Abstract
Bronchopulmonary lophomoniasis (BPL) is an emerging disease of potential importance. BPL is presented by non-specific clinical picture and is usually accompanied by immunosuppression. Culture of Lophomonas blattarum is difficult and its molecular diagnosis has not yet been developed. Therefore, microscopic examination of respiratory samples, e.g., bronchoalveolar lavage (BAL) or sputum, is the mainstay of BPL diagnosis. Creola bodies and ciliocytophthoria are two forms of bronchial cells which occur in chest diseases with non-specific clinical picture like that of BPL. Both forms could be misrecognized as multi-flagellates because of their motile cilia in the wet mounts and due to shape variability of L. blattarum in stained smears. The aim of the study is to compare different staining techniques for visualizing L. blattarum to improve the recognition and diagnosis of BPL, to distinguish respiratory epithelial cells from L. blattarum and to decide which stain is recommended in suspected cases of BPL. BAL samples from patients which contain L. blattarum, creola bodies, and ciliocytophthoria were collected then wet mounts were examined. The BAL samples were also stained by Papanicolaou (PAP), Giemsa, hematoxylin and eosin (H & E), trichrome, Gram, and Diff-Quik (DQ) stains. The different staining techniques were compared regarding the stain quality. In wet mounts, the ciliary movement was coordinate and synchronous while the flagellar movement was wavy and leaded to active swimming of L. blattarum. In stained slides, bronchial cells were characterized by the presence of basal nucleus and the terminal bar from which the cilia arise. Trichrome was the best stain in demonstration of cellular details of L. blattarum. H & E, PAP, and Giemsa stains showed good quality of stains. Gram and DQ stains showed only pale hues of L. blattarum. We recommended adding Wheatley’s trichrome staining to the differential diagnosis workup of cases of non-specific chest infections, especially when BPL is suspected, to avoid overdiagnosis or underdiagnosis of it.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lophomonas blattarum belongs to Lophomonas of Lophomonadae in Hypermastigida and Lophomonadina of Mastigophora in protozoa (Farmer 1980). L. blattarum is a multi-flagellated parasite which inhabits the hindgut of termites and Dictyopteris (cockroaches) as an endocommensal (Strand and Brooks 1977). It is subsequently eliminated from the hindgut in feces and it can encyst in adverse external environment conditions, as has been shown for other flagellated protozoa (Chavez-Munguya et al. 2007; Bittencourt-Silvestre et al. 2010; Zaragatzki et al. 2010). L. blattarum can pollute numerous things, including dust through the crawling of termites and cockroaches. L. blattarum can parasitize the lung of the patient through inhalation of dust (Wang et al. 2006). The existence of various species of protozoa, pathogenic and nonpathogenic, was reported in the hindgut of cockroaches (Brugerolle et al. 2003; Pai et al. 2003).
Bronchopulmonary lophomoniasis (BPL) is an emerging disease of potential importance (Yao 2008; Wu and Liu 2010; Zhang et al. 2011). About 70 cases were identified in the literature. The majority of these reports were from China, with some cases from Peru and Spain. Immunosuppression was a feature in a number of the case reports. Clinical presentation was non-specific, including symptoms such as fever, cough, and breathlessness. All the cases in the reviewed literature had evidence of previous and/or concomitant respiratory disease. These cases showed pulmonary infiltrate in chest computed tomography (CT). Around 35 % of patients had eosinophilia. Antiprotozoal therapy was generally effective (Martinez-Giron and Doganci 2010; Zerpa et al. 2010; Martinez-Giron 2013; Martinez-Giron and van Woerden 2013).
Lophomonas blattarum is difficult to culture than many other protozoa living in the gut of cockroaches. However, it has been grown in three mediums utilized by Chen (1933) and in a medium which contained 0.8 % salt solution with yeast added as food (Kirby 1950). Moreover, no molecular characterization of L. blattarum was developed. Therefore, the identification of this multi-flagellate in human samples has been based on the identification of morphological features under light microscopy using fresh and stained samples from the airways including sputum, bronchoalveolar lavages (BAL), bronchial brushings, and tracheal aspirates (Ribas et al. 2007; Martinez-Giron et al. 2011).
