Abstract
Insecticide resistance has been a major public health challenge. It is impendent to study the mechanism on insecticide resistance. In our previous study, 14 differentially accumulated insect cuticle proteins (ICPs) based on insecticide resistance proteomes and transcriptomes were found in the deltamethrin-resistant (DR) and -susceptible (DS) strains of Culex pipiens pallens. To investigate if these ICPs are associated with deltamethrin resistance, different transcriptional levels of the 14 ICPs were detected in the DS and DR strains from laboratory and field populations by using quantitative real-time polymerase chain reaction (qRT-PCR). The expression levels of the 14 ICPs were also measured after short-term exposure of the DS strain to deltamethrin. The full-length complementary DNA (cDNA) of CpCPLCG5 gene, which encodes one of the 14 ICPs, was cloned from Cx. pipiens pallens. Homology analysis and phylogenetic analysis were carried out with some other insects. Furthermore, small interfering RNA (siRNA) was used to knockdown the expression level of CpCPLCG5 gene for characterizing its contribution to deltamethrin resistance. The results showed that the expression level of CpCPLCG5 gene was higher in DR strain than in DS strain both in laboratory and field populations while the other 13 ICPs were downregulated. The full-length cDNA of CpCPLCG5 gene was 732 bp, with the ORF of 390 bp and deduced 129 amino acids (GenBank/KF723314,2013). Knockdown of CpCPLCG5 gene increased the susceptibility of the DR strain while the expression level of the other 13 ICPs elevated. Our findings indicate that the cuticle proteins are associated with deltamethrin resistance in Cx. pipiens pallens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mosquito control represents a major public health challenge, because mosquitoes transmit several human diseases, including malaria, West Nile fever, dengue fever, and filariasis (Hemingway and Ranson 2000). Since 1930s, chemical control has been the main management strategy applied worldwide. Deltamethrin has been widely used since the 1970s due to its low toxicity in humans and rapid killing of the insects. Unfortunately, the large-scale use of insecticides has led to an undesired insecticide resistance in mosquito populations (Nauen 2007), which has become the main obstacle to vector control (Benelli 2015; Jinfu 1999; Sun et al. 2007).
It is particularly important to study the insecticide resistance mechanism and to understand how to control mosquito populations efficiently. Driven by the widespread resistance of mosquitoes to insecticides, researchers have made significant progress in the study of the mechanisms of insecticide resistance (Nkya et al. 2013). One mechanism closely related to insecticide resistance is knockdown resistance (kdr) (Burton et al. 2011; Chen et al. 2010). Other factors, including detoxification enzyme systems based on cytochrome P450 monooxygenases, non-specific esterases, and glutathione-S transferases, may also play important roles in insecticide resistance (Hemingway and Karunaratne 1998; Gong et al. 2005; Usmani and Knowles 2001; Vontas et al. 2000; Matambo et al. 2007; Pedra et al. 2004). Furthermore, mosquito insecticide resistance is also attributed to the chitinized cuticle, which may impede the absorbance of the insecticide (Ahmad and McCaffery 1991; Kostaropoulos et al. 2001). Recently, a large number of insect cuticle proteins (ICPs) have been identified in genomic, proteomic, and transcriptomic studies (Dittmer et al. 2012; Futahashi et al. 2008; Jones et al. 2013a; Karouzou et al. 2007; Vontas et al. 2007; Kucharski et al. 2007; Tang et al. 2010; Togawa et al. 2007; Willis 2010). In our previous work, based on insecticide resistance proteomes and transcriptomes, 14 ICPs were found differentially expressed in DS and DR strains of Culex pipiens pallens (Wang et al. 2015). However, there is little research on the relationship between ICPs and insecticide resistance in mosquito (Lertkiatmongkol et al. 2010; Nkya et al. 2014; Paris et al. 2012).
The insect cuticle is formed from ICPs together with chitin, which serves as a barrier to the external environment. According to the different motifs, ICPs are divided into 12 families, such as CPR, CPF, CPFL, CPLCG, and CPG, with unique structures and distinguishing characteristics (Andersen 2000; Bouhin et al. 1992; Cornman and Willis 2009; He et al. 2007).
In this study, the expression levels of 14 ICPs were detected from deltamethrin-resistant (DR) and -susceptible (DS) Cx. pipiens pallens both in laboratory and field populations by real-time polymerase chain reaction (qRT-PCR). Next, the expression pattern of these ICPs after induction by deltamethrin was also characterized by qRT-PCR. In addition, the full-length cDNA of the CpCPLCG5 gene, which belongs to ICP family, was cloned from Cx. pipiens pallens, and the sequence alignment and phylogenetic tree were analyzed. Small interfering RNA (siRNA) was injected into the female mosquitoes to inhibit the expression level of CpCPLCG5, and the viability of the injected mosquitoes was analyzed after exposed to deltamethrin by WHO insecticide susceptibility bioassay. The aim of this research was to determine if the cuticle proteins could alter the deltamethrin sensitivity.
