as to mechanism of action. Folia Parasitol 57:95–104
Acknowledgments
This study was funded by grants from the Spanish Ministry of Economy and Competition (CGL2009-08976 and CGL2012-36665) and the Regional Government of Extremadura (GRU: 10134). Luz Garcia-Longoria was supported by a PhD grant from Ministry of Economy and Competition of Spain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garcia-Longoria, L., Møller, A.P., Balbontín, J. et al. Do malaria parasites manipulate the escape behaviour of their avian hosts? An experimental study. Parasitol Res 114, 4493–4501 (2015). https://doi.org/10.1007/s00436-015-4693-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4693-7