Abstract
Parasite-host cell interaction can be modulated by a dynamic communication between extracellular vesicles (EVs). They should play key roles in cell-cell communications transferring biomolecules (miRNA, proteins, soluble factors) from one cell to another cell. While many names have been used to denominate EVs, a better comprehension to understand these vesicles is raised when we classify it according to biogenesis: originated from multivesicular bodies, named exosomes, and from plasmatic membranes, denominated microvesicles. Here, we have reviewed EV participation during the protozoan-host cell interaction and reinforced the differences and similarities between exosomes and microvesicles, suggesting different intracellular routes and functions. We also discussed perspectives to study EVs and the role of EVs in diagnosis and chemotherapies of infectious diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Extracellular vesicles (EVs) are being considered a new element during parasite-host cell interaction. EVs are released by a wide number of cells including blood cells, immune system cells, tumour cells, epithelial and endothelial cells, and adult and embryonic stem cells. Extracellular vesicles are a heterogeneous kind of vesicles (size ranging between 40 and 1000 nm) known by several different names including microparticles, microvesicles, ectosomes, shedding vesicles, exosomes, and oncosomes (Tetta et al. 2012).
An interesting global initiative (The International Society for Extracellular Vesicles or ISEV) started in 2012 with a clear focus of boosting stimulation with the aim of enhancing research in this field. The ISEV meets every year, and much significant progress has been made in the study of EVs from the ideas exchanged from the members of the community. In addition to the contributions of the society, many reviews have been published in general and specific journals to summarize aspects of biogenesis, functions, and applications of extracellular vesicles in many kinds of cell (Deolindo et al. 2013; Raposo and Stoorvogel 2013; Ratajczak et al. 2006).
The participation of extracellular vesicles during parasite-host cell interaction has been the focus in recent reviews highlighting communications between parasites and host cells (Barteneva et al. 2013; Marcilla et al. 2014; Schara et al. 2009; Williams and Urbe 2007).
In this review, we have concentrated our efforts to define biological concepts explaining differences in the biogenesis of extracellular vesicles. Moreover, we have summarized most of the findings involving microvesicles and exosomes in protozoans. We also discussed new perspectives to study the role of EVs during parasites and host cell interaction and hypothesized that EVs could be useful for diagnostic and chemotherapeutic alternatives to control diseases.
Extracellular vesicle biogenesis
Because of the confusion with the nomenclature of extracellular vesicles, the scientific community has established a consensus to classify them according to the size and origin of three kinds of vesicles: exosomes (around 40–100 nM); microvesicles, also named microparticles, ectosomes, etc. (around 100 nm to 1 μM); and apoptotic bodies (from 2 to 4 μM) as we have summarized in Fig. 1 as extracellular vesicle “world”.
Extracellular vesicle world. The diagram shows three big groups of extracellular vesicles classified by origin and size. Exosomes are from multivesicular bodies and about 40–100 nm. Microvesicles originate from plasmatic membrane with size between 100 nM and 1 μm. Finally, apoptotic bodies are from programmed cell death and about 2–4 μm
Among the vesicles, the apoptotic bodies have well-defined characteristics and are easy to identify. They are of large size and originate from cells in the process of programmed cell death.
Some decades ago, different authors started to identify the product released by parasites in vitro or in vivo as shedding vesicles or secreted protein molecules. In several parasites, exo-antigens were identified, and in some cases, compartmentalized structures as vesicles were described. Later, with the advent of new technologies within genomics and post-genomics ages, the concept of “secretome” was accepted as the total product released for a cell in some period. Proteomics strategies allowed the elucidation of some secreted products and showed the presence of biomolecules (glycolipids, glycoconjugates, glycoproteins, etc.) and extracellular vesicles as an important component of the extracellular material. Nowadays, a lot of information and knowledge have been built around the concept of “extracellular vesicles” as the material released by the cells.
In general, the extracellular vesicles detectable both in vivo and in vitro correspond to a mixed population of exosomes and microvesicles which originated from different pathways and are in some occasions indistinguishable.
The exosomes originate from the endosomal membrane cell compartment where the exocytosis of multivesicular bodies occurs discharging into the extracellular space of intraluminal vesicles after fusion with the plasma membrane in a cytoskeleton-dependent activation and independent of calcium (Schara et al. 2009).
Microvesicle shedding is stimulated by sustained elevation of intracellular calcium, opening of calcium-dependent potassium channels, activation of calcium-dependent proteases like calpain, and activation of the phospholipid transporter scramblase, driving the exposure of phosphatidylserine in the outer leaflet of the bilayer. Cytoskeletal and membrane protein as synexin seems to be involved with the protrusion of the membrane and release of the microvesicle from the plasmatic membrane (Schara et al. 2009; Silverman et al. 2010).