Materials and methods
Cx. pipiens pallens strains
Two laboratory strains of Cx. pipiens pallens were used in this study. The DS strain was obtained from Tangkou, Shandong Province, and then maintained in our laboratory without exposure to any insecticides. The DR strain was selected with deltamethrin more than10 generations from the DS strain. The 50 % larval lethal concentrations (LC50) of DS and DR strains were 0.03 and 0.84 mg/l, respectively. Both strains were reared at 28–30 °C in a 16-h light/8-h dark photoperiod with 70–80 % humidity. For each strain comparison, three biological replicates of 10 adult females (3 day post-emergence) were collected for RNA extraction.
Field strain of Cx. pipiens pallens were collected from DP (Dongpin, Shandong Province, N35.94, E116.47). The field studies did not involve endangered local species, and no permits were required for insect field collections. Mosquitoes were reared directly from field-collected larvae. Twenty-five non-blood-fed female mosquitoes, 3 day post-emergence, were tested with 0.05 % deltamethrin-treated papers by using the insecticide susceptibility bioassay (WHO 1970). After exposure for 1 h, the knockdown mosquitoes (recorded as DP-DS strain) and survivors (DP-DR strains) were collected for the following RNA extraction. Three replications were performed for the experiment.
Short-term exposure to deltamethrin of Cx. pipiens pallens
The 12–18-h-old female DS mosquitoes were exposed to a sublethal dose (0.05 %) of deltamethrin for 15 min by using insecticide susceptibility bioassay. The tubes were held in a horizontal position to avoid underdosing of early knockdowns. The mosquitoes were then placed into control tubes with sugar and water, and the survivors were collected at 6, 12, 24, and 48 h post-exposure for subsequent RNA extraction and expression analysis. Three replications were performed for each group.
RNA extraction and cDNA synthesis
Total RNA was extracted from 10 collected female mosquitoes for each group using TRIzol (Invitrogen, USA), and contaminant genomic DNA was removed by DNase I treatment. cDNA was synthesized from 450 ng total RNA with PrimeScript™RT Reagent Kit (TaKaRa, Japan) according to the manufacturer’s protocol. All analyses were repeated three times with different preparations of messenger RNA (mRNA) samples.
Cloning and sequencing of CpCPLCG5 gene
The full-length cDNA of the CpCPLCG5 gene, which belongs to the ICPs family, was cloned from Cx. pipiens pallens. The fragment including opening reading frame (ORF) was cloned with the specific primers: CpCPLCG5-F, 5′-TCAAACATCTCAAACAACCAACT-3′; CpCPLCG5-R, 5′-TCCTGCTGTGATCCTGTAA-3′. The primers were designed based on Cx. pipiens pallens transcriptomes we previously obtained and referred to the sequence of Culex quinquefasciatus cuticle protein (vector base ID: CPIJ003476). The PCR conditions were as follows: initial denaturation for 5 min at 94 °C, followed by 35 cycles at 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 20 s, with a 10-min extension at 72 °C. The 3′-RACE and 5′-RACE specific primers (5′-GAAGGTCGCCATTCTTGCCGTTG-3′ and 5′-AGGTAGCTCCGGGAACAACTCCG-3′) were based on the cloned fragment. The 3′-RACE and 5′-RACE adaptor primers (5′-CTAATACGACTCACTATAGGGC-3′ and 5′-CTAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT-3′) were provided by SMARTer™ RACE cDNA Amplification Kit (TaKaRa, Japan). PCR conditions were as follows: initial denaturation for 5 min at 94 °C, followed by 35 cycles of 94 °C for 30 s, 62 °C for 30 s, and 72 °C for 75 s, with a final 7-min extension at 72 °C. PCR products were separated by 1 % agarose gel electrophoresis and purified using a QIAquick Gel Extraction Kit (Qiagen, Germany). Products were then cloned overnight into the pMDTM19-T Simple plasmid (TaKaRa, Japan) at 16 °C. This ligated mixture was transformed into Escherichia coli TOP10 competent cells and cultured on LB plates containing ampicillin (70 μg/ml). Positive clones were validated and sequenced at BGI, Shenzhen. The sequences of the three fragments were assembled to generate a putative full-length cDNA. The sequence was verified using following primers: 5′-ACATGGGACCATAAGCAGTTC-3′ and 5′-CGTACAAACACAGAACTTCGGT-3′.
