Abstract
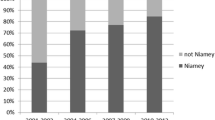
In 2000, the World Health Organization (WHO) published an ultrasound field protocol for assessing morbidity due to schistosomiasis. The present study aims to review the acceptance of the WHO protocol for Schistosoma haematobium. A PubMed literature research using the keywords “ultrasound OR ultrasonography (US) AND schistosomiasis,” “US AND S. haematobium,” “US AND urinary schistosomiasis” from 2001 through 2014 was performed. Thirty-eight eligible publications reporting on 17,861 patients from 13 endemic and 2 non-endemic countries were analysed. Of these, 33 referred to field studies on 17,317 patients. The Niamey protocol was applied to 15,367/17,317 (88.74 %) patients in 23/33 (69.70 %) of field studies (all studies: 15,649/17,861 [87.61 %] patients (25/38 [68.42 %] studies). The acceptance of the protocol by single country in field studies varied from 0 to 100 %. It varied over time between 55.56 % (5/9) in the period from 2001 to 2004, to 87.50 % (7/8) from 2005 to 2008, to 62.50 % (5/8) from 2009 to 2011 and 75.00 % (6/8) from 2012 through 2014 (all studies: 50 % [5/10], 88.89 % [8/9], 62.50 % [5/8], 63.64 % [7/11], respectively). The Niamey protocol was applied also in 2/5 hospital studies in 282/544 (51.84 %) patients.
The usefulness of the WHO protocol for S. haematobium infections is confirmed by its worldwide acceptance. Some simplifications might facilitate its use also for focused ultrasound examinations performed by less skilled examiners. Organ abnormalities due to schistosomiasis detectable by ultrasonography not yet covered by the WHO protocol should be added to the additional investigations section.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ultrasonography has been applied to characterize the typical morphologic features of schistosomiasis-induced organ pathology since 1978 (Abdel-Wahab et al. 1978). Ultrasonography compared positively to cystoscopy (Degremont et al. 1985; Burki et al. 1986) and was soon used for morbidity assessment in the field (Doehring et al. 1985; Hatz 2001).
In 1991, a group of experts was convened by the World Health Organization (WHO) in order to formulate a standardized ultrasonography protocol with the aim to enable the comparison of data obtained in different endemic foci (Cairo Working Group 1991; CairoWorking Group and Jenkins 1992). This protocol was reviewed and modified during a second meeting which took place in 1996 in Niamey, Niger, in order to revise and improve the WHO methodology and published in 2000 (Niamey Working Group 2000; Hatz 2001). The aim of the present study was to analyse the ultrasonographic studies performed since the publication of the Niamey protocol in terms of worldwide acceptance and the practical experiences of those examiners and study groups who applied this protocol. Furthermore, studies with potential implications for a further amendment of the protocol were evaluated.
Materials and methods
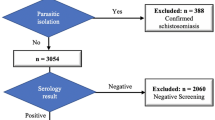
The literature research process is described in detail in Fig. 1. Since the present study aims at investigating the acceptance of the protocol, contrary to a classical meta-analysis, every publication regardless of its scientific quality was considered. Exclusion criteria were as follows: letters, reviews, meta-analysis, case reports, publications referring to Schistosoma species other than S. haematobium and localisations other than the urinary tract. Further searches were performed for studies on possible additional ultrasonographic investigations.
The main analysis criterion was whether or not the Niamey protocol was applied. Further analysis focused on the populations investigated, e.g. on pre-school-age children, school-age children or adults and on the study type (e.g. morbidity assessment prior to interventions or studies on the evolution after therapy such as regression and subsequent reappearance of lesions in populations continuously exposed to reinfection but not retreated). Moreover, it was analysed if intra- and or inter-observer agreement of ultrasound results was tested, if possible confounders such as sufficient bladder filling for bladder examination, or voiding prior to examination of the upper urinary tract were mentioned. Further searches examined in how many studies additional investigations such as residual urine or kidney pyelon fibrosis which were proposed in the WHO protocol were performed. Furthermore, searches were performed for other manifestations due to S. haematobium detectable by ultrasonography which in future could be proposed as to be included into the additional investigations list of the WHO protocol.
