Abstract
High levels of protective immunity can be induced in different animals immunized with radiation-attenuated (RA) Schistosoma cercariae or schistosomula. However, the schistosome-derived molecules responsible for the strong protective effect elicited by RA schistosome larvae have not been identified or characterized. The 70-kDa heat shock proteins of schistosomes are considered major immunogens, and may play an important role in stimulating high levels of innate and adaptive immune responses in an RA schistosome vaccine model. Here, we demonstrate the immunobiological functions of Schistosoma japonicum heat shock protein 70 (SjHSP70) by investigating its expression profile in RA-schistosomula-derived cells, evaluating the protection induced by recombinant SjHSP70 (rSjHSP70) against cercarial challenge, and assaying the humoral and cellular immune responses to rSjHSP70 in BALB/c and C57BL/6 mice. The expression of SjHSP70 on the surfaces of cells from RA or normal schistosomula was determined with flow cytometry. Its expression was significantly higher on early RA schistosomula cells than on the cells from normal parasites. The protection afforded both BALB/c and C57BL/6 mice vaccinated with rSjHSP70 alone, rSj22.6 (a membrane-anchoring protein of S. japonicum) alone, or a combination of rSj22.6 and rSjHSP70 without adjuvant was evaluated. rSjHSP70 alone induced the highest protective effect against S. japonicum cercarial challenge, followed by the rSj22.6 plus rSjHSP70 combination and then rSj22.6 alone, in both mouse strains. Like ISA206 adjuvant, rSjHSP70 enhanced the protective efficacy induced by rSj22.6 in the C57BL/6 mouse strain. Antigen-specific IgG1 and IgG2a responses were detected with enzyme-linked immunosorbent assays in mice immunized with rSjHSP70 alone, rSj22.6 alone, or the rSj22.6 plus rSjHSP70 combination. Immunization with rSjHSP70 or the rSj22.6 plus rSjHSP70 combination induced mixed Th1/Th2-type antibody responses in BALB/c mice and a Th2-type antibody response in C57BL/6 mice. The profiles of cytokine production by splenic lymphocytes in both strains of mice immunized with the antigens described above were detected in vitro using a Cytometric Bead Array. The profiles of the proinflammatory cytokines interferon γ, tumor necrosis factor α, interleukin 6 (IL-6), and IL-17A and the regulatory cytokine IL-10 induced by the rSj22.6 plus rSjHSP70 combination were similar to those induced by rSj22.6 emulsified with the ISA206 adjuvant control. Like the ISA206 adjuvant, rSjHSP70 protein enhanced the proinflammatory and Th2-type or regulatory cytokine production induced by the rSj22.6 antigen. These results indicate that SjHSP70 is exposed on the surfaces of cells from RA schistosomula, and that rSjHSP70 protein is a promising protective antigen with a potential adjuvant function. Thus, SjHSP70 protein might play a key role in the protective immunity elicited by the RA schistosome vaccine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schistosoma japonicum is a zoonotic organism that is endemic in seven provinces of China (Lei et al. 2011). Highly effective and safe prophylactic vaccines are urgently required for the sustainable control of schistosomiasis. Previous studies have shown that high levels of protective immunity can be induced in animals such as mice, rats, cattle, pigs, and rhesus monkeys, immunized with radiation-attenuated (RA) Schistosoma cercariae or schistosomula (Majid et al. 1980; Jwo and LoVerde 1989; Ford et al. 1984; Harrison et al. 1990; Lin et al. 2011). However, the application of RA vaccines is unfeasible because they are unsafe and resources of schistosome larvae are unavailable. Therefore, the development of a molecular vaccine against schistosomiasis is proposed based on the effective mechanisms of protective immunity induced by an RA vaccine (Bickle 2009; Wilson and Coulson 2009). Further analysis of the mechanisms underlying the protective responses induced in mice vaccinated with RA cercariae suggest that this protective immunity is mediated by Th1-type immune responses, and that both CD4+ T-helper (Th) cells and antibodies play major roles in this model (Wilson and Coulson 2009). It has been reported that lung-stage schistosomula antigens are potent inducers of Th1 cellular immune responses, and that candidate vaccine antigens, such as the integral membrane protein Sm32, glutathione S-transferase, and cathepsin B, are recognized by antibodies from mice vaccinated with RA schistosome cercariae (Richter and Harn 1993; Richter et al. 1993; Mountford et al. 1988). However, none of the candidate Schistosoma vaccine antigens identified and/or natural infections are equivalent in efficacy to vaccination with the RA vaccine. This suggests that there is a quantitative and/or qualitative difference between the immune responses elicited by the RA Schistosoma larval vaccine and by a defined molecular vaccine or normal parasites. The enhanced immunogenicity of the RA parasite may be attributable to the delayed and truncated pattern of larval migration, changes in the antigens present on the surface of the parasite, or the ablation of certain proteins present in the normal parasite that downregulate the immune responses (Mountford 1998; Ganley-Leal et al. 2005; Hewitson et al. 2005). However, the schistosome-derived molecules responsible for the strong protective effects elicited by RA schistosome larvae remain unclear.
The 70-kDa heat shock proteins are highly immunogenic, evolutionarily conserved proteins. They play an important primary role as intracellular molecular chaperones, preventing the misfolding and aggregation of proteins, and promoting their folding and translocation of others (Mayer and Bukau 2005). However, recent studies have shown that the 70-kDa heat shock proteins also have immunological functions, including the activation and regulation of innate immunity, depending on their locations in the extracellular milieu or on the cell surface (Radons and Multhoff 2005; Schmitt et al. 2007; Vega et al. 2008). The capacity of heat shock protein 70 (HSP70)–peptide complexes to elicit specific CD4+ and CD8+ T cell immune responses to the bound peptide makes HSP70 an attractive candidate for vaccination against cancers and infectious diseases, and also as an adjuvant (Tobian et al. 2004, 2005). Previous studies have shown that pathogen-derived HSP70 can induce protective immunity, not only as a vaccine antigen, but also as an adjuvant, promoting the level of protection against the antigen of interest (Planelles et al. 2001; Hartmann et al. 2014; Jaiswal et al. 2014). These results suggest that pathogen-derived HSP70 is a potent inducer of the innate and antigen-specific immune responses.
In schistosomiasis research, several studies, including a proteomic analysis, have demonstrated that the RA-Schistosoma-derived 70-kDa heat shock protein (SjHSP70) is upregulated after RA (Yang et al. 2009; Tian et al. 2013), and the DNA vaccine developed from SjHSP70 induces partial protection against experimental infection (He et al. 2010). In a preliminary study, we found that RA schistosomula cultured in vitro showed a necrotic phenotype. Therefore, we speculated that parasite-derived stress proteins, such as HSP70, calreticulin, and high-mobility group box 1 protein (HMGB1), may be released into the extracellular milieu or expressed on the cell surface, and may play important roles in stimulating high levels of innate and adaptive immune responses to a RA vaccine. Therefore, in this study, we focused on the immunobiological function of SjHSP70, including the expression profile of SjHSP70 in RA-schistosomula-derived cells, the protective efficacy induced by recombinant SjHSP70 (rSjHSP70) against cercaria challenge in mice, and the humoral and cellular immune responses to rSjHSP70. We show that the expression of SjHSP70 on the surfaces of cells derived from early RA schistosomula was higher than that on the cells from normal larvae, and that SjHSP70 alone (without adjuvant) can induce protection against cercarial challenge.