Multi-flagellate protozoa are difficult to differentiate from ciliated bronchial epithelial cells, and misidentification under light microscopy is a significant risk. In fresh samples, the motile cilia of the ciliated respiratory epithelial cells could be easily misidentified as flagellated protozoa. In stained smears, the creola bodies (small groups of ciliated bronchial cells) and ciliocytophthoria (detached ciliary tufts with cytoplasmic remnants) are misrecognized as multi-flagellates (Ribas et al. 2007; Martinez-Giron et al. 2011; Martinez-Giron and van Woerden 2014).
BAL samples sent to laboratory are most probably subjected to Papanicolaou (PAP), Giemsa, hematoxylin and eosin (H & E), Gram, Wheatley trichrome and Diff-Quik (DQ) staining. Therefore, the aim of our study is to compare different staining techniques for visualizing L. blattarum to improve the recognition and diagnosis of BPL, to distinguish respiratory epithelial cells from L. blattarum, and to decide which stain is recommended in suspected cases of L. blattarum infection.
Materials and methods
Parasite material and design of the study
Parasite material was collected from BAL samples, of a patient with an end-stage renal disease who complained of fever, severe cough, and expectoration. The blood picture showed eosinophilia and the CT scan showed severe lung infiltration. The case was not improved with regular antibiotic treatment and showed complete rapid improvement on metronidazol.
BAL samples that contain creola bodies and ciliocytophthoria were also collected. Informed consents were taken from the patients. Samples were subjected to wet mount examination and different staining techniques.
Staining procedures
Cytospins and smears were prepared, allowed to dry, and stained by the following stains:
-
1.
Papanicolaou (PAP) stain (Koss 1992):
Immediately fixed in 95 % ethanol, rinse in water (10 dips), Gill’s hematoxylin for 1 min, rinse in water until water is clear, 0.5 % ammonia water for 1 min, rinse in water, modified orange G for 1 min, two changes of 95 % ethanol (10 dips each), EA 50 for 1 min, two changes of 95 % ethanol (10 dips each), two changes of 100 % ethanol 2 min each, two changes of xylene 2 min each, and two changes of xylene 5 min each.
-
2.
Giemsa stain (El-Sayed and Hikal 2014):
Absolute methanol for 30 s (for fixation) and 20 % fresh Giemsa solution in pH 7.2 of phosphate buffer for 20 min.
-
3.
Hematoxylin and eosin (H & E) stain (Kiernan 2008):
Two changes of xylene for 10 min each, two changes of absolute ethanol for 5 min each, 95 % ethanol for 2 min, 70 % ethanol for 2 min, rinse in water, stain in hematoxylin solution for 8 min, rinse in water for 5 min, 1 % acid alcohol for 30 %, rinse water for 1 min, 0.2 % ammonia water for 1 min, rinse in water for 5 min, 95 % ethanol (10 dips), eosin for 1 min, 95 % ethanol for 5 min, two changes of absolute ethanol for 5 min each, and two changes of xylene for 5 min each.
-
4.
Wheatley’s trichrome stain (Garcia 2007):
Schaudinn’s fixative for 30 min, 70 % ethanol for 5 min, 70 % iodine alcohol (70 %) ethanol for 1 min, two changes of 70 % ethanol for 5 min, trichrome stain for 10 min, acetic acid alcohol (90 % ethanol) for 1 to 3 s, dipping in 100 % ethanol, two changes of 100 % ethanol for 3 min each, and two changes of xylene for 5 min each.
-
5.
Gram stain (York 2004):
Heat fixation, crystal violet for 1 min, rise in water, Gram’s iodine for 1 min, rinse in water, 95 % ethanol for 30 s, rinse in water, 0.25 % safranine for 1 min, and rinse in water.
-
6.
Diff-Quik stain (DQ, modified Giemsa stain) (Skipper and DeStephano 1989):
Air-dried slides were fixed in Diff-Quik fixative (1.8 mg/L triarylmethane in methyl alcohol) for 30 s, rinse in water, Diff-Quik solution 2: made of thiazine dye mixture: 1.25 g/L of pure dye (0.625 g/L Azure A and 0.625 g/L methylene blue) and phosphate buffer (pH 6.6) for 30 s, Diff-Quik solution 1: made of xanthene dye, 1 g/L of pure dye, phosphate buffer (pH 6.6), and sodium azide (0.01 %) as preservative for 30 s, and rinse in water.
Identification criteria and evaluation of different staining techniques
All stained slides, as well as wet mounts of BAL samples, were examined with Leica DM 2500 microscope at a magnification of ×400 and ×1000. Photographs were obtained using a Leica DFC 500 digital camera.