Sequence alignment and phylogenetic tree
The standard protein/protein BLAST sequence comparison programs (http://beta.uniprot.org/?tab0blast) were used for alignment of the translated sequence of CpCPLCG5 gene in the SWISSPROT databases. The phylogenic tree was constructed using the neighbor-joining method of the MEGA5.1 program (Tamura et al. 2011) using the ClustalW2 computer program (http://www.ebi.ac.uk/Tools/clustalw2/index.html).
qRT-PCR analysis
The transcripts of 14 cuticle proteins (Table S1) were carried out by qRT-PCR. qRT-PCR was performed on the ABI Prism 7300 HT Sequence Detection system (Applied Biosystem, CA, USA) using FastStart® SYBR Green (Roche) according to the manufacturer’s protocol. The sequences of forward and reverse primers used for the 14 cuticle proteins and their product sizes were listed in Table S2. Another pair of primers was used for β-actin: 5′-AGCGTGAACTGACGGCTCTTG-3′ and 5′-ACTCGTCGTACTCCTGCTTGG-3′, with a product size of 153 bp. We used the following procedure: at 50 °C for 2 min, at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. A melting curve program was run immediately after the PCR reaction, and the data were analyzed with 7300 System SDS Software v1.2.1 (Applied Biosystems). The raw threshold cycle (Ct) values of ICPs were normalized against Ct value of β-actin, which were then used to calculate relative expression levels in the samples using the 2−ΔΔCt method (Livak and Schmittgen 2001). The expression levels of 14 ICPs were detected in the laboratory and field mosquito populations, along with the short-term exposure to deltamethrin. The qRT-PCR analysis was performed three times using independent purified RNA samples with three replicates for each sample.
RNAi of CpCPLCG5 gene
To investigate the role of CpCPLCG5 gene in Culex mosquitoes, 350 ng siRNA of CpCPLCG5 gene (siCpCPLCG5) and negative control (siCtrol) (GenePharma, Shanghai) were injected into 1-day-old DR female mosquitoes, respectively. The SiCpCPLCG5 and SiCtrl sequences are presented in Table 1. RNAi assays were carried out according to standard methodology (Blandin et al. 2002; Dong et al. 2006). Both siCpCPLCG5 and siCtrol groups were kept in the insectary at 28–30 °C in a 16-h light/8-h dark photoperiod with 70–80 % humidity for 3 days. Total RNA was then extracted from 10 injected female mosquitoes, and gene silencing efficiency was determined by using qRT-PCR. Each experiment was repeated three times with independent injection and RNA isolation.
WHO insecticide susceptibility bioassay
Three days after injection, the other injected mosquitoes were tested by WHO insecticide susceptibility bioassay with 0.05 % deltamethrin-treated papers. Twenty female mosquitoes for each population were used. After exposure for 24 h, the number of dead and alive mosquitoes was recorded. The experiment was carried out for three replicates.
Statistical analysis
All the experiments were carried out for three replicates, and the results were means of three experiments. The data were analyzed using a Student’s t test for all two-sample comparisons and a one-way analysis of variance (ANOVA) for multiple sample comparisons with SPSS software. A p value of <0.05 was considered significant.
Results
Differentially expressed genes in laboratory and field strains
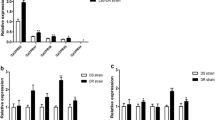
The expression level of CpCPLCG5 gene was 8.58-fold higher in laboratory DR strain than in DS strain. On the other hand, the expression levels of the other 13 ICP genes were lower in the DR strain compared with the DS strain respectively (Fig. 1).
Relative expression of 14 ICP genes detected by qRT-PCR in laboratory DR and DS strains. Data presented were the ratio of each ICP gene expression in DR strain compared with DS strain. β-actin was used as internal control. The data from three independent experiments were analyzed. The results were shown as the mean ± SE. Significant differences were indicated by *p < 0.05 and **p < 0.01
For field strain, the mRNA level of CpCPLCG5 gene was 2.58-fold higher in DP-DR strain than in DP-DS strain. The expression levels of the other 13 ICP genes were lower in the DP-DR compared with the DP-DS strain, respectively (Fig. 2).
Relative expression of 14 ICP genes detected by qRT-PCR in field DP-DR and DP-DS strains. Data presented were the ratio of each ICP gene expression in DP-DR strain compared with DP-DS strain. β-actin was used as internal control. The data from three independent experiments were analyzed. The results were shown as the mean ± SE. Significant differences were indicated by *p < 0.05 and **p < 0.01
Response of DS mosquitoes to deltamethrin
In this experiment, we focused on the short-term response (6–48 h after treatment), using a preliminary time course experiment of pair-wise hybridizations (treated vs. non-treated DS mosquitoes), which indicated some changes in the global expression pattern of the 14 ICPs at 6, 12, 24, or 48 h after exposure to deltamethrin.