Materials and methods
Parasites and animals
BALB/c and C57BL/6 mice, 6–8 weeks old, were purchased from the Shanghai Experimental Animal Center, Chinese Academy of Sciences. Oncomelania hupensis (a Chinese mainland snail strain) carrying S. japonicum cercariae was purchased from the Jiangsu Institute of Parasitic Diseases (Wuxi, China). The study was approved by the Shanghai Veterinary Research Institute Animal Ethics Committee, Chinese Academy of Agricultural Sciences (Shanghai, China).
Cloning, expression, and purification of SjHSP70
Based on GenBank sequence AY813185, the full-length complementary DNA (cDNA) of SjHSP70 was amplified by polymerase chain reaction (PCR) from an S. japonicum egg cDNA template using specific primers: sense 5′-CGGGATCCATGTCTCGTAACTTGTTGTTA-3′ and antisense 5′-CCGGAATTCTTACTGCTTCTCCTGTTTAG-3′, with a BamHI site and EcoRI site, respectively. The PCR was performed as follows: initial denaturation at 95 °C for 4 min, followed by 35 cycles of 94 °C for 45 s, 55 °C for 45 s, and 72 °C for 2 min, with a final extension for 10 min at 72 °C. The PCR products were identified by electrophoresis on 1 % agarose gel and cloned into the pET28a(+) vector (Invitrogen™) using T4 ligase (Takara) after digestion with BamHI and EcoRI (Takara). Positive clones were screened by transformation into Escherichia coli DH5α cells. The recombinant plasmids were confirmed with PCR, restriction digestion, and sequence analyses. Escherichia coli BL21(DE3) cells transformed with the recombinant plasmid pET28a(+)–SjHSP70 were cultured in Luria–Bertani (LB) medium containing 1 mmol/L kanamycin at 37 °C. When the cells reached an of optical density at 600 nm (OD600) of 0.6–0.8, protein expression was induced with 1.0 mM isopropyl-β-d-thiogalactoside (IPTG). To purify the proteins, the transformed E. coli BL21(DE3) cells were cultured in 500 mL of LB broth containing kanamycin (1 mg/mL). At OD600 = 0.6–0.8, IPTG (1.0 mM/L) was added for 5–6 h at 37 °C. The cells were harvested by centrifugation at 10,000×g at 4 °C for 15 min, and broken with three freeze–thaw cycles and ultrasound on ice to release the proteins using a sonicator (Sonics & Materials Inc., USA). Inclusion bodies of SjHSP70 were enriched and purified with His-bind resin (Novagen, San Diego, USA), according to the manufacturer’s protocol and identified with sodium dodecyl sulfate-polyacrylamide gel (12.0 %) electrophoresis (SDS-PAGE). The recombinant SjHSP70 (rSjHSP70) protein was refolded by slow dialysis in phosphate-buffered saline (PBS, pH 7.4), containing decreasing concentrations of urea (6, 5, 4, 3, 2, 1, and 0 M).
Preparation of polyclonal antibodies directed against SjHSP70
Female BALB/c mouse, 6–8 weeks old, were housed in a specific-pathogen-free (SPF) environment. All the mice were divided into two groups. All the animals in one group were injected subcutaneously with 100 μg of recombinant SjHSP70 (rSjHSP70) emulsified in an equal volume of complete Freund’s adjuvant (FA) per mouse, and then immunized twice with 100 μg of rSjHSP70 emulsified with incomplete Freund’s adjuvant (IFA) at 2-week intervals. The animals in the other group were injected with PBS emulsified with FA and IFA as the controls. Seven days after the third immunization, serum samples were collected and stored at −80 °C until analysis.
Immunoblotting
Immunoblotting was performed with the standard procedure to determine the immunogenicity of SjHSP70. Crude adult worm antigens and purified rSjHSP70 were loaded into 12 % SDS-PAGE gels, separated, and transferred onto 0.22-μm nitrocellulose membranes. The membranes were blocked with 5 % skim milk powder in PBS containing Tween 20 (PBST) overnight at 4 °C and washed three times for 5 min each with PBST. They were then incubated for 1 h at room temperature in serum (diluted 1:100) from mice injected with rSjHSP70, from mice infected with S. japonicum or from SPF mice. After the membranes were washed three times as mentioned above, they were incubated with 1:15,000-diluted IRDye800CW (Odyssey)-conjugated goat anti-mouse IgG (eBioscience) for 40 min at room temperature. After the membranes were washed three times, as above, they were photographed with the Odyssey Infrared Imaging System, according to the manufacturer’s instructions.
Expression of SjHSP70 on the surfaces of cells from RA and normal schistosomula
Schistosoma cercariae shed from the infected Oncomelania snails were collected in distilled deionized H2O containing 5 % lactoalbumin hydrolysate, incubated on ice for 20 min, washed twice with RPMI 1640 medium (Hyclone) containing 1 % penicillin and streptomycin, and centrifuged at 500×g at 4 °C for 5 min. These cercariae were divided into the RA and normal groups. For irradiated attenuation, the cercarial bodies were exposed to a UV source (Cole-Parmer, USA) at a wavelength of 254 nm and an intensity of 400 μW/cm2 for 1 min. The cercarial tails were detached by repeatedly passing the cercarial suspension through a 22-G needle. The cercarial suspension was collected and RPMI 1640 medium was added to final volume of 10 mL. The samples were centrifuged at 500×g at 4 °C for 5 min. All but the bottom 2–3 mL of fluid was withdrawn and discarded. The pellet was resuspended in the residual solution and RPMI 1640 medium was added to a final volume of 10 mL. The samples were centrifuged at 500×g at 4 °C for 5 min. The pellets were washed twice, each time by resuspending them in 10 mL of RPMI 1640 medium, centrifugation at 100×g for 5 min at 4 °C, and removing the supernatant. The final pellet was placed in 2–3 mL of RPMI 1640 medium in a 30-mm diameter Petri dish and incubated at 37 °C under 5 % CO2 for 4, 7, 10, or 14 days. The schistosomula were collected and washed three times with 0.25 % trypsin–EDTA. After the schistosomula were cut with surgical scissor, schistosomula were digested at 4 °C overnight. The schistosomula cells (2 × 106) from the RA and normal groups were collected and washed with precooled PBST and blocked with 5 % goat serum for 10 min. The cells from RA and normal group were incubated with anti-SjHSP70 mouse serum (diluted 1:200 in PBST) for 40 min on ice. The cells as control were from digested normal schistosomula (cercarial detached tails by repeatedly passing the cercarial suspension through a 22-G needle) incubated at 37 °C under 5 % CO2 for 4, 7, 10, or 14 days and were incubated with negative mouse serum (diluted 1:200 in PBST). After the cells were washed with PBST, they were incubated with 1:2000-diluted Alexa-Fluor®-488-conjugated goat anti-mouse IgG (H+L) antibody for 40 min. After the cells were washed with PBST, they were resuspended in 500 μL of PBS and analyzed with flow cytometry.