Communication between cells
Cells alone and within tissues have communication between them. A natural means to have communication is the physical contact by cell junctions, adhesion molecules, and soluble factors. From the endocrine point of view, the soluble factors can be autocrine acting on the same cells from which they were produced, or paracrine affecting neighbouring cells or even work a long distance. The emerging extracellular vesicle world has been considered a new mechanism of cell communication. As mentioned before, EVs are released by numerous cell types and also found in body fluids such as serum, urine, amniotic fluid, and breast milk in physiological and pathological conditions. The release of EVs is being considered a universal communication between cells. In this way, cells can modify the behaviour of other cells, passing material and information through proteins, lipids, nucleic acids, and other components (Deolindo et al. 2013). EVs are cytosol fragments surrounded by a lipid bilayer and proteins derived from the plasmatic membrane. They are released constitutively in vitro or in vivo by cells or upon activation by an agonist or physical or chemical stress, like hypoxia, oxidative stress, etc. (Silverman and Reiner 2011). Another possible inductor is the physical contact of a protozoan circulating in the blood or in direct contact with host cells (Cestari et al. 2012).
New talk in protozoan-host cell interaction
Protozoa are a wide group of unicellular eukaryotic organisms that have a complex life cycle; 10,000 species of protozoa have been described, and less than 1 % produce diseases in humans. EVs can carry receptors, biomolecules, proteins, mRNAs, and miRNA which deliver information to recipient cells. Within the context of the protozoan-host cell interaction, the release of EVs is able to modify the phenotype and function of target cells. This kind of communication could reformulate concepts in parasitism and modulate the innate immune system.
Here we have pointed out the main findings of EVs in some protozoan-related diseases.
In protozoan-host cell interaction, it has long been hypothesized that secretion of effector molecules should be important for pathogenesis. Many research have been described showing putative molecules released by pathogens (secretome) (Da Silveira et al. 1979; Goncalves et al. 1991; Barteneva et al. 2013).
Advances in high-throughput mass spectrometry allowed secretome analysis from several single-cell pathogens. Some works have been done identifying extracellular vesicles as the product of secretome. The enormous amount of data has contributed to understanding some strategies used by protozoans to interact with other cells. However, the assays have showed a high diversity of secreted proteins according with the complexity of the infection process. Moreover, information on well-defined and identified proteins involved with translation, carbohydrate and lipid metabolism, and DNA packaging is varied regarding the function and localization within the cells. Maybe more controlled experiments and a new generation of mass spectrometry could give more accurate and reliable results (Thery et al. 2009). We have listed different manuscripts published in recent years on some protozoa, regarding extracellular vesicles and secretome (Table 1).
Intracellular protozoans
Plasmodium spp.
Malaria is a disease caused by apicomplexan protozoa of the genus Plasmodium and is transmitted to humans through the bite of the insect mosquito anopheles. Four species of Plasmodium spp. produce the disease: Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale. The World Health Organization (WHO) considers the disease as the highest produced by protozoans in the world with more than 200 million cases and with a third of the cases reaching death (Coltel et al. 2006). There are reports showing EV release during murine models and human infections. Several studies have described the circulation of EVs during P. falciparum and P. vivax infections in humans (Regev-Rudzki et al. 2013; Combes et al. 2005). In general, the first work named EVs as microparticles and not microvesicles, and recently, authors have started to identify the vesicles as exosomes and microvesicles. Important research have suggested a role of EVs at the pathogenesis of the disease because levels of circulating EVs have coincided with fever and cerebral dysfunctions (Nantakomol et al. 2011). Murine malaria caused by Plasmodium berghei represents the clearest protozoan model where EVs are producing an effect at pathogenesis. EVs isolated from infected animals induced a potent activation of macrophages through Toll-like receptors, while microvesicles (MVs) from native animals have no effect over macrophages. Moreover, when the ABCA transporter gene that modulates the presence of phosphatidylserine on the surface of the plasmatic membrane was knocked out from animals, the animals were protected from cerebral malaria (CM), indicating a relation between microvesicles and CM (Combes et al. 2005). Recently, an important report has showed that exosomes derived from infected reticulocytes are able to modulate the immune system. In fact, it is the first evidence that exosomes derived from reticulocytes could be used as a vaccine for malaria infections. Immunizations of balb/c with exosomes from infections raised IgG antibodies capable of recognizing Plasmodium yoelii-infected red blood cells. Moreover, in immunizations combined with CpG oligodeoxynucleotides, they have found a complete protection against lethal infections in more than 85 % of the mice infected (Martin-Jaular et al. 2011).