Results
Forty publications on studies performed in 13 endemic and 2 non-endemic countries were eligible. Five publications referred to the same ultrasonographic results of two studies, whereas one publication reported the results of two different studies so that eventually 38 studies were analysed (Fig. 1) (Campagne et al. 2001; King et al. 2001, 2002; Keita et al. 2001, 2005; Lougue-Sorgho et al. 2002; El Baz et al. 2003; Brouwer et al. 2003; Wamachi et al. 2004; Poda et al. 2004; Garba et al. 2004; Ouma et al. 2005; Shiff et al. 2006; Koukounari et al. 2006, 2009, 2010; Nmorsi et al. 2007; Sayed et al. 2007; Nayama et al. 2007; Stothard et al. 2009; Tohon et al. 2008; Ramarakoto et al. 2008; Naples et al. 2009; Sousa-Figueiredo et al. 2009; Ekwunife et al. 2009; Lyons et al. 2009; Bonnard et al. 2011; Qazi et al. 2012; Meurs et al. 2012, 2013; Salas-Coronas et al. 2013; Elmadani et al. 2013; Gouvras et al. 2013; Zhong et al. 2013; Strahan et al. 2013; Gasmelseed et al. 2014; Njaanake et al. 2014; Ahmed et al. 2014). Out of 17,861 patients examined, 17,317 of whom were examined in the field. The Niamey protocol was applied to 15,367/17,317 (88.74 %) patients in 23/33 (69.70 %) field studies (all studies: 15,649/17,861 [87.61 %] patients (25/38 [68.42 %] studies). The acceptance of the protocol by single country in field studies varied from 0 to 100 % (Table 1). It varied over time between 55.56 % (5/9) in the period from 2001 to 2004, to 87.50 % (7/8) from 2005 to 2008, to 62.50 % (5/8) from 2009 to 2011 and 75.00 % (6/8) from 2012 through 2014 (all studies: 50 % [5/10], 88.89 % [8/9], 62.50 % [5/8], 63.64 % [7/11] respectively) (Fig. 2). The Niamey protocol was applied also in 2/5 hospital studies including 282/544 (51.84 %) patients (Nayama et al. 2007; Salas-Coronas et al. 2013). Other methods applied were either descriptive not following any published protocol or followed the former Cairo protocol (Cairo Working Group 1991; Cairo Working Group and Jenkins 1992).
The Niamey protocol was applied in all studies including pre-school-age children (8/8 [100.0 %]), in 16/23 (69.57 %) studies regarding school-age children and in 14/20 (70 %) studies on adults (7 studies comprised both children and adults. In 2 studies, no data on the age of patients were provided) (King et al. 2002, 2004; Wamachi et al. 2004; Tohon et al. 2008; Meurs et al. 2012; Meurs et al 2013; Strahan et al. 2013; Njaanake et al. 2014). Using the Niamey protocol, 27/38 (71.05 %) studies on morbidity assessment prior to therapy were performed. Six out of 10 (60 %) studies on regression of lesions after therapy referred to the Niamey protocol (Ouma et al. 2005; Tohon et al. 2008; Ramarakoto et al. 2008; Ekwunife et al. 2009; Qazi et al. 2012; Strahan et al. 2013). Two out of 10 studies examined subsequent reappearance; however, none of the two referred to Niamey.
All four studies on inter-observer agreement referred to the Niamey protocol (King et al. 2001; Wamachi et al. 2004; Ouma et al. 2005; Bonnard et al. 2011). Since ultrasonography was not the main issue in most of these studies, inter-observer agreement results were not systematically reported on. King et al. (2001) reported that ultrasound results were confirmed by a second blinded examiner; Wamachi et al. (2004) and Ouma et al. (2005) did not specifically report on inter-observer ultrasound data, but compared ultrasonography data obtained by two blinded examiners with results of other investigations thereby suggesting that inter-observer agreement was adequate.
Inter-observer agreement was found excellent in a Senegalese study (Bonnard et al. 2011) where the learning curve of a learning clinician was compared to the results obtained by a teaching radiologist: The frequency of normal ultrasound images was 58 % for the teacher and 59 % for the learner. Mean score of teacher was 0.94 ± 2.01 [0–17], and mean score of learner was 1.11 ± 2.77 [0–19] without any statistical difference between them (p = 0.08). Of the 132 ultrasound examinations, only 12 discrepancies (9 %) were found. Among these 12 discordant ultrasound images, 8 scores had 1 point difference (67 %), 2 had 2 points difference and 2 were found to have a discrepancy of more than 2 points (8 and 10 points). Moreover, in nine of these discordant ultrasound images, it was the learner who had not seen the lesions seen by the teacher (underestimation) and in three cases it was the learner who reported lesions not seen by the teacher. By the third day of the study, after 50 examinations conducted by the learner and teacher, the learner found the same result as the teacher in over 90 % of the cases. Furthermore, the sensitivity/specificity analysis showed that after day 3, the learner screened 100 % of healthy subjects, and within two more days of learning, he managed to find all the lesions seen by the teacher without making any mistake. Finally, this learning appeared to persist over time. The other three studies including inter-observer testing reported reliable inter/observer agreement, too. Possible confounders such as insufficient bladder filling during ultrasound examinations or examination of the upper urinary tract before voiding, both possibly leading to an overestimation of organ pathology were mentioned mostly in studies where the Niamey protocol was applied: in 7/12 (58.33 %) of publications mentioning adequate bladder filling (Brouwer et al. 2003; Keita et al. 2005; Shiff et al. 2006; Koukounari et al. 2006; Nmorsi et al. 2007; Ekwunife et al. 2009; Gouvras et al. 2013) and in 7/9 (77.78 %) of publications mentioning the necessity of voiding before examining the upper urinary tract (Brouwer et al. 2003; Keita et al. 2005; Koukounari et al. 2006; Nmorsi et al. 2007; Ekwunife et al. 2009; Gouvras et al. 2013; Njaanake et al. 2014).