Challenge experiments and antibody response analyses
For the vaccination/challenge trial, two independent experiments were performed. In each experiment, female BALB/c and C57BL/6 mice were divided into five groups each, with six mice per group. The mice in the SjHSP70 + Sj22.6 group were immunized with a combination of rSj22.6 plus rSjHSP70; the mice in the SjHSP70 group were immunized with rSjHSP70 alone; the mice in the Sj22.6 group were immunized with rSj22.6 alone; the mice in the Sj22.6 + ISA206 group were immunized with rSj22.6 emulsified with ISA206 adjuvant; and the control group mice were immunized with PBS alone. Each mouse was immunized with a subcutaneous injection of 40 μg of rSjHSP70 or rSj22.6 antigen, individually or in combination. The control group was immunized with an equal volume of PBS. All the mice were boosted twice at 14-day intervals with the same amount of antigen. Two weeks after the final vaccination, all the mice were challenged percutaneously with 40 ± 1 cercariae, using the cover slip method. Forty-two days after challenge, the mice were killed and perfused to determine their worm burdens. The reduction in the worm burden is expressed as a percentage of the burden recorded in the control group.
Cytokine analysis
To characterize the cytokine response, female BALB/c and C57BL/6 mice were divided into different treatment groups, as shown in Table 1. The mice were immunized with the appropriate antigen preparation. The immune procedure was described as above. On day 10 after the final immunization, the mice were killed to characterize their cytokine responses. The spleens of the mice were removed aseptically to prepare splenocyte suspensions. The spleens were ground through a 300-μm mesh and the filtered products were centrifuged at 800×g for 5 min. The cells were incubated with 2 mL of red cell break resin (TIANGEN) for 5 min, then resuspended in 10 mL of RPMI 1640 medium (HyClone). After centrifugation at 800×g for 5 min, the spleen cells were cultured in 96-well plates at 1 × 107 cells/mL in the presence of the appropriate antigen for each group at 37 °C under 5 % CO2 for 72 h. The restimulated cell culture supernatants were collected and stored at −20 °C. They were then assayed for interleukin 2 (IL-2), IL-4, IL-6, interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), IL-10, and IL-17a with flow cytometry using the BD™ Cytometric Bead Array (BD Biosciences).
Statistical analyses
Groups were compared using the two-tailed student t test and analysis of variance (ANOVA) with GraphPad Prism 5 software. Results were considered significant at a P value of <0.05 or 0.01.
Results
Expression of SjHSP70 and its identification with immunoblotting
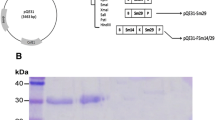
The full-length SjHSP70 nucleotide sequence determined by us contains 1962 nucleotides and encodes a 653-amino acid protein with a predicated molecular mass of approximately 72 kDa. As shown in Fig. 1, a band of ∼72 kDa was observed in the gels, corresponding to the predicted molecular size of rSjHSP70. After purification with His-resin affinity chromatography, a single band of ∼72 kDa was recovered (Fig. 1a, lane 5). Immunoblotting demonstrated that the recombinant protein was recognized by S. japonicum-infected mouse serum (Fig. 1b, lane 1), and a crude adult worm antigen of ∼72 kDa was recognized by the antiserum from mice immunized with rSjHSP70 (Fig. 1c, lane 1), indicating that rSjHSP70 shows good antigenicity and that sera directed against rSjHSP70 can be used to detect the expression of SjHSP70 in cells from S. japonicum.
Expression, purification, and immunogenicity of rSjHSP70. a Expression and purification of rSjHSP70. M protein molecular weight maker, lane 1 lysate of bacteria carrying the pET28a vector, lane 2 lysate of bacteria carrying the pET28a vector after IPTG induction, lane 3 lysate of bacteria carrying the pET28a–SjHSP70 vector before IPTG induction, lane 4 lysate of bacteria carrying the pET28a-SjHSP70 vector after IPTG induction, lane 5 purified SjHSP70 protein. b Analysis of the reactiogenicity of rSjHSP70. M protein molecular weight marker, lane 1 rSjHSP70 is recognized by negative serum of SPF mice, lane 2 rSjHSP70 is recognized by sera of mice infected with Schistosoma japonicum. c Analysis of the immunogenicity of rSjHSP70. M protein molecular weight marker, lane 1 Negative serum from SPF mice recognizes the crude worm antigen of Schistosoma japonicum, lane 2 rSjHSP70 antiserum recognizes the crude adult worm antigen of Schistosoma japonicum
Expression profile of SjHSP70 on the surfaces of cells from different stages of RA and normal schistosomula
To investigate whether the expression profiles of SjHSP70 differ in RA and normal schistosomula, the expression levels of SjHSP70 on the surfaces of cells from different stages of schistosomula were determined with flow cytometry. As shown in Fig. 2, the expression level (mean fluorescence intensity, MFI) of SjHSP70 on the surfaces of cells from RA schistosomula was significantly higher than that of normal parasites on days 4, 7, and 10 (P < 0.01) after treatment (Fig. 2a1–4, b1–4). There was no significant difference in the expression levels of SjHSP70 on the surfaces of cells from the RA and normal groups on day 14 (Fig. 2b4). These data suggest that SjHSP70 is expressed at higher levels on the surfaces of cells from early stage RA schistosomula.
SjHSP70 expression on cells from normal and RA schistosomula at different stages. Cercariae were exposed or not exposed to a UV source (254 nm) at an intensity of 400 μW/cm2 for 1 min and the tails were detached before culture for 4, 7, 10, or 14 days. Schistosomula were digested and the cells were collected to determine SjHSP70 expression with flow cytometry. Data illustrating representative experiments and derived from triplicate experiments are shown as a graph (a1–4) and mean fluorescence intensities (MFIs; b1–4). Statistically significant differences compared with the normal group are shown as *P < 0.05
Determination of the protection against S. japonicum cercariae challenge in mice immunized with rSjHSP70 or rSj22.6 plus rSjHSP70 without adjuvant
To measure the immunostimulatory capacity of SjHSP70, we determined whether rSjHSP70 induces protective immunity or enhances the protection induced by other antigens. To this end, we selected the rSj22.6 protein as the control antigen. Sj22.6 is annotated as a membrane-anchoring protein, located in the tegument of S. japonicum. The protection induced by immunization with the rSjHSP70 and rSj22.6 antigens (alone or in combination) were evaluated after cercarial challenge in both BALB/c and C57BL/6 mice. As shown in Table 2, immunization with rSjHSP70 alone induced the highest level of protection in BALB/c mice, followed by the rSj22.6 plus rSjHSP70 combination, rSj22.6 alone, and rSj22.6 emulsified with ISA206 adjuvant. There was no significant difference in the levels of protection afforded by rSj22.6 plus rSjHSP70, rSj22.6 emulsified with ISA206, and rSj22.6 alone. A replicated experiment showed similar results. These results suggest that immunization with rSjHSP70 alone induced a high level protection, and like ISA206 adjuvant, rSjHSP70 was unable to enhance the protection afforded by rSj22.6 against S. japonicum cercaria challenge in BALB/c mice.