Important concepts regarding the co-participation of EV production between parasite and host cells were demonstrated by some authors (Regev-Rudzki et al. 2013; Coltel et al. 2006; Bhatnagar et al. 2007) in P. falciparum infection. Regel-Rudzkel et al. have showed the release of exosome-like vesicles (80 nm) by ring-stage parasites using an atomic force microscope. Moreover, they have demonstrated the transference of genetic material between infected red blood cells (iRBCs). Transgenic parasites which are resistant to one drug were co-cultured in the presence of two drugs, and doubly resistant parasites were obtained in a few days. The participation of exosomes was demonstrated by transwell assays where exosomes from one population were transferred to another, inducing a new resistance to the drug for the recipient population. In another study, some authors have shown larger-sized vesicles between 150 and 250 nm at the schizogony stage. They also showed that iRBCs produce 10 times more EVs than uninfected RBCs (Mantel et al. 2013).
Toxoplasma gondii
Toxoplasma gondii is an intracellular protozoan that causes toxoplasmosis which affects humans and domestic animals worldwide. In humans, it is associated with complications during pregnancy and in immunocompromised patients, causing severe clinical complications including death. The first report on the use of exosomes in therapy was related to Toxoplasma. Dendritic cells were pulsed in vitro with antigens and exosomes from T. gondii. Later, the exosomes from T. gondii were used in immunizations of mice that were challenged with lethal doses of parasites, and nearly 70 % of protection was done. Moreover, exosomes obtained from infected macrophages were able to stimulate a pro-inflammatory response (Aline et al. 2004). Some authors also reported exosomes being released from macrophages infected with Toxoplasma. These vesicles contain an array of miRNA (Pope and Lasser 2013).
Trypanosoma cruzi
In Trypanosoma cruzi, da Silveira et al. have demonstrated for the first time the release of MVs from non-infective epimastigote forms where parasites were cultured with acid pH buffers and other reagents (da Silveira et al. 1979). Later, it was described in infective cultured trypomastigotes the shedding of surface molecules in extracellular vesicles with a size smaller than 80 nm (Torrecilhas et al. 2012). Some years ago, the concept of the shedding of vesicles was used again from the same group in an interesting description. Trocoli Torrecilhas et al. have reported that mammalian tissue culture cell-derived trypomastigotes (TCTs) release vesicles (ranking from less than 100 nm to approximately 500 nm) carrying virulence factors involved in Chagas disease pathogenesis by increasing heart parasitism and inflammation (Trocoli Torrecilhas et al. 2009). Maybe this heterogeneous description from extracellular vesicles in T. cruzi could be due to differences of the experimental conditions to obtain vesicles as methodology divergences among other factors. Moreover, several reports have been showing excreted/secreted proteins by T. cruzi, involving the shedding of vesicles (Bayer-Santos et al. 2012; da Silveira et al. 1979; Goncalves et al. 1991; Neves et al. 2014). Recently, a comprehensive proteomic analysis of T. cruzi showed the existence of two different extracellular vesicle populations in non-infective epimastigote and infective metacyclic trypomastigote forms. Bayer-Santos et al. have demonstrated that both forms release exosomes and microvesicles. Moreover, the infective metacyclic forms release vesicles carrying virulence factors such as GP82 glycoproteins and mucins suggesting that the parasite could use extracellular vesicles to deliver modulatory molecules into host cells (Bayer-Santos et al. 2012).
Recently, some authors have showed that T. cruzi in trypomastigote stage from Y and CL-Brener strains are able to release vesicles with acid phosphatase activities that increase the adhesion of the parasite to the host cell (Neves et al. 2014). The importance of the microvesicle release during T. cruzi-host interaction as an evasion mechanism was demonstrated before (Cestari et al. 2012). The authors have showed that microvesicles released during metacyclic forms and THP1 cell line interaction are able to inhibit the complement system by C3 convertase inhibition and increase the invasion of metacyclic forms to host cells. The role of extracellular vesicles modulating host cells seems to be associated with the presence of small RNAs as indicated recently by different groups (Bayer-Santos et al. 2012; Ghildiyal and Zamore 2009; Garcia-Silva et al. 2014; Liu et al. 2010; Twu et al. 2013). Bayer-Santos et al. show that the small RNAs contained in extracellular vesicles originate from multiple sources, including tRNAs. Their results reveal that the variety and expression of small RNAs are different between parasite stages, suggesting diverse functions. Garcia-Silva et al. analysed the effects induced by T. cruzi shed vesicles and their associated small tRNA cargo on gene expression of susceptible HeLa cells. The ability to transfer genetic information between different cells has provided support to understand the modulation of innate immunity and cellular communications.