The Niamey-Belo Horizonte protocol proposes additional optional investigations such as the determination of residual urine after voiding and the assessment of kidney pyelon fibrosis. Results on the performance of additional investigations are listed in Table 2. Of the 10 publications retrieved by PubMed search, only two were eligible (Nmorsi et al. 2007; Watanabe et al. 2011); other results comprised eight studies (five before 2000) which do not refer to ultrasonography. Watanabe et al. (2011) compared parasitological data and symptomatology using the International Prostate Symptoms Score (I-PSS) with bladder voiding function as assessed by ultrasound and uroflowmetry. Results of this study suggested that the feeling of incomplete bladder emptying and hesitancy were better indicators for urinary schistosomiasis than the presence of residual urine. Unexpectedly, no significant residual urine post voiding and no decline in urine flow rates were found in S. haematobium-infected boys. Contrary, volume-corrected maximum and average flow rates in S. haematobium infected boys were higher than in those not infected. These results suggest that cystitis associated with S. haematobium infection causes irritation and hypercontractility of the bladder. The second study also reported that the finding of residual urine after voiding is relatively rare (27.9 %) as compared to abnormal bladder wall thickening (55.8 %) and abnormal bladder shape (69.8 %) (Nmorsi et al. 2007).
PubMed search did not yield any study on schistosomiasis which would have reported the occurrence of kidney pyelon fibrosis. The non-occurrence of pyelon fibrosis is further confirmed by a case series and a review on kidney pathology due to schistosomiasis where kidney pyelon fibrosis was also not reported (Barsoum 2003; Shebel et al. 2012). On the other hand, five classes of glomerulonephritis have been reported to be related to not only to S. haematobium but more frequently to Schistosoma mansoni: mesangioproliferative; exudative; mesangiocapillary (membranoproliferative); focal segmental sclerosis; and amyloidosis (Barsoum 2003; Duarte et al. 2014).
Searches for ultrasonography AND schistosomiasis or S. haematobium AND calcifications retrieved only seven publications, of which only three were published after 2001, only two of them were eligible (Nmorsi et al. 2007; Salas-Coronas et al. 2013). These studies confirmed the notion that radiology is more sensitive than ultrasonography for detecting urinary tract calcifications (Burki et al. 1986; King 2002).
Although searches for urinary calculi possibly related to urinary schistosomiasis revealed 84 hits, only 12 of them were published since 2001, of which 5 publications were eligible (Holman et al. 2004; Hussein et al. 2006; Abdelrahim et al. 2008; Coulibaly et al. 2011; Khalaf et al. 2012). All studies were hospital based. The different frequency of occurrence of calculi of the urinary tract in schistosomiasis patients is likely to be due to the different age as calculi having been only exceptionally seen in children, who are the usual target of field surveys.
After the detailed report on ultrasonographic detection of schistosomiasis-related ureter pathology by Kardorff and Döhring (2001), 18 further studies (14 eligible) on schistosomiasis were published, mostly referring to schistosomiasis or bladder cancer-induced strictures and urological management of calculi (El-Nahas et al. 2003; Neal 2004; Ajit et al. 2005; Hussein et al. 2006; Wong-You-Cheong et al. 2006; Gilligan and Dreicer 2007; Abdelrahim et al. 2008, Badmos et al. 2009; Abou-Elela et al. 2010; Oranusi et al. 2011; Shebel et al. 2012; Bakari et al. 2012; Vancauwenberghe et al. 2013; Hasegawa et al. 2014). These studies indicate that ultrasonography has a particular potential for the detection of ureter pathology not yet explored by the Niamey protocol.
A particular challenge for ultrasonography is the early detection of schistosomiasis-related bladder cancer, with special emphasis on its detection during field surveys. Seven ultrasound studies on this issue have been published since 2001, one of these being an interesting field study indicating that molecular bladder cancer markers are increased in early urinary schistosomiasis (Shiff et al. 2006; see also Figueiredo et al. 2014b). Two other studies were hospital observations (Lougue-Sorgho et al. 2002; Salas-Coronas et al. 2013), 1 a review (Richter 2003), 1 reported genetic abnormalities induced by schistosomiasis and 2 referred to exclusion of bladder cancer in schistosomiasis patients.