In the C57BL/6 mice, immunization with rSjHSP70 alone also induced the highest level of protection, followed by rSj22.6 emulsified with ISA206 adjuvant, the rSj22.6 plus rSjHSP70 combination, and rSj22.6 alone. The protection afforded by immunization with rSj22.6 alone was negligible. There was no significant difference in the protective effects of immunization with rSj22.6 plus rSjHSP70 and rSj22.6 plus ISA206. A replicate experiment showed similar results. These data indicate that immunization with rSjHSP70 alone also induced a high level of protection, and both rSjHSP70 and ISA206 adjuvant enhanced the protection induced by rSj22.6 against S. japonicum cercarial challenge in C57BL/6 mice.
Taken together, these experimental results demonstrate that immunization with rSjHSP70 alone (without adjuvant) induced the highest level of protection against S. japonicum cercariae in both mouse strains. When combined with the rSj22.6 antigen, SjHSP70 might also exert an immunostimulatory effect as an adjuvant, like ISA206.
rSjHSP70 immunization induced a Th2-biased antibody response in both BALB/c and C57BL/6 mice
To clarify the mechanism underlying the protective immunity induced by rSjHSP70, sera from mice immunized with rSjHSP70 or rSj22.6 antigen (alone or in combination) or a control agent were tested with ELISAs to detect specific anti-rSjHSP70/rSj22.6 IgG1 and IgG2a antibody responses. The data are shown in Fig. 3. After the second and the third immunizations, the BALB/c mice immunized with the four antigen preparations (rSjHSP70, rSj22.6, the rSj22.6 plus rSjHSP70 combination, or rSj22.6 emulsified with ISA206) showed significantly higher levels of antigen-specific IgG1 than were elicited in the control animals treated with PBS (P < 0.01; Fig. 3a2). Immunization with rSjHSP70 or the rSj22.6 plus rSjHSP70 combination also induced significantly higher levels of anti-rSjHSP70/rSj22.6 + rSjHSP70-specific IgG2a than the control treatment (P < 0.01; Fig. 3a1). These data suggest that immunization with rSjHSP70 induces both Th1-type and Th2-biased responses, and that rSj22.6 also elicits a Th2-type response in BALB/c mice. After the second and third immunizations in the C57BL/6 mice, both rSjHSP70 alone and the rSj22.6 plus rSjHSP70 combination induced significantly higher levels of antigen-specific IgG1 than were detected in the control group (P < 0.01; Fig. 3b2). However, both rSj22.6 alone and rSj22.6 emulsified with ISA206 also induced an antigen-specific IgG1 response (Fig. 3b1). The levels of IgG1 induced by them were significantly lower than those induced by rSjHSP70 alone or the rSj22.6 plus rSjHSP70 combination. However, the levels of IgG2a induced by these four antigen preparations did not differ significantly from the control group after the second and third immunizations. These data suggest that immunization with rSjHSP70 induced both Th2-type antibody responses in the C57BL/6 mice.
Kinetics of specific anti-rSj22.6 and/or anti-rSjHSP70 responses in BALB/c mice (a1–2) and C57BL/6 mice (b1–2). Mice were immunized with three doses of rSj22.6 (40 μg/mouse) or rSjHSP70 (40 μg/mouse), alone or in combination. Serum samples were collected from six individual mice per group on days 7, 21, and 35 after the first immunization and assayed for IgG1 and IgG2 with ELISAs. The results are presented as mean absorbances measured at 450 nm. Data are shown as the mean ± SD of two independent experiments. Statistically significant differences compared with the control group are shown as *P < 0.05 or **P < 0.01
These experimental results suggest that the high levels of protection induced by immunization with rSjHSP70 correlated with the high levels of Th2-type anti-rSjHSP70 antibodies in both the BALB/c and C57BL/6 mice. This was also the case for the protection induced by the immunization of BALB/c mice with rSj22.6 alone. However, the levels of antigen-specific IgG antibodies did not correlate positively with the protection induced by rSj22.6 plus rSjHSP70 or by rSj22.6 emulsified with ISA206 adjuvant.
Cytokine response patterns of splenic lymphocytes of mice immunized with rSjHSP70 or rSj22.6
To further evaluate the immunostimulatory properties of SjHSP70, we determined the cytokine production patterns in mice immunized with rSjHSP70 or rSj22.6 (alone and in combination). The splenic lymphocytes of mice immunized with the rSjHSP70 or rSj22.6 antigen (alone or in combination) or with a control agent were restimulated in vitro with the corresponding antigen preparations (as shown in Table 1). The cytokines were detected with flow cytometry using a Cytometric Bead Array. The results are shown in Fig. 4. In the BALB/c mice, the splenocytes of mice immunized with rSjHSP70 or rSj22.6 emulsified with ISA206 produced much higher levels of IL-2 than the cells of the control mice (Fig. 4a7), which probably contributed to the higher levels of antigen-specific antibodies induced by these two antigens. The splenocytes of mice immunized with rSj22.6, rSj22.6 emulsified with ISA206, or rSj22.6 plus rSjHSP70 produced higher levels of proinflammatory cytokines (IFN-γ, TNF-α, IL-6, and IL-17A); a Th2-type cytokine (IL-4); and a regulatory cytokine (IL-10) than the cells from the control or rSjHSP70-immunized mice (Fig. 4a1–6). This suggests that these three antigen preparations (rSj22.6, rSj22.6 emulsified with ISA206, or rSj22.6 plus rSjHSP70) induced similar patterns of cytokine production, which might explain the results of the present experiment because similar levels of protection were induced in BALB/c mice by immunization with each individual antigen preparation.
Production of cytokines by splenocytes from BALB/c mice and C57BL/6 mice. Splenocytes from BALB/c mice (a1–7) and C57BL/6 mice (b1–7) were restimulated with rSj22.6 and/or rSjHSP70 for 72 h at 37 °C. The levels of IL-2, IL-4, IL-6, IFN-γ, TNF-α, IL-10, and IL-17a were measured. Data are shown as the mean ± SD of triplicate experiments. Statistically significant differences compared with the control group are shown as *P < 0.05 or **P < 0.01; statistically significant differences compared with the SjHSP70 (70) group and the control group are shown as **P < 0.01 and significant differences compared with the Sj22.6 + ISA206 group are shown as # P < 0.01
The splenocytes of the C57BL/6 mice immunized with rSj22.6 emulsified with ISA206, rSj22.6 plus rSjHSP70, or rSjHSP70 alone produced much higher levels of IL-2 than the cells of the control mice (Fig. 4b7). This was probably partially responsible for the antigen-specific antibody response observed. Immunization with rSj22.6 plus SjHSP70 or Sj22.6 emulsified with ISA206 also induced similar patterns of cytokine production, although the levels of proinflammatory cytokines (IFN-γ, TNF-α, IL-6, and IL-17A) and regulatory cytokines (IL-10) induced by rSj22.6 + rSjHSP70 were higher than those induced by Sj22.6 + ISA206 (Fig. 4b1–4, 6). Immunization with rSj22.6 alone induced the secretion of no other proinflammatory or regulatory cytokines, except a low level of the Th2-type cytokine IL-4, which probably resulted in a very low level of protection conferred by the immunization of the C57BL/6 mouse strain with this antigen alone.