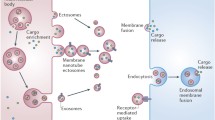
We have summarized in Fig. 2 the dynamics of microvesicle release during parasite-host cell interactions. The different kinds of interaction between extracellular vesicles derived from protozoans or eukaryotic cells during the parasite-host cell relationship are shown indicating biogenesis, release of autocrine message, fusion events, and modulation of neighbouring host cells.
Extracellular vesicle release during protozoan-host cell interaction. 1 The protozoan has reached the mammalian host. 2 The protozoan has started to release EVs. 3 The protozoan contacts with the host cell releasing EVs that can be received and internalized by the host cell, and also the proper host cell could start to release EVs. 4 Different kinds of EVs could participate in the dynamic contact between the protozoan and host cell: EVs from the protozoan, EVs from host cells, and fusion between the protozoan and host cell EVs that can produce an effect in the protozoan or host cells
Leishmania spp.
In leishmaniasis, the release of exosomes and microvesicles during interaction with mammalian cells has also been described (Silverman et al. 2010). Indeed, the authors have concentrated their efforts to analyse the impact of extracellular vesicles on intracellular stages of Leishmania spp., indicating that changes in the environment seem to define the kind of release and cargo. Proteomic analysis has supported the idea that in neutral pH, the exosomes were enriched in kinase activity, while in acidic pH, they were enriched with phosphatase activity. Extracellular stages submitted to heat shock stress treatment have showed a similar kind of enzymes. Interestingly, Leishmania respond to specific environment changes with the release of extracellular vesicles containing a group of virulence factors such GP63, membrane proteins, and redox and heat shock proteins that should modulate the parasite-host cell interaction (Silverman and Reiner 2011).
A large number of research groups have shown that extracellular vesicles can function to modulate immune responses, including immune stimulation and immune suppression during intracelullar parasite infection (reviewed by Marcilla et al. 2014; Schorey et al. 2015). The role of extraparasitic material release during the parasite-host cell interaction could be critical to maintaining parasite infection by aiding colonization and modulating the host immune response. Our current knowledge of extracellular biogenesis does not allow for specific inhibition of extracellular release; therefore, an insight to evaluate the importance of these extraparasitic materials during in vivo infection is currently not possible
Extracellular protozoans
The ability of protozoa to respond to environmental changes has been found in Giardia intestinalis, an extracellular parasite that produces diarrheal illness with global prevalence. Giardia is considered the earliest branching protist. The parasite presents a secretory pathway, including organelle biogenesis, that is limited or completely understood (Embley and Hirt 1998; Tovar et al. 2003). Some authors have described secretory vesicles associated with encystation process (Benchimol 2004; Gottig et al. 2006). Moreover, recently it was shown that G. intestinalis is able to release microvesicles in response to environmental changes (e.g. pH changes, bilis presence, calcium concentration, etc.). Deolindo et al. have suggested that this response could be a mechanism from the parasite to adapt to different changing conditions during the course of infection and to avoid innate immunity.
Another extracellular parasite that releases extracellular vesicles during contact with the host is Trichomonas vaginalis (de Miguel et al. 2010; Twu et al. 2013). Twu et al. have described that exosome secretion increases during parasite attachment to the host and modulation of the innate immunity of the host cell reducing the IL-8 expression of the host ectocervical cells. The authors have suggested that extracellular vesicles could control the regulation of IL-6 and IL-8 secretion and exosomes could be important for the establishment of chronic infection at the urogenital tract (Twu et al. 2013).
Trypanosoma brucei is the parasite that causes sleeping sickness transmitted by tsetse flies. The illness can be lethal and affect more than 60 million people in sub-Saharan Africa. T. brucei spends its life as an extracellular parasite, and when exposed to innate immunity, it must develop evasion mechanisms such as antigen variation and extracellular vesicle release (Brun and Blum 2012). Proteomic analysis of the secretome in T. brucei has allowed the characterization of proteins released by the bloodstream forms of different strains. More than 400 proteins have been identified from the secretome of T. brucei, and curiously, a higher percentage of proteins lack targeting for a secretory pathway. The authors have described microvesicles containing markers and proteases that could affect host cells (Geiger et al. 2010).
Similarities and differences between exosomes and microvesicles during the protozoan-host cell interaction: clarifying concepts
Many descriptions have been made in different biological systems showing an intense extracellular vesicle release of exosomes and microvesicles. This activity also has been shown at the parasite-host cell interaction and seems to be a new kind of communication between the cells. In several parasites, exo-antigens were identified, and in some cases, compartmentalized structures as vesicles were described. Later, with the advent of new technologies within genomics and post-genomics ages, the concept of “secretome” was accepted as the total product released for a cell in some period. Proteomics strategies allowed elucidation of some secreted products and showed the presence of biomolecules (glycolipids, glycoconjugates, glycoproteins, etc.) and extracellular vesicles as an important component of the extracellular material. Nowadays, a lot of information and knowledge has been built around the concept of “extracellular vesicles” as the material released by the cells.