These 14 ICPs were differentially expressed in the female mosquitoes of DS strain 6–48 h after exposure to a sublethal dosage of deltamethrin, compared with unexposed mosquitoes (Table S3). The highest expression level for the genes occurred at 12 or 24 h after exposure and then decreased rapidly over the next few hours (Fig. 3a–d).
Specificity of induction of the 14 ICPs after short-term exposure to deltamethrin in the DS strain, as detected by qRT-PCR. a CpCPR5,6,24,117; b CpCPR118,123,130,147; c CpCPLCG14,15,16,5; d CpCPFL1,3. Data presented were ICP gene expression in DS strain induced by deltamethrin at different time. Each value was normalized to that of untreated (0 h) set at the value 1. β-actin was used as internal control. The data from three independent experiments were analyzed. The results were shown as the mean ± SE. *p < 0.05;**p < 0.01; n.s. non-significant p value
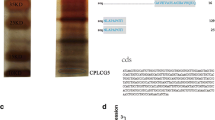
Cloning the full-length cDNA of CpCPLCG5 gene from Cx. pipiens pallens
The full-length cDNA of CpCPLCG5 gene from DR strain of Cx. pipiens pallens was amplified and sequenced. The 390 bp fragment including ORF was amplified by PCR, and the fragments of 268 and 74 bp were obtained from 3′-RACE and 5′-RACE, respectively. The sequencing results were then assembled to generate a putative full-length CpCPLCG5 cDNA. The start codon ATG was found to be nucleotides 75–77 of the gene, and a stop codon TAA was at 462–464 with tailing signal sequence AATAAA presented at the 3′-untranslated region, which confirms that it is the full-length cDNA sequence of CpCPLCG5. The full-length cDNA was deposited in GenBank under accession number KF723314. The deduced peptide was composed of 129 amino acids. Homology analysis of the Cx. pipiens pallens CpCPLCG5 sequence was compared with other insect species, including Cx. quinquefasciatus, Anopheles gambiae, Aedes aegypti, Anopheles darlingi, etc. (Fig. 4a). The phylogenetic analysis showed that Cx. pipiens pallens, Cx. quinquefasciatus, An. gambiae, Ae. aegypti, and An. darlingi share the most recent common ancestry (Fig. 4b). Structure analysis showed that there is a conserved domain G-x(2)-Hx-A-P-x(2)-G-H at the C terminus of CpCPLCG5 which is a characteristic of CPLCG subfamily in cuticle protein family (Willis 2010).
Analysis of the deduced amino acid sequence of CpCPLCG5 gene from Cx. pipiens pallens. a An amino acid sequence alignment of Cx. pipiens pallens CpCPLCG5 gene with other insect species (Cx. quinquefasciatus, Aedes aegypt, Anopheles darlingi, An. gambiae, Drosophila grimshaw, D. mojavensis, Musca domestica, Ceratitis capitata, D. virilis, D. ananassae, D. erecta, D. willistoni, D. sechellia, D. melanogaster, D. pseudoobscura, D. simulans, D. yakuba). b Phylogenetic relationships of CpCPLCG5 among Cx. pipiens pallens and other insect species
RNAi increases the sensitivity of the DR strain to deltamethrin treatment
qRT-PCR showed that the knockdown efficiency of CpCPLCG5 gene was 73.4 % compared with NC group (Fig. 5). WHO insecticide susceptibility bioassay showed the mortality rate of siCpCPLCG5 group increased to 46.7 % (Fig. 6). The number of tested mosquitoes and their mortality rates are presented in Table S4. Interestingly, the other 13 ICPs were highly expressed in siCpCPLCG5 group compared with the NC group (Fig. 7). Our results showed that knockdown of CPLCG5 gene increased the sensitivity of Culex mosquitoes to deltamethrin.