Different staining techniques were compared regarding the stain quality and the ability to demonstrate the flagella and the cellular content. A set of morphological criteria to differentiate L. blattarum from ciliated cell fragments in wet mount and stained slides were also described.
Results
All results are shown in Figs. 1, 2, 3, 4, and 5; Tables 1 and 2; and videos 1 and 2.
PAP-stained slides. a L. blattarum; irregular long flagella (fl) arise from the anterior part. Nucleus is not well apparent, only nuclear shadow (ns) appears. Some cytoplasmic vacuoles (v) are visible. b Bronchial cell; regular short cilia (ci) arise from the terminal bar (tb) at the luminal border of the cell. The nucleus (nc) is at the base of the cell. Some RBCs (r) appear in the field. (×1000)
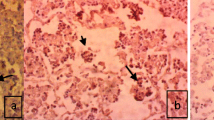
H & E-stained slides. a L. blattarum; irregular long flagella (fl) arise from anterior part. Nucleus is not well apparent, only nuclear shadow (ns) appears. Some RBCs (r) appear in the field. b Bronchial cell; regular short cilia (ci) arise from the terminal bar (tb) at the luminal border of the cell. The nucleus (nc) is at the base of the cell. c Creola body; cluster of detached bronchial cells which are ciliates (ci). d Ciliocytophthoria; degenerated bronchial cell in which a pinching off occurs between the cytoplasm bearing the cilia (ci) which arise from the terminal part, and the nucleated cytoplasm, resulting in a mass of cytoplasm-bearing cilia without a nucleus, and a degenerating nucleus (dn) with cytoplasm. In some degenerated bronchial cells, the cilia-bearing cytoplasm was completely separated from the degenerating nucleus. Some RBCs (r) appear in the field. (×1000)
Trichrome-stained slides. a L. blattarum; irregular long flagella (fl) arise over the anterior part which are longer at center and shorter at sides. The vesicular nucleus (nc) is well apparent. Some cytoplasmic vacuoles (v) are visible among the granular cytoplasm. b Bronchial cell; regular short cilia (ci) arise from the terminal bar (tb) at the luminal border of the cell. The nucleus (nc) is at the base of the cell. (×1000)
Discussion
Clinical picture of BPL is not specific, and therefore should be differentially diagnosed from other chest diseases. BPL should be suspected in cases of immunosuppression with severe chest infection, eosinophilia, and failure of response to regular antibiotic treatment.
Two morphological forms of respiratory epithelial cells, creola bodies and ciliocytophthoria, could be mistaken for L. blattarum. Creola bodies are clusters of desquamated epithelial cells that were reported in cases of bronchial allergy and infections (Yoshihara et al. 2006; Yamada and Yoshihara 2010). Ciliocytophthoria (or detached ciliary tufts) is a peculiar degeneration of the ciliated respiratory epithelium in which a pinching off occurs between the cilia-bearing cytoplasm and the nucleated cytoplasm, resulting in an anucleated mass of cytoplasm-bearing cilia and a degenerating nucleus with cytoplasm which are called ciliocytophthoria (Johnston and Elson 2008).
In wet mounts of our study, bronchial cells, ciliocytophthoria and creola bodies showed motility of cilia and therefore could be misdiagnosed as L. blattarum. Shakoor et al. (2011) and Khan et al. (2015) reported the presence of motile ciliary tufts that can mimic the motility of flagellated amoebae in wet mounts of CSF and presents a diagnostic dilemma. Kuritzkes et al. (1988) reported that these detached ciliary tufts retain the mitochondria and therefore remain motile for days.
Wet preparation has no permanent record unlike the permanent stained smears which can be used for consultations with specialists (Clavel et al. 1999; Garcia 2007). Moreover, morphological details are more readily seen by permanent stained smears (Aykan et al. 2005).
In stained slides of our study, all forms of bronchial cells could be differentiated from L. blattarum by the presence of the basal nucleus and/or terminal bar. Similar findings were reported by Martinez-Giron and van Woerden 2013.
In the present study, L. blattarum was reported to have different shapes varying from oval, rounded, to pyriform. This may be due to its plasticity or because it has different life stages forms (Kudo 1954; Brugerolle and Lee 2000). The shape variability of L. blattarum leads to more confusion in its identification in the stained smears. We found that the nucleus of L. blattarum is not usually visible. Similar observation was reported by Brugerolle and Lee (2000). Kessel and Beams (1990) reported that the nucleus of L. blattarum is usually hidden inside a funnel-shaped space, which is formed by axial filaments.