Attention has increased towards the genital manifestations of schistosomiasis where 53 works have been published since 2001.
In men, some abnormalities possibly indicating schistosomiasis have been described including hyperechogenic spots in the prostate and hydrocele (Richter et al. 2002; Ramarakoto et al. 2008; Figueiredo et al. 2014b). Searches for hydrocele AND schistosomiasis yielded seven hits (four since 2001): three publications referred to filariasis, one to schistosomiasis (Rambau et al. 2011). Another publication is not listed in PubMed (Richter et al. 2002). The search term combination ultrasound AND hydrocele AND schistosomiasis yielded no hit.
On the other hand, so far, ultrasound has not been found to be useful to detect specific abnormalities of the female upper reproductive tract (Richter et al. 1995; Ramarakoto et al. 2008). Ultrasound may, however, be helpful in diagnosing complications related to genital schistosomiasis such as sterility, vesico-vaginal fistulae and pregnancy-related problems (Richter 2000; Richter et al. 1996, 2008; Schanz et al. 2010; Figueiredo et al. 2013; Ben-Chetrit et al. 2014; Schleenvoigt et al. 2014). Searches for ultrasound AND schistosomiasis AND pregnancy yielded nine hits (two since 2001) which unfortunately were all not eligible. Search for ultrasonography AND schistosomiasis AND placenta yielded one hit only where placental schistosomiasis was excluded. The search for placenta AND schistosomiasis yielded 30 hits (15 since 2001). However, placental ultrasonographic abnormalities specifically due to schistosomiasis have not yet been looked for (for more detailed discussion, see Schleenvoigt et al. 2014).
Reports on urogenital manifestations due to Schistosoma spp. other than S. haematobium were limited to eight reports on ectopic S. mansoni infections and glomerulonephritis, six induced by S. mansoni and tow reports on kidney abnormalities presumably associated with Schistosoma intercalatum since 2001. There was no hit for S. guineensis, S. japonicum, nor for S mekongi.
Discussion
Ultrasonography is a pivotal method to assess the morbidity of the urinary tract due to schistosomiasis and its complications. At the time when the Niamey protocol was proposed, the preceding method developed during the WHO meeting in Cairo already had proved useful and reproducible (Hatz 2001) but there is, so far, no experimental proof of the accuracy and usefulness of the protocol as modified in Niamey. Thus, the studies undertaken after the publication of the Niamey protocol constitute the practical evaluation of this modified protocol.
The protocol was accepted by most study groups in the world except some group from Egypt, the Sudan and Niger. This is surprising insofar, that the first WHO protocol had been developed in Cairo (Cairo Working Group 1991) and the second in Niger (Niamey Working Group 2000). Researchers not yet applying the protocol should be encouraged to do so, in order to provide comparable data worldwide. Moreover, although not designed for hospital use, the protocol was found useful also in the hospitals since more than half of the hospitalized patients were also examined using the Niamey protocol. The worldwide adherence to the Niamey protocol for S. haematobium by proportion of patients is, thus, comparable to that of the WHO protocol for S. mansoni-related morbidity (el Scheich et al. 2014). Acceptance of the Niamey protocol proved excellent as compared to another protocol proposed by a WHO expert group, i.e. the WHO protocol for the ultrasonographic assessment of echinococcosis (Tamarozzi et al. 2014).
Inter-observer agreement of ultrasonographic findings using the Niamey protocol was found satisfactory. Possible confounders such as insufficient bladder filling before ultrasound examination and insufficient voiding prior to examination of the upper urinary tract were addressed by the majority of study groups applying the Niamey protocol. The impact of pitfalls on overall results may be minimized further by simplifying the bladder score as well as by keeping the bladder and upper urinary tract scores separate. A simplified bladder scoring was proposed by the group of King and collaborators (King et al. 2001; Wamachi et al. 2004) partly based on a former protocol of Medhat et al. 1997) which we re-propose in a slightly modified way:
-
Grade 0: No abnormality of the urinary bladder wall which is less then to 5 mm thick
-
Grade 1: Any area of bladder wall with minor thickening of the bladder wall 5–7 mm
-
Grade 2: Maximum bladder-wall thickening of 8–9 mm
-
Grade 3: Maximum bladder-wall thickening of 10 mm or more, thus including any polyp, mass or tumor
Another refinement concerning the upper urinary tract could improve its sensitivity (provided the bladder is empty after voiding), i.e. to add the note if there is a fissure of the renal pelvis between 3 and 5 and 6 to 10mm.