Expression level of CpCPLCG5 gene was downregulated in DR mosquitoes by RNAi. Data presented were the ratio of the CpCPLCG5 gene expression in siCpCPLCG5-injected mosquitoes compared with that in siCtrol-injected mosquitoes. The data from three independent experiments were analyzed. The results were shown as the mean ± SE. Significant differences were indicated by **p < 0.01)
Mortality of mosquitoes in WHO insecticide susceptibility bioassay after RNAi of CpCPLCG5 gene. The 3-day siRNA-injected mosquitoes were treated with 0.05 % deltamethrin. Mortality was recorded after 24-h exposure to deltamethrin. DM deltamethrin. The data from three independent experiments were analyzed. The results were shown as the mean ± SE. Significant differences were indicated by **p < 0.01
Expression levels of the other 13 ICPs in siCpCPLCG5 group compared with the siCtrol group. Data presented were the ratio of the 13 ICP gene expression in siCpCPLCG5-injected mosquitoes compared with that in siCtrol-injected mosquitoes. CpCPLCG5 was not included. The data from three independent experiments were analyzed. The results were shown as the mean ± SE. Significant differences were indicated by *p < 0.05 and **p < 0.01
Discussion
The cuticle is the outer surface of insects, and it is a protein-chitin mixture secreted by single epidermal cells. As an external skeleton, insect cuticle can provide mechanical support for the body, such as motion, feeding, perception, flight, and so on (Qiu and Hardin 1995). In addition, it is a physical barrier to prevent evaporation which leads to dehydration. It is well-known that the cuticular proteins are key components of insect cuticle, a structure that plays an important role in insect development and defense (Chu et al. 2013). In recent years, with the rapid development of biological technology and application of new method for transcriptome and proteome, some ICPs such as the CPR, CPLC, and CPLCG subfamily members were found to be differential expressed in some insects between sensitive and resistant strains (Awolola et al. 2009; Nkya et al. 2014; Reid et al. 2012; Silva et al. 2012). However, little is known about the function of these cuticle proteins in the insecticide resistance (Ahmad et al. 2006; Wood et al. 2010; Zhu et al. 2013) . In this study, the expression levels of 14 ICPs of laboratory and field Cx. pipiens pallens between DR and DS strains were examined. The results show that ICPs were differently expressed between resistant and susceptible strains both in laboratory and in field populations, which is consistent with the results in Ae. aegypti and bed bug (Lertkiatmongkol et al. 2010; Mamidala et al. 2012; Paris et al. 2012).
In induction experiment, the transcripts of the 14 ICPs were differently expressed after induced by deltamethrin in DS strain. In RNA interference experiment, knockdown of CpCPLCG5 gene in DR strain by using micro-injection increased mosquitoes’ sensitivity to deltamethrin. The above results showed that there is a relationship between cuticle proteins and insecticide resistance.
We found that the transcript of CpCPLCG5 gene, which encodes one of the 14 ICPs in Cx. pipiens pallens was at a high expression level in both laboratory and field DR strains. This is also consistent with the reports of ICPs in An. gambiae mosquitoes from Kenya, where 19 ICPs showed significant differential accumulation between R and S mosquitoes, and only transcript AGAP006009-RA (CRP30) was accumulated at higher levels in R than in S mosquitoes (Bonizzoni et al. 2012). RNAi of CpCPLCG5 gene showed that the sensitivity of the DR strain of Cx. pipiens pallens increased when CpCPLCG5 gene downregulated. This is the first evidence that CpCPLCG5 may play an important role on insecticide resistance. As CpCPLCG5 belongs to CPLCG subfamily, and CPLCG3, CPLCG4, CPLCG5, and CPLCG15 have been reported overexpressed in insecticide-resistant An. gambiae (Nkya et al. 2014; Vannini et al. 2014); the relationship between CPLCG subfamily and insectcide resistance in mosquitoes warrants further investigation.
Interestingly, changes in expression levels of CpCPLCG5 gene were far greater than the other 13 ICPs in the laboratory- and field-resistant strains. While CpCPCLG5 gene was upregulated in DR strain, the other 13 ICPs were downregulated. And after knockdown of CpCPLCG5 gene, the expression levels of other 13 ICPs were correspondingly increased. These results suggested that CpCPLCG5 gene may act as a key factor in mosquito cuticle protein family which involves in insecticide resistance. There may be some internal adjustment mechanism that maintain a certain level of the whole ICPs and keep the stability of the whole ICPs’ function. However, only 14 ICPs at adult stage were investigated in our study, and there may be other ICPs involved. Further study is needed to investigate the underlying mechanism between CpCPLCG5 and other ICPs.
In the experiment of the effect of short-term exposure to deltamethrin, we found that these 14 ICPs were differentially expressed in the DS strain after short-term exposure. Our results show that all the 14 ICPs were upregulated at an early time, peaked at 12 or 24 h, and then rapidly declined. We cannot exclude the possibility that some genes are induced significantly at different time intervals outside of the 6–48 h window (early or late responses), and these could be of importance in insecticide resistance. In previous work on the susceptibility of the An. gambiae strains after exposure to insecticide, NAP1-P03-H-05 (the coding for an insect cuticle protein) was downregulated at a later time after exposure (Vontas et al. 2005). A potential cause of the insecticide resistance was a change in the cuticle structure (Jones et al. 2013b). Still, it needs further study.