In the present work, the trichrome stain was the best to show morphological details of L. blattarum followed by the H & E stain. Trichrome stain gave uniform results and is effective in confirming the diagnosis of BPL. Satisfactory results were also obtained with PAP stain and Giemsa stain; however, they were of medium quality. L. blattarum were stained irregularly with Gram and DQ stains. Most of them showed only a slight pinkish or violet hue by Gram or DQ stain, respectively.
Deficiency of awareness of the presence of creola bodies and ciliocytophthoria will increase the risk of overdiagnosis of BPL especially when the clinical picture strongly indicates BPL. Moreover, usually, BAL samples sent to the cytology laboratory, are subjected to PAP or Giemsa staining. Both stains showed medium quality in identification of L. blattarum, which may lead to misidentification of L. blattarum by insufficiently experienced personnel. On the other hand, BAL samples sent to microbiological laboratories are usually stained by Gram stain which showed pink hues of L. blattarum. Therefore, we recommended adding Wheatley’s trichrome staining to the differential diagnosis workup of cases of non-specific chest infections, especially when BPL is suspected, to avoid overdiagnosis or underdiagnosis of it.
References
Aykan B, Caglar K, Kustımur S (2005) Evaluation of the protozoa found in fecal samples using the trichrome staining method. Turkiye Parazitol Derg 29(1):34–38
Bittencourt-Silvestre J, Lemgruber L, de Souza W (2010) Encystation process of Giardia lamblia: morphological and regulatory aspects. Arch Microbiol 192:259–265
Brugerolle G, Lee JJ (2000) Phylum parabasalia. In: Lee JJ, Leedale GF, Bradbury P (eds) An illustrated guide to the protozoa (vol. 2), 2nd edn. Society of Protozoologists, Lawrence
Brugerolle G, Silva-Neto ID, Pellens R, Grandcolas P (2003) Electron microscopic identification of the intestinal protozoan flagellates of the xylophagus cockroach Parasphaeria boleiriana from Brazil. Parasitol Res 90:249–256
Chavez-Munguya B, Omana-Molina M, Gonzalez-Lazaro M, Gonzalez-Robles A, Cedillo-Rivera R, Bonilla P, Martynez-Palomo A (2007) Ultrastructure of cyst differentiation in parasitic protozoa. Parasitol Res 100:1169–1175
Chen L (1933) Zuchtungsversuche an parasitischen protozoen vonPeriplaneta orientalis. Z Parasitenkd 6:207–219
Clavel A, Varea M, Doiz O, Lopez L, Quilez J, Castillo FJ, Rubio C, Gomez-Lus R (1999) Visualization of hydatid elements: comparison of several techniques. J Clin Microbiol 37(5):1561–1563
El-Sayed NM, Hikal WM (2014) Several staining techniques to enhance the visibility of Acanthamoeba cysts. Parasitol Res 114(3):823–830
Farmer JN (1980) The protozoa. In: Farmer JN (ed) Introduction to protozoology. The C.V. Mosby Company, London, pp 265–273
Garcia LS (2007) Macroscopic and microscopic examination of fecal specimens. In: Garcia LS (ed) Diagnostic medical parasitology (Part II), 5th edn. ASM Press, Washington, pp 782–830
Johnston WW, Elson CC (2008) Respiratory tract. In: Bibbo M, Wilbur DC (eds) Comprehensive cytopathology, 3rd edn. Saunders, Elsevier, pp 303–361
Kessel RG, Beams HW (1990) Freeze fracture and scanning electron microscope studies on the nuclear envelope and perinuclear cytomembranes (parabasal apparatus) in the protozoan, Lophomonas blattarum. J Submicrosc Cytol Pathol 22:367–378
Khan S, Kumar VA, Venkitachalam A, Vishwam V, Dinesh K, Karim S (2015) Detached ciliary tufts masquerading as free-living amoebae. Int J Infect Dis 30:142–143
Kiernan JA (2008) Histological and histochemical methods: theory and practice, 4th edn. Scion, Oxford
Kirby H (1950) Collection and cultivation of methods for symbiotic protozoa. In: Kirby H (ed) Materials and methods in the study of protozoa. University of California Press, Berkeley