These results demonstrate that rSjHSP70 alone elicited higher levels of IL-2 production in both mouse strains than the cells of the control mice, whereas rSj22.6 alone induced higher levels of proinflammatory and Th2 or regulatory cytokines only in the BALB/c mouse strain. The pattern of cytokine production in response to rSj22.6 plus SjHSP70 was similar to that elicited by rSj22.6 emulsified with ISA206 in both the BALB/c and C57BL/6 mice.
rSjHSP70 significantly enhanced the levels of cytokines induced by rSj22.6 in both the BALB/c and C57BL/6 mouse strains
There was no significant difference in the protection against S. japonicum afforded by Sj22.6 plus SjHSP70 and Sj22.6 emulsified with ISA206 adjuvant in both the BALB/c and C57BL/6 mice. We speculated that the rSjHSP70 protein exerts an adjuvant effect when combined with rSj22.6, and therefore, we evaluated whether rSjHSP70 enhances rSj22.6-induced cytokine production. To this end, the splenic lymphocytes of mice immunized with rSjHSP70 plus rSj22.6 or control antigen/agent were restimulated in vitro with rSj22.6 and rSjHSP70, respectively. The levels of cytokine production were determined with a Cytometric Bead Array (Fig. 4). The splenocytes of BALB/c mice immunized with rSj22.6 plus rSjHSP70 produced much higher levels of proinflammatory (IFN-γ, TNF-α, IL-6, and IL-17A), Th2-type (IL-4), and regulatory cytokines (IL-10) than the cells of mice immunized with rSj22.6 alone when restimulated with rSj22.6 (Fig. 5a1–6). However, these splenocytes showed lower levels of proinflammatory cytokines IFN-γ, TNF-α, and IL-6 or no significant difference in the levels of proinflammatory (IL-17A), Th2-type (IL-4), and regulatory cytokines (IL-10) than the cells of mice immunized with rSjHSP70 when restimulated with rSjHSP70 (Fig. 5a1–6). These data demonstrate that rSjHSP70 facilitates the production of cytokines induced by the rSj22.6 antigen in BALB/c mice.
Profiles of cytokines enhanced by SjHSP70 in splenocytes from BALB/c mice and C57BL/6 mice. Splenocytes from BALB/c mice (a1–7) and C57BL/6 mice (b1–7) were restimulated with rSj22.6 and/or rSjHSP70 for 72 h at 37 °C. The levels of IL-4, IL-6, IFN-γ, TNF-α, IL-10, and IL-17a were measured. Data are shown as the mean ±SD of triplicate experiments. Statistically significant differences compared with the Sj22.6 (22.6) group are shown as **P < 0.01 or *P < 0.05; and significant differences compared with the SjHSP70 (70) group are shown as ## P < 0.01
The splenocytes of C57BL/6 mice immunized with rSj22.6 plus rSjHSP70 also produced much higher levels of proinflammatory (IFN-γ, TNF-α, IL-6, and IL-17A), Th2-type (IL-4), and regulatory cytokines (IL-10) than the cells of mice immunized with rSj22.6 alone when restimulated with rSj22.6 (Fig. 5b1–6). However, the splenocytes of mice immunized with rSj22.6 + rSjHSP70 produced higher levels of IFN-γ, IL-6, Il-4, and IL-10 when restimulated with rSjHSP70 antigen than the cells of mice immunized with rSjHSP70 (Fig. 5b1, 3, 6–7). The concentrations of these cytokines were very low, so rSj22.6 had a negligible effect on the levels of cytokines induced by rSjHSP70. These data demonstrate that rSjHSP70 significantly promotes the production of cytokines induced by the rSj22.6 antigen in C57BL/6 mice.
These results indicate that rSjHSP70 significantly enhances the levels of cytokines induced by rSj22.6 in both BALB/c and C57BL/6 mice, suggesting that rSjHSP70 exerts an adjuvant effect when combined with rSj22.6.
Discussion
It is widely accepted that there is a quantitative and/or qualitative difference between the immune responses elicited by an RA Schistosoma larval vaccine and those elicited by the normal parasite (Ganley-Leal et al. 2005; Hewitson et al. 2005; Wilson and Coulson 2009). However, the Schistosoma-derived molecules that are responsible for this difference remain unclear. Based on the conclusions of related research, we speculated that RA-parasite-derived SjHSP70 might be an antigen that induces a high level of protective immunity. In the underlying mechanism, SjHSP70 molecules probably activate the innate immune system. For example, SjHSP70 might be involved in various inflammatory conditions by affecting cells of the innate immune system and cells that are not part of the immune system but communicate with it. SjHSP70 may also be a potent antigen carrier and natural adjuvant. Whether the SjHSP70 protein has these immunological properties is still unclear. In the present study, we provided further evidence for the immunostimulatory functions of SjHSP70, including its induction of a variety of cytokines and protective immunity against S. japonicum cercarial challenge in both BALB/c and C57BL/6 mice. Importantly, we have shown that the expression of SjMLP/HSP70 on the surfaces of cells derived from early RA schistosomula is significantly higher than on cells from normal parasites, suggesting that SjMLP/HSP70 might be one of the immunogenic molecules responsible for the greater protective efficacy of the RA Schistosoma larval vaccine.
To generate the recombinant protein, a gene encoding a mortalin-like protein from S. japonicum (SjMLP/HSP70) was cloned and recombinant SjMLP/HSP70 (rSjMLP/HSP70) was expressed in E. coli. The rSjMLP/HSP70 preparation was treated with polymixin B-coated beads to remove any lipopolysaccharide. This purified preparation can be used as the material for further research. He et al. previously identified SjMLP/HSP70 as a member of the Schistosoma HSP70 family, and the protein is expressed in the egg, cercaria, schistosomula, and adult worm, localizing predominantly in the mitochondria (He et al. 2010). However, in the present study, we observed that the SjMLP/HSP70 protein is expressed on the surfaces of cells from in vitro-cultured schistosomula. This may be because HSP70 is translocated to the cell surface under stressful conditions, such as UV irradiation in vitro and drug treatment (Schmitt et al. 2007). The SjHSP70 protein was also differentially expressed in RA and normal schistosomula. The levels of SjHSP70 were higher in in vitro-cultured 4-, 7-, and 10-day RA schistosomula than in the corresponding normal parasites. A plausible explanation is that cells are more severely damaged by UV irradiation than by in vitro culture insult, and the increased expression of SjMLP/HSP70 might protect the cells against UV-induced damage. Indeed, it has been reported that HSP70 protects the murine epidermis and fibroblasts from UVB-induced radiation damage (Simon et al. 1995; Matsuda et al. 2010). A hypothesized mechanism is that the translocation of HSP70 to the cell surface stabilizes the integrity of the plasma membrane and elevated HSP70 levels inhibit UV-irradiation-induced cell apoptosis (Nylandsted et al. 2004; Bivik et al. 2007). Our investigation has shown that the percentages of early apoptotic and late apoptotic cells in in vitro-cultured 4- and 7-day RA schistosomula are significantly lower than in the corresponding normal parasites (unpublished data), suggesting that cell apoptosis in early stage RA schistosomula is partly inhibited. This can be attributed to the antiapoptotic function of elevated SjMLP/HSP70 on the surfaces of the cells from RA schistosomula. However, in vitro-cultured 10- and 14-day RA schistosomula display a more severely necrotic phenotype than the corresponding normal parasites (unpublished data). Therefore, it is probable that cells from in vitro-cultured normal schistosomula show more apoptotic characteristics, whereas cells from RA parasites show more necrotic properties. These distinct types of cell death induce different types of responses. Apoptotic cells that are engulfed by dendritic cells may produce a state of antigenic tolerance, whereas necrotic cells may induce an immunogenic response (Zhou et al. 2013; Golden et al. 2014). The molecular mechanisms of immunogenic cell death induced by UV irradiation are not completely understood. However, it is likely that some stress molecules, including members of the heat shock protein family, such as HSP70 and HSP90, and the damage-associated molecular pattern HMGB1 might be exposed on or released onto the cell surface or into the extracellular milieu to play important roles in inducing immunogenic responses (Williams and Ireland 2008). A recent study has demonstrated the ability of membrane-bound and secreted HSP70 to significantly enhance the gag-specific T cell responses and increase the breadth of the T cell responses to include subdominant epitopes. Membrane-bound or secreted HSP70 also significantly improves the multifunctionality of HIV-specific T cells and T cell proliferation (Garrod et al. 2014). Whether membrane-bound or secreted SjHSP70 protein from RA schistosomula displays the immunostimulatory potency described above must be determined experimentally.