This study provides evidence that ICPs are associated with deltamethrin resistance. To better understand how the cuticle protein is associated with insecticide resistance, future work should focus on a better understanding of ICP production and how the ICPs change under different conditions.
References
Ahmad M, McCaffery AR (1991) Elucidation of detoxication mechanisms involved in resistance to insecticides in the third instar larvae of a field-selected strain of Helicoverpa armigera with the use of synergists. Pestic Biochem Physiol 41(1):41–52
Ahmad M, Denholm I, Bromilow RH (2006) Delayed cuticular penetration and enhanced metabolism of deltamethrin in pyrethroid-resistant strains of Helicoverpa armigera from China and Pakistan. Pest Manag Sci 62(9):805–810
Andersen SO (2000) Studies on proteins in post-ecdysial nymphal cuticle of locust, Locusta migratoria, and cockroach, Blaberus craniifer. Insect Biochem Mol Biol 30(7):569–577
Awolola TS, Oduola OA, Strode C, Koekemoer LL, Brooke B, Ranson H (2009) Evidence of multiple pyrethroid resistance mechanisms in the malaria vector Anopheles gambiae sensu stricto from Nigeria. Trans R Soc Trop Med Hyg 103(11):1139–1145
Benelli (2015) Research in mosquito control: current challenges for a brighter future. Parasitol Res 114(8):2801–2805
Blandin S, Moita LF, Kocher T, Wilm M, Kafatos FC, Levashina EA (2002) Reverse genetics in the mosquito Anopheles gambiae: targeted disruption of the Defensin gene. EMBO Rep 3(9):852–856
Bonizzoni M, Afrane Y, Dunn WA, Atieli FK, Zhou G, Zhong D, Li J, Githeko A, Yan G (2012) Comparative transcriptome analyses of deltamethrin-resistant and -susceptible Anopheles gambiae mosquitoes from Kenya by RNA-Seq. PLoS ONE 7(9), e44607
Bouhin H, Charles JP, Quennedey B, Courrent A, Delachambre J (1992) Characterization of a cDNA clone encoding a glycine-rich cuticular protein of Tenebrio molitor: developmental expression and effect of a juvenile hormone analogue. Insect Mol Biol 1(2):53–62
Burton MJ, Mellor IR, Duce IR, Davies TG, Field LM, Williamson MS (2011) Differential resistance of insect sodium channels with kdr mutations to deltamethrin, permethrin and DDT. Insect Biochem Mol Biol 41(9):723–732
Chen L, Zhong D, Zhang D, Shi L, Zhou G, Gong M, Zhou H, Sun Y, Ma L, He J, Hong S, Zhou D, Xiong C, Chen C, Zou P, Zhu C, Yan G (2010) Molecular ecology of pyrethroid knockdown resistance in Culex pipiens pallens mosquitoes. PLoS ONE 5(7), e11681
Chu X, Lu W, Zhang Y, Guo X, Sun R, Xu B (2013) Cloning, expression patterns, and preliminary characterization of AccCPR24, a novel RR-1 type cuticle protein gene from Apis cerana cerana. Arch Insect Biochem Physiol 84(3):130–144
Cornman RS, Willis JH (2009) Annotation and analysis of low-complexity protein families of Anopheles gambiae that are associated with cuticle. Insect Mol Biol 18(5):607–622
Dittmer NT, Hiromasa Y, Tomich JM, Lu N, Beeman RW, Kramer KJ, Kanost MR (2012) Proteomic and transcriptomic analyses of rigid and membranous cuticles and epidermis from the elytra and hindwings of the red flour beetle, Tribolium castaneum. J Proteome Res 11(1):269–278
Dong Y, Aguilar R, Xi Z, Warr E, Mongin E, Dimopoulos G (2006) Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog 2(6), e52
Futahashi R, Okamoto S, Kawasaki H, Zhong YS, Iwanaga M, Mita K, Fujiwara H (2008) Genome-wide identification of cuticular protein genes in the silkworm, Bombyx mori. Insect Biochem Mol Biol 38(12):1138–1146
Gong MQ, Gu Y, Hu XB, Sun Y, Ma L, Li XL, Sun LX, Sun J, Qian J, Zhu CL (2005) Cloning and overexpression of CYP6F1, a cytochrome P450 gene, from deltamethrin-resistant Culex pipiens pallens. Acta Biochim Biophys Sin (Shanghai) 37(5):317–326
He N, Botelho JM, McNall RJ, Belozerov V, Dunn WA, Mize T, Orlando R, Willis JH (2007) Proteomic analysis of cast cuticles from Anopheles gambiae by tandem mass spectrometry. Insect Biochem Mol Biol 37(2):135–146