How this intense cargo of protein, biomolecules, and genetic information (DNA, RNA, small RNA) could be modulating the host cell and the progression of the infection is still not understood. Exosomes and microvesicles have different origins, content, and probably different functions.
Highlighted points
Different biogenesis and complicated purification
Although there are several names given to extracellular vesicles, it is clear to differentiate exosomes (30–80 nm) that are formed from the other larger multivesicular bodies named microvesicles (from 100 nm to 1 μm) (also ectosome, microparticles, oncosomes) that originate from the plasmatic membrane. The ISEV recommendation to name these microvesicles will avoid confusion of vesicle nomenclature and will facilitate the understanding of the phenomenon. MVs are grouped based on size, density, method of purification, and markers. EVs represent a mixture of vesicular fractions of exosomes and microvesicles.
The understanding of extracellular vesicles has been associated with exosomes, and recently, the concept of microvesicles is being used by researchers. The processes that drive exosomes and microvesicles are completely different.
Exosomes are derived from the formation of multivesicular bodies (MVB) that are a late endosome loaded with intraluminal vesicles. The organelles fuse with lysosomes producing a fusion of the vesicles in the lysosomes. This represents a general and slow general degradative lysosomal pathway. This process is physiological and depends on GTPases rab 7, Snare system, and syntaxin (Bucci et al. 2000) and belongs to the exocytosis process. Exosomes are not related to the plasma membrane, and many reports previously published could have included incorrect or wrong information about the location or size of exosomes, while on the other hand, vesicles are larger in size and derived from the plasmatic membrane.
Microvesicles originate from the plasma membrane with the budding of the small plasma membrane domain in an active process with participation of the scramblase enzyme and segregation of phospholipids, cholesterol, ceramide, etc. (Gottig et al. 2006). In contrast to exosomes, the release of microvesicles does not require exocytosis and larger (100–1000 nm) vesicles are released into the extracellular space at a high rate. This process is calcium-dependent where an increase of free Ca++ acts as a second messenger to sustain the release of microvesicles intensively in seconds (Schara et al. 2009).
Purification of the mixture of extracellular vesicles is carried out by pre-cleaning with filtration before centrifugation (differential centrifugation, sucrose gradients). There are also immune affinity beads, gel filtration, etc. Unfortunately, the standardization is not fully understood. Indeed, the physical properties and ability of vesicles to fuse with each other produce a heterogeneous population with variable sizes. There are methods such as nanoparticle tracking and X-ray scattering that are promising and are under investigation.
At least CD63 and CD61 are exosome markers, and flotilin and Annexin V represent good microvesicle markers. The markers are present in most of the subpopulations studied and could be important to distinguish between the different kinds of vesicles. It is accepted that exosomes are CD63(+) and Annexin V(−) and that microvesicles are Annexin V(+) and CD63(−). These markers and the size of the vesicles are the most important point to differentiate between the vesicles.
Different routes and functions
Recently, a manuscript has shown for the first time the differences between exosomes and microvesicles with the ability to transfer genetic information (Kanada et al. 2015). The authors have observed that only microvesicles (MVs), but not exosomes, can functionally transfer loaded reporter molecules to recipient cells. It seems interesting that the membrane prevents soluble factors, proteins, and RNA against degradation. The authors have shown that exosomes and MVs are structurally and functionally distinct and could have different roles during contact with host cells.
Microvesicles as biomarkers
Microvesicles have been described in several diseases including diabetes, hypertension, atherosclerosis, and cancer. In this process, the RNA transference between cells could be involved. Recently, microvesicles have been described as a biomarker for tumour progression (Nakano et al. 2015; Giusti et al. 2013). Recently, Chen et al. have seen the identification of circulating biomarkers in sera of Plasmodium knowlesi-infected malaria patients. Microvesicle detection in serum from the patient could help to diagnose the diseases and also be useful for prognosis of the diseases (Chen et al. 2015).
Content of biomolecules
Maybe at the context of cell communication, the knowledge about proteins, nucleic acids, lipids, and biomolecules would give ideas about the putative phenotypic modification produced by the biomolecules contained in the extracellular vesicles. Many reports (Marcilla et al. 2014; Montaner et al. 2014) have shown the diversity of proteins included in exosomes and microvesicles through proteomic analysis and have been included at the Evpedia (Kim et al. 2015).