To test whether SjHSP70 protein has an immunostimulatory function, we evaluated the protective efficacy of rSjHSP70 alone or in a cocktail of rSj22.6 and rSjHSP70 in a mouse model. Both C57BL/6 and BALB/c mice immunized with rSjHSP70 alone showed protection against experimental infection, and rSjHSP70 protein enhanced the level of protection afforded by the rSj22.6 protein in C57BL/6 mice. This suggests that the SjHSP70 protein is not only an effective protective antigen, but also has a possible adjuvant function. We believe this finding is consistent with clues present in the literatures. For instance, in a recent study, He et al. demonstrated that DNA vaccines of SjHSP70 alone or SjGST combined with SjHSP70 induced 25.94 and 31.31 % reductions in the worm burden in BALB/c mice, respectively (He et al. 2010). HSP-induced protection was also observed in both humans infected with Plasmodium falciparum (Alexandre et al. 1997) and animals infected with Toxoplasma gondii (Mohamed et al. 2003), Leishmania donovani (Kaur et al. 2011), Trichinella spiralis (Wang et al. 2009), or Hantavirus (Li et al. 2008). Previously published studies (He et al. 2010; Kaur et al. 2011) showed that immunization with the antigen of interest (protein or DNA vaccine) together with the HSP70 antigen more significantly reduced the parasite load than the antigen of interest (protein or DNA vaccine) or the HSP70 antigen alone. However, we found that immunization with rSjHSP70 alone induced the highest level of protection in both C57BL/6 and BALB/c mice. Moreover, the protective efficacy of rSj22.6 alone and the adjuvanticity of rSjHSP70 depended on the genetic backgrounds of the mouse strains. Only in the Th2-type mouse strain (BALB/c) (Watanabe et al. 2004) did rSj22.6 alone induce a certain level of protection, whereas only in the Th1-type mouse strain (C57BL/6) (Watanabe et al. 2004) did rSjHSP70 exert its adjuvant effects.
To clarify the mechanisms underlying the phenomena described above, we analyzed the mouse sera for the presence of specific anti-SjHSP70 antibody isotypes. We found that rSjHSP70 vaccination induced a mixed Th1/Th2-type antibody response against SjHSP70 in the Th2-type mice and a Th2-biased type of anti-SjHSP70 antibody response in the Th1-type mice, indicating that the SjHSP70 molecule elicits a strong IgG antibody response in both mouse strains, and that these IgG antibodies probably partly contribute to the high level of protection induced by vaccination with the rSjHSP70 protein alone. Several studies have demonstrated that a strong humoral response can be elicited by different pathogen-derived HSP70 proteins, including Mycobacterium tuberculosis HSP70 (Bonorino et al. 1998; Aosai et al. 2002), Leishmania infantum HSP70 (Rico et al. 2002), and Toxoplasma gondii HSP70 (Aosai et al. 2002). These HSP70 proteins induce the proliferation of B cells, suggesting that they have B cell mitogenic properties. Therefore, we speculated that the SjHSP70 protein might also function as a B ell mitogen, producing a strong antigen-specific IgG response. This hypothesis must be validated experimentally.
An unexpected, but interesting finding of this study was that BALB/c mice immunized with rSj22.6 alone (without adjuvant) showed protection against experimental S. japonicum infection, although the protective efficacy of rSj22.6 alone was lower than that of rSjHSP70 alone. It has been documented that two protein antigens of 74 and 68 kDa, isolated from Schistosoma mansoni worm antigen preparations, can induce high levels of protection (30–76 %) without adjuvant. The ability of antibodies against these antigens to mediate protection in vivo might correlate with the recognition of epitopes on the surfaces of early live schistosomula, which are the target of antibody-mediated damage (King et al. 1987; Attallah et al. 1999). The Sj22.6 protein has also been identified as a tegumental protein and can be expressed in the lung-stage schistosomulum of S. japonicum (Mulvenna et al. 2010; Zhang et al. 2012). Both B cell and Th cell epitopes on the Sj22.6 protein have been identified with different techniques (Waine et al. 1997; Zhang et al. 2012). BALB/c mice immunized with rSj22.6 (with Freund’s complete adjuvant) showed partial protection (32.1 %) and high levels of IgG (Su et al. 1999). In our study, vaccination with rSj22.6 alone (without adjuvant) also conferred partial protection against experimental infection and induced high levels of IgG1 antibodies in BALB/c mice. Immunization with rSj22.6 alone also induced higher levels of Th1- and/or Th2-type cytokine production by lymphocytes in BALB/c mice than in C57BL/6 mice, indicating that a stronger cellular immune response might be induced by rSj22.6 alone. These data suggest that some antigens from the tegument of the Schistosoma schistosomulum might be good target antigens that provide protective immunity. These proteins can directly stimulate the B cells or Th cells responsible for protective immunity, although the protection induced by them is not only dependent on the immunobiological features of the antigens but also on the genetic backgrounds of mouse strains.
Another interesting finding of this study is that the protection afforded by the cocktail of Sj22.6 and SjHSP70 against S. japonicum was lower in both mouse strains than that afforded by rSjHSP70 alone, indicating that rSj22.6 protein mixed with rSjHSP70 reduces the protective efficacy of rSjHSP70 protein. It has been reported that combing different antigens does not result in a more effective protective immunity against Schistosoma infection than when each component is administered individually, and immune interference resulting from immunization with multiple antigens is considered one mechanism responsible for the lack of enhanced protection (Wang et al. 2010). We observed a similar effect in the present study. To explain the phenomenon, we investigated the cytokine production profiles of splenic lymphocytes after immunization with individual antigens and combinations of the antigens. The levels of both proinflammatory and Th2/regulatory cytokines produced after immunization with rSj22.6 together with rSjHSP70 or with rSj22.6 emulsified with ISA206 were significantly increased compared with those induced by immunization with rSjHSP70 alone. It is likely that those cytokines, especially the regulatory cytokines, downregulate the inflammatory response, which is important in protecting mice against S. japonicum infection.