By these modifications, less skilled examiners might use the protocol more easily. The literature available on the transfer of ultrasonographic skills between a radiologist and a clinician in developing countries is scanty. Recently, in analogy of the use of focused ultrasonography in emergency medicine, it has been proposed to establish training curricula for the ultrasonographic assessment of selected pathologies which can be applied by non-specialised medical personal (Heller et al. 2010). This approach has been successfully applied for the rapid assessment of trauma by clinicians, in obstetrical indications, to assess cystic echinococcosis, obstructive uropathy and to identify tuberculosis in HIV-positive patients in South Africa (Kirkpatrick 2007; Heller et al. 2010; Kimberly et al. 2010; Jang et al. 2010a, b; Del Carpio et al. 2012). The successful transfer of ultrasonographic skills form a radiologist to a clinician sufficient for assessing S. haematobium induced urinary tract morbidity has been confirmed in a Senegalese study (Bonnard et al. 2011). As for an on the spot orientation of sufficient bladder filling, it should be reminded that a well-filled bladder changes from an ellipsoid to a approximately cubic shape. This might, however, not occur in a schistosomal bladder with a severely thickened wall.
It must be borne in mind, that transabdominal ultrasonography with portable devices for field use is not yet sufficiently sensitive to detect early ulcerative bladder wall abnormalities, independently from which protocol is used. This explains why results obtained by dipsticks are by far more sensitive for detecting urinary schistosomiasis than ultrasonography (Lougue-Sorgho et al. 2002; van der Werf and de Vlas 2004; Keita et al. 2005; Ahmed et al. 2014). Furthermore, ultrasonography is known to be less sensitive than radiology in detecting urinary tract calcifications (Burki et al. 1986; King 2002).
Evolution of schistosomal morbidity after therapy
Six out of 10 (60 %) studies on the regression of lesions after therapy referred to the Niamey protocol (Ouma et al. 2005; Tohon et al. 2008; Ramarakoto et al. 2008; Ekwunife et al. 2009; Qazi et al. 2012; Strahan et al. 2013). Unfortunately, none of the two studies on re-appearance of lesions following therapy in re-exposed people referred to the Niamey protocol (Campagne et al. 2001; Garba et al 2004)
However, since there is a number of studies where evolution of pathology has been assessed using the former Cairo protocol, it is likely to be applicable in this respect, too (Hatz et al. 1998; Wagatsuma et al. 1999; Richter 2000; Hatz 2001; Richter et al. 2003). By the modifications of the Niamey protocol as proposed above, the assessment of the regression and subsequent reappearance of lesions in constantly exposed individuals might be evaluated more properly. A cystoscopy study on Brazilian soldiers returning from a peacekeeping mission in Mozambique revealed that even after two rounds of adequate praziquantel therapy, 36 % of soldiers who were no more excreting ova in the urine still had viable ova in the bladder mucosa (Silva et al. 2006), an important issue also for travel medicine, considering the potential of schistosomiasis to induce bladder cancer (Mostafa, et al. 1999; Amonkar et al. 2001). Unfortunately; this study missed the opportunity to evaluate ultrasonography in this respect. It cannot be overemphasized that a bladder mass or polyp persisting for 6 months or more in spite of antihelminthic therapy is highly suspicious for bladder cancer and that the affected patient must be referred to a adequately equipped hospital for further diagnostic investigations.
Additional investigations
The Niamey-Belo Horizonte protocol also included optional investigations such as the determination of residual urine after voiding and the assessment of kidney pyelon fibrosis. The issue of residual urine after voiding has been addressed by an intriguing study of Watanabe et al. (2011), who compared parasitological findings and symptoms using an international questionnaire developed for prostate disease with results of ultrasonography and uroflowmetry. Results of this study indicated that the feeling of incomplete bladder emptying and hesitancy were better indicators for urinary schistosomiasis than the presence of residual urine (Watanabe et al. 2011), a result which additionally to the presence of cloudy urine should be taken into account for the questionnaires designed for identifying highly endemic communities for urinary schistosomiasis (Lengeler et al. 1991; Red Urine Study Group 1995; Wagatsuma et al. 2003; Campagne et al. 2001; Chippaux et al. 2001).
As part of the morbidity due to schistosomiasis detectable by ultrasonography is not yet covered by the WHO protocol, it should be included in the additional investigations section since the detection of calculi and calcifications does not cost additional examination time and their presence should be noted. Ureter, at least at their distal end might be investigated systematically for dilatation, wall irregularities, thickenings and strictures. A glance to the prostate also in adolescents could reveal early prostate abnormalities (Ramarakoto et al. 2008; Figueiredo et al. 2014b). Depending on the cultural and investigational context, e.g. hospital or health care centre settings, ultrasound examinations of boys and men could include the scrotum to detect genital abnormalities and hydrocele. Although usually attributed to filariasis, it is known for a long time that schistosomiasis not unfrequently causes hydrocele also in the absence of filariasis coinfection (Malik and Ibrahim 1982; Richter et al. 2002).