Hemingway J, Karunaratne SH (1998) Mosquito carboxylesterases: a review of the molecular biology and biochemistry of a major insecticide resistance mechanism. Med Vet Entomol 12(1):1–12
Hemingway J, Ranson H (2000) Insecticide resistance in insect vectors of human disease. Annu Rev Entomol 45:371–391
Jinfu W (1999) Resistance to deltamethrin in Culex pipiens pallens (Diptera: Culicidae) from Zhejiang, China. J Med Entomol 36(3):389–393
Jones CM, Haji KA, Khatib BO, Bagi J, Mcha J, Devine GJ, Daley M, Kabula B, Ali AS, Majambere S, Ranson H (2013a) The dynamics of pyrethroid resistance in Anopheles arabiensis from Zanzibar and an assessment of the underlying genetic basis. Parasit Vectors 6:343
Jones CM, Haji KA, Khatib BO, Bagi J, Mcha J, Devine GJ, Daley M, Kabula B, Ali AS, Majambere S, Ranson H (2013b) The dynamics of pyrethroid resistance in Anopheles arabiensis from Zanzibar and an assessment of the underlying genetic basis. Parasit Vectors 6(1):343
Karouzou MV, Spyropoulos Y, Iconomidou VA, Cornman RS, Hamodrakas SJ, Willis JH (2007) Drosophila cuticular proteins with the R&R Consensus: annotation and classification with a new tool for discriminating RR-1 and RR-2 sequences. Insect Biochem Mol Biol 37(8):754–760
Kostaropoulos I, Papadopoulos AI, Metaxakis A, Boukouvala E, Papadopoulou-Mourkidou E (2001) Glutathione S-transferase in the defence against pyrethroids in insects. Insect Biochem Mol Biol 31(4-5):313–319
Kucharski R, Maleszka J, Maleszka R (2007) Novel cuticular proteins revealed by the honey bee genome. Insect Biochem Mol Biol 37(2):128–134
Lertkiatmongkol P, Pethuan S, Jirakanjanakit N, Rongnoparut P (2010) Transcription analysis of differentially expressed genes in insecticide-resistant Aedes aegypti mosquitoes after deltamethrin exposure. J Vector Ecol 35(1):197–203
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408
Mamidala P, Wijeratne AJ, Wijeratne S, Kornacker K, Sudhamalla B, Rivera-Vega LJ, Hoelmer A, Meulia T, Jones SC, Mittapalli O (2012) RNA-Seq and molecular docking reveal multi-level pesticide resistance in the bed bug. BMC Genomics 13:6
Matambo TS, Abdalla H, Brooke BD, Koekemoer LL, Mnzava A, Hunt RH, Coetzee M (2007) Insecticide resistance in the malarial mosquito Anopheles arabiensis and association with the kdr mutation. Med Vet Entomol 21(1):97–102
Nauen R (2007) Insecticide resistance in disease vectors of public health importance. Pest Manag Sci 63(7):628–633
Nkya TE, Akhouayri I, Kisinza W, David JP (2013) Impact of environment on mosquito response to pyrethroid insecticides: facts, evidences and prospects. Insect Biochem Mol Biol 43(4):407–416
Nkya TE, Poupardin R, Laporte F, Akhouayri I, Mosha F, Magesa S, Kisinza W, David JP (2014) Impact of agriculture on the selection of insecticide resistance in the malaria vector Anopheles gambiae: a multigenerational study in controlled conditions. Parasit Vectors 7:480
Paris M, Melodelima C, Coissac E, Tetreau G, Reynaud S, David JP, Despres L (2012) Transcription profiling of resistance to Bti toxins in the mosquito Aedes aegypti using next-generation sequencing. J Invertebr Pathol 109(2):201–208
Pedra JH, McIntyre LM, Scharf ME, Pittendrigh BR (2004) Genome-wide transcription profile of field- and laboratory-selected dichlorodiphenyltrichloroethane (DDT)-resistant Drosophila. Proc Natl Acad Sci U S A 101(18):7034–7039
Qiu J, Hardin PE (1995) Temporal and spatial expression of an adult cuticle protein gene from Drosophila suggests that its protein product may impart some specialized cuticle function. Dev Biol 167(2):416–425
Reid WR, Zhang L, Liu F, Liu N (2012) The transcriptome profile of the mosquito Culex quinquefasciatus following permethrin selection. PLoS ONE 7(10), e47163
Silva AX, Jander G, Samaniego H, Ramsey JS, Figueroa CC (2012) Insecticide resistance mechanisms in the green peach aphid Myzus persicae (Hemiptera: Aphididae) I: A transcriptomic survey. PLoS ONE 7(6), e36366
Sun L, Qian J, Zhou H, Li J, Shen B, Gao Q, Shen B, Zhu C (2007) Continuous, mixed, and alternating exposure to deltamethrin and fenthion affect development of resistance in Culex pipiens pallens in China. J Am Mosq Control Assoc 23(3):330–334