Interestingly, we also found that the protective efficacy of the cocktail of Sj22.6 and SjHSP70 against S. japonicum was approximately equal to that of Sj22.6 emulsified with ISA206 in both mouse strains. Therefore, we speculated that the rSjHSP70 protein, when combined with rSj22.6 antigen, exert an adjuvant effect rather than induces protective immunity. To test this hypothesis, we determined the cellular immune response against the rSj22.6 antigen in mice vaccinated with different immunization protocols. rSjHSP70 exceeded the efficacy of the ISA206 adjuvant in promoting the production of both proinflammatory (IFN-γ, TNF-α, IL-6, and IL-17A) and Th2/regulatory (IL-4, IL-10) cytokines in the C57BL/6 mice. The secretion of proinflammatory cytokines (TNF-α and IL-17A) was also elevated and the production of Th2/regulatory cytokines (IL-4, IL-10) was boosted in BALB/c mice, but less than by ISA206 adjuvant. This suggests that rSjHSP70 effectively induces an rSj22.6-specific cellular immune response, and might be a potent adjuvant. Many studies have described the ability of mouse HSP70s to enhance the immunogenicity of associated antigens or peptides (Roman and Moreno 1997; Tobian et al. 2004, 2005). It is likely that the HSP70–antigen/peptide complex is effectively internalized into antigen-presenting cells by receptor-mediated endocytosis. Once internalized, the associated antigen or peptide is released, processed, and made available for assembly into major histocompatibility molecules, increasing their presentation to naïve T cells (Tobian et al. 2004, 2005). Whether SjHSP70 can facilitate the presentation of associated antigens or peptides warrants further investigation.
In conclusion, we have demonstrated that higher levels of SjHSP70 are presented on the surfaces of RA schistosomula cell than on the cells of normal parasites, and that the recombinant SjHSP70 protein is not only a good protective antigen, but also has a potential adjuvant function. This study provides preliminary data for the further clarification of the molecular mechanisms underlying the protective immunity afforded by the RA schistosomal vaccine.
References
Alexandre CO, Camargo LM, Mattei D, Ferreira MU, Katzin AM, Camargo EP, da Silva LH (1997) Humoral immune response to the 72 kDa heat shock protein from Plasmodium falciparum in populations at hypoendemic areas of malaria in western Brazilian Amazon. Acta Trop 64(3–4):155–166
Aosai F, Chen M, Kang HK, Mun HS, Norose K, Piao LX, Kobayashi M, Takeuchi O, Akira S, Yano A (2002) Toxoplasma gondii-derived heat shock protein HSP70 functions as a B cell mitogen. Cell Stress Chaperones 7(4):357–364
Attallah AM, Attia H, Ismail H, Yones E, El-Nashar EM, Abd El-Kader K, Tabll A, Saad A, Sultan A (1999) Vaccination against Schistosoma mansoni infection using 74 kDa Schistosoma protein antigen. Vaccine 17(22):2786–2791
Bickle QD (2009) Radiation-attenuated schistosome vaccination—a brief historical perspective. Parasitology 136(12):1621–1632
Bivik C, Rosdahl I, Ollinger K (2007) Hsp70 protects against UVB induced apoptosis by preventing release of cathepsins and cytochrome c in human melanocytes. Carcinogenesis 28(3):537–544
Bonorino C, Nardi NB, Zhang X, Wysocki LJ (1998) Characteristics of the strong antibody response to mycobacterial Hsp70: a primary, T cell-dependent IgG response with no evidence of natural priming or gamma delta T cell involvement. J Immunol 161(10):5210–5216
Ford MJ, Bickle QD, Taylor MG (1984) Immunization of rats against Schistosoma mansoni using irradiated cercariae, lung schistosomula and liver-stage worms. Parasitology 89:327–344
Ganley-Leal LM, Guarner J, Todd CW, Da’Dara AA, Freeman GL Jr, Boyer AE, Harn DA, Secor WE (2005) Comparison of Schistosoma mansoni irradiated cercariae and Sm23 DNA vaccines. Parasite Immunol 27(9):341–349
Garrod TJ, Grubor-Bauk B, Gargett T, Li Y, Miller DS, Yu W, Major L, Burrell CJ, Wesselingh S, Suhrbier A, Gowans EJ (2014) DNA vaccines encoding membrane-bound or secreted forms of heat shock protein 70 exhibit improved potency. Eur J Immunol 44(7):1992–2002
Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC (2014) Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 3:e28518
Harrison RAI, Bickle QD, Kiare S, James ER, Andrews BJ, Sturrock RF, Taylor MG, Webbe G (1990) Immunization of baboons with attenuated schistosomula of Schistosoma haematobium: levels of protection induced by immunization with larvae irradiated with 20 and 60 krad. Trans Royal Soc Trop Med Hyg 84:89–99
Hartmann W, Singh N, Rathaur S, Brenz Y, Liebau E, Fleischer B, Breloer M (2014) Immunization with Brugia malayi Hsp70 protects mice against Litomosoides sigmodontis challenge infection. Parasite Immunol 36(4):141–149
He S, Yang L, Lv Z, Hu W, Cao J, Wei J, Sun X, Yang J, Zheng H, Wu Z (2010) Molecular and functional characterization of a mortalin-like protein from Schistosoma japonicum (SjMLP/hsp70) as a member of the HSP70 family. Parasitol Res 107(4):955–966
Hewitson JP, Hamblin PA, Mountford AP (2005) Immunity induced by the radiation-attenuated schistosome vaccine. Parasite Immunol 27(7–8):271–280
Jaiswal AK, Khare P, Joshi S, Kushawaha PK, Sundar S, Dube A (2014) Th1 stimulatory proteins of Leishmania donovani: comparative cellular and protective responses of rTriose phosphate isomerase, rProtein disulfide isomerase and rElongation Factor-2 in combination with rHSP70 against visceral Leishmaniasis. PLoS ONE 9(9):e108556
Jwo J, LoVerde PT (1989) The ability of fractionated sera from animals vaccinated with irradiated cercariae of Schistosoma mansoni to transfer immunity to mice. J Parasitol 75(2):252–260
Kaur T, Sobti RC, Kaur S (2011) Cocktail of gp63 and Hsp70 induces protection against Leishmania donovani in BALB/c mice. Parasite Immunol 33(2):95–103
King CH, Lett RR, Nanduri J, el Ibiary S, Peters PA, Olds GR, Mahmoud AA (1987) Isolation and characterization of a protective antigen for adjuvant-free immunization against Schistosoma mansoni. J Immunol 139(12):4218–4224
Lei ZL, Zheng H, Zhang LJ, Zhu R, Guo JG, Li SZ, Wang LY, Chen Z, Zhou XN (2011) Schistosomiasis status in People’s Republic of China in 2010. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 23:599–604
Li J, Li KN, Gao J, Cui JH, Liu YF, Yang SJ (2008) Heat shock protein 70 fused to or complexed with Hantavirus nucleocapsid protein significantly enhances specific humoral and cellular immune responses in C57BL/6 mice. Vaccine 26(25):3175–3187
Lin D, Tian F, Wu H, Gao Y, Wu J, Zhang D, Ji M, McManus DP, Driguez P, Wu G (2011) Multiple vaccinations with UV-attenuated cercariae in pig enhance protective immunity against Schistosoma japonicum infection as compared to single vaccination. Parasit Vector 4:103