Unfortunately, even using transvaginal probes, specific lesions of the female reproductive tract are difficult to diagnose ultrasonographically. However, complications of genital schistosomiasis, such as ectopic pregnancy possibly associated with intraabdominal bleeding are readily diagnosed ultrasonographically (Richter 2000). The same applies to pregnant women with schistosomiasis, where so far, studies on placenta ultrasound have not been published, although it is known that schistosomiasis also involves the placenta and that the placenta is an organ which is relatively easy to scan (Kimberly et al. 2010; Ben-Chetrit et al. 2014; Schleenvoigt et al. 2014). The best documented case series on the pregnancy outcome was published by Ben-Chetrit et al. (2014) on 10 pregnant women: the course and outcome of pregnancy in 4 patients who were diagnosed and treated with praziquantel was uneventful whereas 13 babies born to 6 asymptomatic women not diagnosed during pregnancy were significantly smaller as compared with those of the infants of the women who were treated during their pregnancy (median 2.8 vs 3.5 kg). One baby was born preterm. One infected woman had three miscarriages.
As another possible limitation of ultrasonography, one could put forward that urogenital tuberculosis may sometimes mimic schistosomiasis. Ultrasonographic differential diagnostic criteria for schistosomiasis are that upper urinary tract obstruction is due to bladder and ureter pathology and thus non-specific whereas in tuberculosis hypoechogenic lesions initiate in the kidney parenchyma also affecting the papillae. In doubtful cases, parasitological and bacteriological means are required for differential diagnosis (Das et al. 1992; Tikkakoski et al. 1993; Benchekroun et al. 1997; Hsieh et al. 2006; Singh et al. 2011).
Finally, especially in a Public Health and control context aiming at assessing the morbidity due to S. haematobium, it must be borne in mind that ultrasound just reveals the tip of the iceberg of S. haematobium induced morbidity, since micro- and macrohaematuria, symptomatology and extraurinary localizations may occur also in the absence of ultrasound-detectable lesions (van der Werf and de Vlas 2004). Furthermore, genital and ectopic localizations may occur in the absence of urinary egg excretion (Poggensee et al. 1998; Ramarakoto et al. 2008; Figueiredo et al. 2014a) and indirect not appreciated morbidity, i.e. anemia due to microhaematuria is also not captured by ultrasonography (Tohon et al. 2008).
Conclusions
In general, the WHO-Niamey-Belo protocol was well accepted since its publication. However, in some regions, the protocol is still not applied regularly. Researchers of some highly endemic regions such as Egypt, the Sudan and Niger should be encouraged to use the protocol so that their data can be compared on an international scale. The scoring of bladder wall and upper urinary tract might be slightly modified to simplify its application. The Niamey protocol proved applicable for focused ultrasound assessment by less specialized personnel. A similar protocol is also needed for assessing the morbidity due to S. japonicum and Schistosoma mekongi which should be developed shortly.
References
Abdelrahim AF, Abdelmaguid A, Abuzeid H, Amin M, Mousa el-S, Abdelrahim FJ (2008) Rigid ureteroscopy for ureteral stones: factors associated with intraoperative adverse events. Endourology 22(2):277–280
Abdel-Wahab MF, Abdel-Latif Z, El Kady NM, Arafa NM (1978) The use of ultrasonography in the diagnosis of different schistosomal syndromes. Proceedings of the third International Workshop on Diagnostic Ultrasound Imaging. Al-Ahram Press, Cairo, Egypt 458–463
Abou-Elela A, Torky M, Salah E, Morsy A, Elsherbiny M (2010) Inverted ureteral nipple as antireflux technique in surgical management of bilharzial ureteral strictures. Urology 76(4):983–987
Ahmed FO, Hamdan HZ, Abdelgalil HB, Sharfi AA (2014) A comparison between transabdominal ultrasonographic and cystourethroscopy findings in adult Sudanese patients presenting with haematuria. Int Urol Nephrol 6. [Epub ahead of print]
Ajit MK, Groenewald EA, Speakman M (2005) An unusual presentation of genitourinary schistosomiasis in a Caucasian woman in the UK. Ann R Coll Surg Engl 87(6):481