Probably, exosomes and microvesicles carry part of the cytoplasm and protein which are not specific for each kind of vesicles. Differences in the nucleic acid content should indicate the different routes between the vesicles and the probable modulatory effect in neighbouring cells. The analysis of phospholipids in extracellular vesicles could indicate the redistribution of the plasma membrane and phospholipids triggered by increased cytosolic Ca++ (Zhou et al. 1998).
Methods for the measurement of phospholipids scrambling under different conditions and analysis of phosphatidylserine, phosphatidylethanolamine, phosphatidylcholine, and other phospholipids at the extracellular vesicles and at the plasmatic membrane from protozoan and host cells will help to understand the mechanism of biogenesis, dynamics of lipid remodelling,and interchange between the cells.
Microvesicles at therapy
Extracellular vesicles represent a promising therapeutic delivery tool. Microvesicles have the ability to act as bioactive cargoes carrying secretory molecules such as cytokines, chemokines, and growth factors as genetic material. In cardiovascular diseases, microvesicles are reduced to normal levels in order to reduce associated deleterious signalling (Martinez et al. 2011).
In parasitic diseases, at least in toxoplasmosis, this has been used with success (Aline et al. 2004).
The understanding of the exosome or microvesicle mechanism could allow to design strategies to block the induction of exosomes/microvesicles through inhibitors. The release of microvesicles could be inhibited with RNA interference targeting the genes involved with the pathway (flipase/scramblase).
The mechanism of the releasing extracellular vesicles, intracellular trafficking, and signal transduction could be important to modulate the host cell and produce phenotype transformation of the cells. This important issue of cell biology should be a focus to be investigated in the future. Moreover, investigation of the host cell target of small RNA could be important to understand how the extracellular vesicles could modify gene regulation and modulate the host cell.
Concluding remarks
In this review, we have summarized information about extracellular vesicles during protozoan-host cell interactions. We have focused on defining the differences between the main extracellular vesicles—exosomes and microvesicles—and on reinforcing the role of these vesicles in cell communication. The findings including the transference of biological material (microRNA, proteins, and soluble factors) from one cell to another change the concepts in parasite-host cell interactions.
We have pointed on the biogenesis and route of the vesicles during the parasite-host cell interaction. Indeed, the huge increase in the research of extracellular vesicles is a reason to think that new advances will come in the next few years. These may include understanding the mechanism of release, content, phenotypic effect, and communications between the cells. More knowledge in the physiology and pathology of extracellular vesicles will allow the exploration of the rational use of exosomes and microvesicles as biomarkers in the diagnosis and therapy of infectious diseases.
Extracellular vesicles during protozoan-host cell interactions are a fascinating issue with impact in cell biology, pharmacology, immunology, and medicine joining basic and applied sciences. New findings will help to understand the role of EVs in parasitism and cell communication.
References
Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I (2004) Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect Immun 72:4127–4137
Barteneva NS, Maltsev N, Vorobjev IA (2013) Microvesicles and intercellular communication in the context of parasitism. Front Cell Infect Microbiol 3:49
Bayer-Santos E, Aguilar-Bonavides C, Rodrigues SP, Cordero EM, Marques AF, Varela-Ramirez A, Choi H, Yoshida N, da Silveira JF, Almeida IC (2012) Proteomic analysis of Trypanosoma cruzi secretome: characterization of two populations of extracellular vesicles and soluble proteins. J Proteome Res 12:883–897
Benchimol M (2004) The release of secretory vesicle in encysting Giardia lamblia. FEMS Microbiol Lett 235:81–87
Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS (2007) Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 110:3234–3244
Brun R, Blum J (2012) Human African trypanosomiasis. Infect Dis Clin N Am 26:261–273
Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B (2000) Rab7: a key to lysosome biogenesis. Mol Biol Cell 11(2):467–480
Cestari I, Ansa-Addo E, Deolindo P, Inal JM, Ramirez MI (2012) Trypanosoma cruzi immune evasion mediated by host cell-derived microvesicles. J Immunol 188:1942–1952
Chen Y, Chan CK, Kerishnan JP, Lau YL, Wong YL, Gopinath SC (2015) Identification of circulating biomarkers in sera of Plasmodium knowlesi-infected malaria patients--comparison against Plasmodium vivax infection. BMC Infect Dis 15:49
Coltel N, Combes V, Wassmer SC, Chimini G, Grau GE (2006) Cell vesiculation and immunopathology: implications in cerebral malaria. Microbes Infect 8:2305–2316
Combes V, Coltel N, Alibert M, van Eck M, Raymond C, Juhan-Vague I, Grau GE, Chimini G (2005) ABCA1 gene deletion protects against cerebral malaria: potential pathogenic role of microparticles in neuropathology. Am J Pathol 166:295–302
da Silveira JF, Abrahamsohn PA, Colli W (1979) Plasma membrane vesicles isolated from epimastigote forms of Trypanosoma cruzi. Biochim Biophys Acta 550:222–232
de Miguel N, Lustig G, Twu O, Chattopadhyay A, Wohlschlegel JA, Johnson PJ (2010) Proteome analysis of the surface of Trichomonas vaginalis reveals novel proteins and strain-dependent differential expression. Mol Cell Proteomics 9:1554–1566
Deolindo P, Evans-Osses I, Ramirez MI (2013) Microvesicles and exosomes as vehicles between protozoan and host cell communication. Biochem Soc Trans 41:252–257
Embley TM, Hirt RP (1998) Early branching eukaryotes? Curr Opin Genet Dev 8:624–629
Garcia-Silva MR, Cabrera-Cabrera F, das Neves RF, Souto-Padron T, de Souza W, Cayota A (2014) Gene expression changes induced by Trypanosoma cruzi shed microvesicles in mammalian host cells: relevance of tRNA-derived halves. Biomed Res Int 2014:305239
Geiger A, Hirtz C, Becue T, Bellard E, Centeno D, Gargani D, Rossignol M, Cuny G, Peltier JB (2010) Exocytosis and protein secretion in Trypanosoma. BMC Microbiol 10:20
Ghildiyal M, Zamore PD (2009) Small silencing RNAs: an expanding universe. Nat Rev Genet 10:94–108
Giusti I, D’Ascenzo S, Dolo V (2013) Microvesicles as potential ovarian cancer biomarkers. Biomed Res Int 2013:703048
Goncalves MF, Umezawa ES, Katzin AM, de Souza W, Alves MJ, Zingales B, Colli W (1991) Trypanosoma cruzi: shedding of surface antigens as membrane vesicles. Exp Parasitol 72:43–53
Gottig N, Elias EV, Quiroga R, Nores MJ, Solari AJ, Touz MC, Lujan HD (2006) Active and passive mechanisms drive secretory granule biogenesis during differentiation of the intestinal parasite Giardia lamblia. J Biol Chem 281:18156–18166
Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, Sylvester MD, Schmidt TL, Kaspar RL, Butte MJ, Matin AC, Contag CH (2015) Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci U S A 112(12):E1433–42
Kim DK, Lee J, Kim SR, Choi DS, Yoon YJ, Kim JH, Go G, Nhung D, Hong K, Jang SC, Kim SH, Park KS, Kim OY, Park HT, Seo JH, Aikawa E, Baj-Krzyworzeka M, van Balkom BW, Belting M, Blanc L, Bond V, Bongiovanni A Borràs FE, Buée L, Buzás EI, Cheng L, Clayton A, Cocucci E, Dela Cruz CS, Desiderio DM, Di Vizio D, Ekström K, Falcon-Perez JM, Gardiner C, Giebel B, Greening DW, Gross JC, Gupta D, Hendrix A, Hill AF, Hill MM, Nolte-'t Hoen E, Hwang do W, Inal J, Jagannadham MV, Jayachandran M, Jee YK, Jørgensen M, Kim KP, Kim YK, Kislinger T, Lässer C, Lee DS, Lee H, van Leeuwen J, Lener T, Liu ML, Lötvall J, Marcilla A, Mathivanan S, Möller A, Morhayim J, Mullier F, Nazarenko I, Nieuwland R, Nunes DN, Pang K, Park J, Patel T, Pocsfalvi G, Del Portillo H, Putz U, Ramirez MI, Rodrigues ML, Roh TY, Royo F, Sahoo S, Schiffelers R, Sharma S, Siljander P, Simpson RJ, Soekmadji C, Stahl P, Stensballe A, Stępień E, Tahara H, Trummer A, Valadi H, Vella LJ, Wai SN, Witwer K, Yáñez-Mó M, Youn H, Zeidler R, Gho YS (2015) EVpedia: a community web portal for extracellular vesicles research. Bioinformatics 31(6):933–939
Liu Q, Tuo W, Gao H, Zhu XQ (2010) MicroRNAs of parasites: current status and future perspectives. Parasitol Res 107:501–507
Mantel PY, Hoang AN, Goldowitz I, Potashnikova D, Hamza B, Vorobjev I, Ghiran I, Toner M, Irimia D, Ivanov AR, Barteneva N, Marti M (2013) Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe 13:521–534
Marcilla A, Martin-Jaular L, Trelis M, de Menezes-Neto A, Osuna A, Bernal D, Fernandez-Becerra C, Almeida IC, Del Portillo HA (2014) Extracellular vesicles in parasitic diseases. J Extracell Vesicles 3:25040