Majid AA, Bushara HO, Saad AM, Hussein MF, Taylor MG, Dargie JD, Marshall TF, Nelson GS (1980) Observations on cattle schistosomiasis in the Sudan, a study in comparative medicine. III. Field testing of an irradiated Schistosoma bovis vaccine. Am J Trop Med Hyg 29:452–455
Matsuda M, Hoshino T, Yamashita Y, Tanaka K, Maji D, Sato K, Adachi H, Sobue G, Ihn H, Funasaka Y, Mizushima T (2010) Prevention of UVB radiation-induced epidermal damage by expression of heat shock protein 70. J Biol Chem 285(8):5848–5858
Mayer MP, Bukau B (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62(6):670–684
Mohamed RM, Aosai F, Chen M, Mun HS, Norose K, Belal US, Piao LX, Yano A (2003) Induction of protective immunity by DNA vaccination with Toxoplasma gondii HSP70, HSP30 and SAG1 genes. Vaccine 21(21–22):2852–2861
Mountford AP (1998) Vaccination against schistosomiasis: the case for lung-stage antigens. Parasitol Today 14:109–114
Mountford AP, Coulson PS, Wilson RA (1988) Antigen localization and the induction of resistance in mice vaccinated with irradiated cercariae of Schistosoma mansoni. Parasitology 97(Pt 1):11–25
Mulvenna J, Moertel L, Jones MK, Nawaratna S, Lovas EM, Gobert GN, Colgrave M, Jones A, Loukas A, McManus DP (2010) Exposed proteins of the Schistosoma japonicum tegument. Int J Parasitol 40(5):543–554
Nylandsted J, Gyrd-Hansen M, Danielewicz A, Fehrenbacher N, Lademann U, Høyer-Hansen M, Weber E, Multhoff G, Rohde M, Jäättelä M (2004) Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med 200(4):425–435
Planelles L, Thomas MC, Alonso C, López MC (2001) DNA immunization with Trypanosoma cruzi HSP70 fused to the KMP11 protein elicits a cytotoxic and humoral immune response against the antigen and leads to protection. Infect Immun 69(10):6558–6563
Radons J, Multhoff G (2005) Immunostimulatory functions of membrane-bound and exported heat shock protein 70. Exerc Immunol Rev 11:17–33
Richter D, Harn DA (1993) Candidate vaccine antigens identified by antibodies from mice vaccinated with 15- or 50-kilorad-irradiated cercariae of Schistosoma mansoni. Infect Immun 61(1):146–154
Richter D, Incani RN, Harn DA (1993) Isotype responses to candidate vaccine antigens in protective sera obtained from mice vaccinated with irradiated cercariae of Schistosoma mansoni. Infect Immun 61(7):3003–3011
Rico AI, Gironès N, Fresno M, Alonso C, Requena JM (2002) The heat shock proteins, Hsp70 and Hsp83, of Leishmania infantum are mitogens for mouse B cells. Cell Stress Chaperones 7(4):339–346
Roman E, Moreno C (1997) Delayed-type hypersensitivity elicited by synthetic peptides complexed with Mycobacterium tuberculosis hsp 70. Immunology 90(1):52–56
Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C (2007) Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol 81(1):15–27
Simon MM, Reikerstorfer A, Schwarz A, Krone C, Luger TA, Jäättelä M, Schwarz T (1995) Heat shock protein 70 overexpression affects the response to ultraviolet light in murine fibroblasts. Evidence for increased cell viability and suppression of cytokine release. J Clin Invest 95(3):926–933
Su C, Ma L, Wang R, Hu X, Chen S, Shao L, Wu H, Shen L, Zhang Z, Wu G (1999) Studies on immunoprotection in mice after immunization with Schistosoma japonicum 22.6 kDa recombinant protein. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 17(5):288–291
Tian F, Hou M, Chen L, Gao Y, Zhang X, Ji M, Wu G (2013) Proteomic analysis of schistosomiasis japonica vaccine candidate antigens recognized by UV-attenuated cercariae-immunized porcine serum IgG2. Parasitol Res 112(8):2791–2803
Tobian AA, Canaday DH, Harding CV (2004) Bacterial heat shock proteins enhance class II MHC antigen processing and presentation of chaperoned peptides to CD4+ T cells. J Immunol 173(8):5130–5137
Tobian AA, Harding CV, Canaday DH (2005) Mycobacterium tuberculosis heat shock fusion protein enhances class I MHC cross-processing and -presentation by B lymphocytes. J Immunol 174(9):5209–5214
Vega VL, Rodríguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De Maio A (2008) Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol 180(6):4299–4307
Waine GJ, Mazzer DR, Brandt ER, McManus DP (1997) A dominant B-cell epitope on the 22 kDa tegumental membrane-associated antigen of Schistosoma japonicum maps to an EF-hand calcium binding domain. Parasite Immunol 19(8):337–345
Wang S, Zhu X, Yang Y, Yang J, Gu Y, Wei J, Hao R, Boireau P, Cui S (2009) Molecular cloning and characterization of heat shock protein 70 from Trichinella spiralis. Acta Trop 110(1):46–51
Wang X, Zhang L, Chi Y, Hoellwarth J, Zhou S, Wen X, He L, Liu F, Wu C, Su C (2010) The nature and combination of subunits used in epitope-based Schistosoma japonicum vaccine formulations affect their efficacy. Parasite Vector 3:109
Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A (2004) Innate immune response in Th1- and Th2-dominant mouse strains. Shock 22(5):460–466
Williams JH, Ireland HE (2008) Sensing danger–Hsp72 and HMGB1 as candidate signals. J Leukoc Biol 83(3):489–492
Wilson RA, Coulson PS (2009) Immune effector mechanisms against schistosomiasis: looking for a chink in the parasite’s armour. Trends Parasitol 25(9):423–431
Yang LL, Lv ZY, Hu SM, He SJ, Li ZY, Zhang SM, Zheng HQ, Li MT, Yu XB, Fung MC, Wu ZD (2009) Schistosoma japonicum: proteomics analysis of differentially expressed proteins from ultraviolet-attenuated cercariae compared to normal cercariae. Parasitol Res 105(1):237–248
Zhang YL, Jia K, Zhao BP, Li Y, Yuan CX, Yang JM, Lin JJ, Feng XG (2012) Identification of Th1 epitopes within molecules from the lung-stage schistosomulum of Schistosoma japonicum by combining prediction analysis of the transcriptome with experimental validation. Parasitol Int 61(4):586–593
Zhou F, Lauretti E, di Meco A, Ciric B, Gonnella P, Zhang GX, Rostami A (2013) Intravenous transfer of apoptotic cell-treated dendritic cells leads to immune tolerance by blocking Th17 cell activity. Immunobiology 218(8):1069–1076
Acknowledgments
This study was supported by the Fund on Basic Scientific Research Project of Nonprofit Central Research Institutions of China (grant no. 2013JB15) and the Fund on Sci & Tech Innovation Program of the Chinese Academy of Agricultural Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ming Ming Duan and Rui Min Xu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Duan, M.M., Xu, R.M., Yuan, C.X. et al. SjHSP70, a recombinant Schistosoma japonicum heat shock protein 70, is immunostimulatory and induces protective immunity against cercarial challenge in mice. Parasitol Res 114, 3415–3429 (2015). https://doi.org/10.1007/s00436-015-4567-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4567-z