Amonkar P, Murali G, Krishnamurthy S (2001) Schistosoma induced squamous cell carcinoma of the bladder. Indian J Pathol Microbiol 44:363–364
Badmos KB, Popoola AA, Buhari MO, Abdulkadir AY (2009) Ureteric schistosomiasis with obstructive uropathy. J Coll Physicians Surg Pak 19(7):456–458
Bakari AA, Gadam IA, Aliyu S, Suleiman I, Ahidjo AA, Pindiga UH (2012) Use of Mitrofanoff and Yang-Monti techniques as ureteric substitution for severe schistosomal bilateral ureteric stricture: a case report and review of the literature. Niger J Surg 18(1):30–33
Barsoum RS (2003) Schistosomiasis and the kidney. Semin Nephrol 23(1):34–41
Benchekroun TS, Kriouil A, Belkacem A, Jorio-Benkhraba M, el Fakir Y, Benhammou M, Outarahout O, el Malki-Tazi A (1997) Urogenital tuberculosis in children. Arch Pediatr 4(9):857–861
Ben-Chetrit E, Lachish T, Mørch K, Atias D, Maguire C, Schwartz E (2014) Schistosomiasis in pregnant travelers: a case series. J Travel Med 13. doi: 10.1111/jtm.12165. [Epub ahead of print]
Bonnard P, Boutouaba S, Diakhate I, Seck M, Dompnier JP, Riveau G (2011) Learning curve of vesico-urinary ultrasonography in Schistosoma haematobium infection with WHO practical guide: a "simple to learn" examination. Am J Trop Med Hyg 85:1071–1074
Brouwer KC, Ndhlovu PD, Wagatsuma Y, Munatsi A, Shiff CJ (2003) Urinary tract pathology attributed to Schistosoma haematobium: does parasite genetics play a role? Am J Trop Med Hyg 68(4):456–462
Burki A, Tanner M, Burnier E, Schweizer W, Meudt R, Degrémont A (1986) Comparison of ultrasonography, intravenous pyelography and cystoscopy in detection of urinary tract lesions due to Schistosoma haematobium. Acta Trop 43(2):139–151
Cairo Working Group (1991) Meeting on the use of ultrasound for the assessment of pathology due to schistosomiasis. World Health Organization, Geneva
Cairo Working Group Hatz C, Jenkins J (eds) (1992) The use of diagnostic ultrasound in schistosomiasis-attempts at standardization of methodology. Acta Trop 51:45–61
Campagne G, Garba A, Barkiré H, Vera C, Sidiki A, Chippaux JP (2001) Continued ultrasonic follow-up of children infected with Schistosoma haematobium after treatment with praziquantel. Trop Med Int Health 6(1):24–30
Chippaux JP, Campagne G, Garba A, Véra C (2001) [Significance of rapid evaluation indicators during the monitoring of graduated treatment against Schistosoma haematobium]. [Article in French]. Bull Soc Pathol Exot 94(1):36–41
Coulibaly Y, Ouattara Z, Togo A, Konate M, Ouattara M, Ouattara K (2011) [Bilharziasis and urinary lithiasis: a study of 23 cases at the Gabriel Toure Hospital] [Article in French]. Mali Med 26(1):26–28, French
Das KM, Indudhara R, Vaidyanathan S (1992) Sonographic features of genitourinary tuberculosis. Am J Roentgenol 158(2):327–329
Degremont A, Burki A, Burnier E, Schweizer W, Meudt R, Tanner M (1985) Value of ultrasonography in investigating morbidity due to Schistosoma haematobium infection. Lancet 23(1):662–665
Del Carpio M, Mercapide CH, Salvitti JC, Uchiumi L, Sustercic J, Panomarenko H, Moguilensky J, Herrero E, Talmon G, Volpe M, Araya D, Mujica G, Calabro A, Mancini S, Chiosso C, Labanchi JL, Saad R, Goblirsch S, Brunetti E, Larrieu E (2012) Early diagnosis, treatment and follow-up of cystic echinococcosis in remote rural areas in Patagonia: impact of ultrasound training of non-specialists. PLoS Negl Trop Dis 6:1444
Doehring E, Ehrich JH, Reider F, Dittrich M, Schmidt-Ehry G, Brodehl J (1985) Morbidity in urinary schistosomiasis: relation between sonographical lesions and pathological urine findings. Trop Med Parasitol 36(3):145–149
Duarte DB, Vanderlei LA, Bispo RK, Pinheiro ME, Silva Junior GB, Daher EF (2014) Acute kidney injury in Schistosomiasis: a retrospective cohort of 60 patients in Brazil. J Parasitol. 2014 Nov 13. [Epub ahead of print]
Ekwunife CA, Okafor FC, Nwaorgu OC (2009) Ultrasonographic screening of urinary schistosomiasis infected patients in Agulu community, Anambra state, southeast Nigeria. Int Arch Med 2(1):34. doi:10.1186/1755-7682-2-34