Martinez MC, Tual-Chalot S, Leonetti D, Andriantsitohaina R (2011) Microparticles: targets and tools in cardiovascular disease. Trends Pharmacol Sci 32(11):659–665
Martin-Jaular L, Nakayasu ES, Ferrer M, Almeida IC, Del Portillo HA (2011) Exosomes from Plasmodium yoelii-infected reticulocytes protect mice from lethal infections. PLoS One 6:e26588
Montaner S, Galiano A, Trelis M, Martin-Jaular L, Del Portillo HA, Bernal D, Marcilla A (2014) The role of extracellular vesicles in modulating the host immune response during parasitic infections. Front Immunol 5:433
Nakano I, Garnier D, Minata M, Rak J (2015) Extracellular vesicles in the biology of brain tumour stem cells--Implications for inter-cellular communication, therapy and biomarker development. Semin Cell Dev Biol 40:17–26
Nantakomol D, Dondorp AM, Krudsood S, Udomsangpetch R, Pattanapanyasat K, Combes V, Grau GE, White NJ, Viriyavejakul P, Day NP, Chotivanich K (2011) Circulating red cell-derived microparticles in human malaria. J Infect Dis 203:700–706
Neves RF, Fernandes AC, Meyer-Fernandes JR, Souto-Padron T (2014) Trypanosoma cruzi-secreted vesicles have acid and alkaline phosphatase activities capable of increasing parasite adhesion and infection. Parasitol Res 113:2961–2972
Pope SM, Lasser C (2013) Toxoplasma gondii infection of fibroblasts causes the production of exosome-like vesicles containing a unique array of mRNA and miRNA transcripts compared to serum starvation. J Extracellular Vesicles 2
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383
Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ (2006) Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20:1487–1495
Regev-Rudzki N, Wilson DW, Carvalho TG, Sisquella X, Coleman BM, Rug M, Bursac D, Angrisano F, Gee M, Hill AF, Baum J, Cowman AF (2013) Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 153:1120–1133
Schara K, Jansa V, Sustar V, Dolinar D, Pavlic JI, Lokar M, Kralj-Iglic V, Veranic P, Iglic A (2009) Mechanisms for the formation of membranous nanostructures in cell-to-cell communication. Cell Mol Biol Lett 14:636–656
Schorey JS, Cheng Y, Singh PP, Smith VL (2015) Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep 16(1):24–43
Silverman JM, Reiner NE (2011) Leishmania exosomes deliver preemptive strikes to create an environment permissive for early infection. Front Cell Infect Microbiol 1:26
Silverman JM, Clos J, Horakova E, Wang AY, Wiesgigl M, Kelly I, Lynn MA, McMaster WR, Foster LJ, Levings MK, Reiner NE (2010) Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J Immunol 185:5011–5022
Tetta C, Ghigo E, Silengo L, Deregibus MC, Camussi G (2012) Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine 44:11–19
Thery C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9:581–593
Torrecilhas AC, Schumacher RI, Alves MJ, Colli W (2012) Vesicles as carriers of virulence factors in parasitic protozoan diseases. Microbes Infect 14:1465–1474
Tovar J, Leon-Avila G, Sanchez LB, Sutak R, Tachezy J, van der Giezen M, Hernandez M, Muller M, Lucocq JM (2003) Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature 426:172–176
Trocoli Torrecilhas AC, Tonelli RR, Pavanelli WR, da Silva JS, Schumacher RI, de Souza W, E Silva NC, de Almeida Abrahamsohn I, Colli W, Manso Alves MJ (2009) Trypanosoma cruzi: parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes Infect 11:29–39
Twu O, de Miguel N, Lustig G, Stevens GC, Vashisht AA, Wohlschlegel JA, Johnson PJ (2013) Trichomonas vaginalis exosomes deliver cargo to host cells and mediate hostratioparasite interactions. PLoS Pathog 9:e1003482
Williams RL, Urbe S (2007) The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol 8:355–368
Zhou Q, Sims PJ, Wiedmer T (1998) Identity of a conserved motif in phospholipid scramblase that is required for Ca2+-accelerated transbilayermovement of membrane phospholipids. Biochemistry 37(8):2356–2360
Acknowledgments
We would like to thank grants from Programa Parasitologia Basic (CAPES), CNPq, and Faperj. We also thank Instituto Oswaldo Cruz and Universidade Federal de Parana for hosting the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Evans-Osses, I., Reichembach, L.H. & Ramirez, M.I. Exosomes or microvesicles? Two kinds of extracellular vesicles with different routes to modify protozoan-host cell interaction. Parasitol Res 114, 3567–3575 (2015). https://doi.org/10.1007/s00436-015-4659-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4659-9