Koss LG (1992) Diagnostic cytology and its histopathologic bases (vol. 2). JB Lippincott, Philadelphia, pp 1452–1509
Kudo RR (1954) Protozoology, 4th edn. Charles C Thomas Publisher, Springfield
Kuritzkes D, Rein M, Horowitz S, Droege G, Waldon MA, Bell DA, Fuller AF, Ellman LL, Dickersin GR, Swartz MN, Wolfson JS (1988) Detached ciliary tufts mistaken for peritoneal parasites: a warning. Rev Infect Dis 10:1044–1047
Martinez-Giron R (2013) Protozoal infections. In: Barrios R, Haque AK (eds) Parasitic diseases of the lungs. Springer, New York, pp 47–68
Martinez-Giron R, Doganci L (2010) Lophomonas blattarum: a bronchopulmonary pathogen. Acta Cytol 54(5):1050–1051
Martinez-Giron R, van Woerden HC (2013) Lophomonas blattarum and bronchopulmonary disease. J Med Microbiol 62(11):1641–1648
Martinez-Giron R, van Woerden HC (2014) Bronchopulmonary lophomoniasis: emerging disease or unsubstantiated legend? Parasit Vectors 23(7):284
Martinez-Giron R, van Woerden HC, Doganci L (2011) Lophomonas misidentification in bronchoalveolar lavages. Intern Med 50(21):2721, author reply 2723
Pai HH, Ko YC, Chen ER (2003) Cockroaches (Periplaneta americana and Blatella germanica) as potential mechanical disseminators of Entamoeba histolytica. Acta Trop 87:355–359
Ribas A, Martinez-Giron R, Ponte-Mittelbrum C, Alonso-Cuervo R, Iglesias-Llaca F (2007) Immunosupression, flagellated protozoa in the human airways and metronidazole: observations on the state of the art. Transpl Int 20:811–812
Shakoor S, Beg M, Mahmood S, Bandea R, Sriram R, Noman F, Ali F, Visvesvara GS, Zafar A (2011) Primary amebic meningoencephalitis caused by Naegleria fowleri, Karachi, Pakistan. Emerg Infect Dis 17:258–261
Skipper R, DeStephano DB (1989) A rapid stain for Campylobacter pylori in gastrointestinal tissue sections using Diff-Quik®. J Histotechnol 12:303–304
Strand MA, Brooks MA (1977) Pathogens of Blattidae (cockroaches). Bull World Health Organ 55(1):289–296
Wang Y, Tang Z, Ji S, Zhang Z, Chen J, Cheng Z, Cheng D, Liu Z, Li L (2006) Pulmonary Lophomonas blattarum infection in patients with kidney allograft transplantation. Transpl Int 19:1006–1013
Wu Z, Liu Y (2010) Blattarum lophomoniasis: a newly discovered parasitosis. J Pathog Biol 7:21
Yamada Y, Yoshihara S (2010) Creola bodies in infancy with respiratory syncytial virus bronchiolitis predict the development of asthma. Allergol Int 59(4):375–380
Yao G (2008) Bronchopulmonary infection with Lophomonas blattarum: two cases report and literature review. J Med Col PLA 23:176–182
York MK (2004) Gram stain. In: Isenberg HD (ed) Clinical microbiology procedures handbook. American Society of Microbiology, Washington
Yoshihara S, Yamada Y, Abe T, Linden A, Arisaka O (2006) Association of epithelial damage and signs of neutrophil mobilization in the airways during acute exacerbations of paediatric asthma. Clin Exp Immunol 144(2):212–216
Zaragatzki E, Hess M, Grabensteiner E, Abdel-Ghaffar F, Al-Rasheid KAS, Mehlhorn H (2010) Light and transmission electron microscopic studies on the encystation of Histomonas meleagridis. Parasitol Res 106:977–983
Zerpa R, Ore E, Patino L, Espinoza YA (2010) Lophomonas sp. in respiratory tract secretions in hospitalized children with severe lung disease. Rev Peru Med Exp Salud Publ 27:575–577
Zhang X, Xu L, Wang LL, Liu S, Li J, Wang X (2011) Bronchopulmonary infection with Lophomonas blattarum: a case report and literature review. J Int Med Res 39:944–949
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alam-Eldin, Y.H., Abdulaziz, A.M. Identification criteria of the rare multi-flagellate Lophomonas blattarum: comparison of different staining techniques. Parasitol Res 114, 3309–3314 (2015). https://doi.org/10.1007/s00436-015-4554-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4554-4