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739
Tang L, Liang J, Zhan Z, Xiang Z, He N (2010) Identification of the chitin-binding proteins from the larval proteins of silkworm, Bombyx mori. Insect Biochem Mol Biol 40(3):228–234
Togawa T, Augustine Dunn W, Emmons AC, Willis JH (2007) CPF and CPFL, two related gene families encoding cuticular proteins of Anopheles gambiae and other insects. Insect Biochem Mol Biol 37(7):675–688
Usmani KA, Knowles CO (2001) DEF sensitive esterases in homogenates of larval and adult Helicoverpa zea, Spodoptera frugiperda, and Agrotis ipsilon (Lepidoptera: Noctuidae). J Econ Entomol 94(4):884–891
Vannini L, Reed TW, Willis JH (2014) Temporal and spatial expression of cuticular proteins of Anopheles gambiae implicated in insecticide resistance or differentiation of M/S incipient species. Parasit Vectors 7:24
Vontas JG, Small GJ, Hemingway J (2000) Comparison of esterase gene amplification, gene expression and esterase activity in insecticide susceptible and resistant strains of the brown planthopper, Nilaparvata lugens (Stal). Insect Mol Biol 9(6):655–660
Vontas J, Blass C, Koutsos AC, David JP, Kafatos FC, Louis C, Hemingway J, Christophides GK, Ranson H (2005) Gene expression in insecticide resistant and susceptible Anopheles gambiae strains constitutively or after insecticide exposure. Insect Mol Biol 14(5):509–521
Vontas J, David JP, Nikou D, Hemingway J, Christophides GK, Louis C, Ranson H (2007) Transcriptional analysis of insecticide resistance in Anopheles stephensi using cross-species microarray hybridization. Insect Mol Biol 16(3):315–324
Wang W, Lv Y, Fang F, Hong S, Guo Q, Hu S, Zou F, Shi L, Lei Z, Ma K, Zhou D, Zhang D, Sun Y, Ma L, Shen B, Zhu C (2015) Identification of proteins associated with pyrethroid resistance by iTRAQ-based quantitative proteomic analysis in Culex pipiens pallens. Parasit Vectors 8:95
Willis JH (2010) Structural cuticular proteins from arthropods: annotation, nomenclature, and sequence characteristics in the genomics era. Insect Biochem Mol Biol 40(3):189–204
Wood O, Hanrahan S, Coetzee M, Koekemoer L, Brooke B (2010) Cuticle thickening associated with pyrethroid resistance in the major malaria vector Anopheles funestus. Parasit Vectors 3:67
Zhu F, Gujar H, Gordon JR, Haynes KF, Potter MF, Palli SR (2013) Bed bugs evolved unique adaptive strategy to resist pyrethroid insecticides. Sci Rep 3:1456
Acknowledgments
This work was supported by the National Institutes of Health of US (NIH) (Grant No. 2R01AI075746), the National Natural Science Foundation of China (Grant No. 30901244, 81171610, and 81301458), the National S & T Major Program (Grant No. 2012ZX10004-219 and 2012ZX10004-220) and Priority Academic Program Development of Jiangsu Higher Education Institutions.
We thank Maoqing Gong from Shandong Institute of Parasitic Diseases and Xuelian Chang from BengBu Medical College for their help with mosquito collection and Dr. Rongfeng Li and Qiang Liu for their expert technical assistance in gene injection.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Summary of the 14 ICPs identified based on insecticide-resistance proteomes and transcriptomes in the deltamethrin-resistant (DS) and –susceptible (DR) strains of Cx. pipiens pallens. (DOC 42 kb)
Table S2
Sequences of primers used in this study. (DOC 46 kb)
Table S3
Relative expression ratios of the 14 ICPs in DS strain after exposure to a sublethal dosage of deltamethrin. (DOC 42 kb)
Table S4
Mortality of the gene-injected mosquitoes in WHO insecticide susceptibility bioassay. (DOC 36 kb)
Rights and permissions
About this article
Cite this article
Fang, F., Wang, W., Zhang, D. et al. The cuticle proteins: a putative role for deltamethrin resistance in Culex pipiens pallens . Parasitol Res 114, 4421–4429 (2015). https://doi.org/10.1007/s00436-015-4683-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4683-9