El Baz MA, Morsy TA, El Bandary MM, Motawea SM (2003) Clinical and parasitological studies on the efficacy of Mirazid in treatment of schistosomiasis haematobium in Tatoon, Etsa Center, El Fayoum Governorate. J Egypt Soc Parasitol 33(3):761–776
Elmadani AE, Hamdoun AO, Monis A, Karamino NE, Gasmelseed N (2013) Ultrasound findings in urinary schistosomiasis infection in school children in the Gezira State Central Sudan. Saudi J Kidney Dis Transpl 24(1):162–167
El-Nahas AR, Shoma AM, El-Baz M (2003) Bilharzial pyelitis: a rare cause of secondary ureteropelvic junction obstruction. J Urol 170(5):1946–1947
el Scheich, T, Holtfreter MC, Ekamp H, Singh DD, Mota R, Hatz C, Richter J (2014) The WHO ultrasonography protocol for assessing hepatic morbidity due to Schistosoma mansoni. Acceptance and evolution over 12 years. Parasitol Res 113(11):3915–3925
Figueiredo J, Richter J, Belo S, Grácio MA (2013) Urogenital schistosomiasis presenting genital and urinary tract lesions and abdominal discomfort in a sterile Angolan woman. J Genit Syst Disord\ 2(3):1–4
Figueiredo J, Santos Â, Clemente H, Lourenço A, Costa S, Grácio MA, Belo S (2014a) Schistosomiasis and acute appendicitis. Acta Med Port 27(3):396–399, Portuguese
Figueiredo JC, Richter J, Borja N, Balaca A, Costa S, Belo S, Grácio MA (2014b). Prostate adenocarcinoma associated with prostatic infection due to Schistosoma haematobium. Case report and systematic review. Parasitol Res Dec 30 Epub ahead of print]
Garba A, Campagne G, Tassie JM, Barkire A, Vera C, Sellin B, Chippaux JP (2004) Long-term impact of a mass treatment by praziquantel on morbidity due to Schistosoma haematobium in two hyperendemic villages of Niger. Bull Soc Pathol Exot 97(1):7–11
Gasmelseed N, Karamino NE, Abdelwahed MO, Hamdoun AO, Elmadani AE (2014) Genetic diversity of Schistosoma haematobium parasite is not associated with severity of disease in an endemic area in Sudan. BMC Infect Dis 14(469). doi: 10.1186/1471-2334-14-469
Gilligan T, Dreicer R (2007) The atypical urothelial cancer patient: management of bladder cancers of non-transitional cell histology and cancers of the ureters and renal pelvis. Semin Oncol 34(2):145–153
Gouvras AN, Kariuki C, Koukounari A, Norton AJ, Lange CN, Ireri E, Fenwick A, Mkoji GM, Webster JP (2013) The impact of single versus mixed Schistosoma haematobium and S. mansoni infections on morbidity profiles amongst school-children in Taveta, Kenya. Acta Trop 128(2):309–317
Hasegawa Y, Nishiii M, Masui S, Yoshio Y, Kanda H, Kanai M, Yamada Y, Arima K, Sugimura Y (2014) Urinary schistosomiasis: report of a case. Hinyokika Kiyo 60(2):91–94, Japanese
Hatz CF (2001) The use of ultrasound in schistosomiasis. Adv Parasitol 48:225–284
Hatz CF, Vennervald BJ, Nkulila T, Vounatsou P, Kombe Y, Mayombana C, Mshinda H, Tanner M (1998) Evolution of Schistosoma haematobium-related pathology over 24 months after treatment with praziquantel among school children in southeastern Tanzania. Am J Trop Med Hyg 59(5):775–781
Heller T, Wallrauch C, Lessells RJ, Goblirsch S, Brunetti E (2010) Short course for focused assessment with sonography for human immunodeficiency virus/tuberculosis: preliminary results in a rural setting in South Africa with high prevalence of human immunodeficiency virus and tuberculosis. Am J Trop Med Hyg 88:512–515
Holman E, Khan AM, Flasko T, Toth C, Salah MA (2004) Endoscopic management of pediatric urolithiasis in a developing country. Urology 63(1):159–162
Hsieh HC, Lu PL, Chen YH, Chen TC, Tsai JJ, Chang K, Chen TP (2006) Genitourinary tuberculosis in a medical center in southern Taiwan: an eleven-year experience. J Microbiol Immunol Infect 39(5):408–413
Hussein A, Rifaat E, Zaki A, Abol-Nasr M (2006) Stenting versus non-stenting after non-complicated ureteroscopic manipulation of stones in bilharzial ureters. Int J Urol 13(7):886–890
Jang TB, Casey RJ, Dyne P, Kaji A (2010a) The learning curve of resident physicians using emergency ultrasonography for obstructive uropathy. Acad Emerg Med 17:1024–1027
Jang TB, Ruggeri W, Dyne P, Kaji AH (2010b) Learning curve of emergency physicians using emergency bedside sonography for symptomatic first-trimester pregnancy. J Ultrasound Med 29:1423–1428
Kardorff R, Döhring E (2001) Ultrasound diagnosis of bilharziasis. Ultraschall Med 22(3):107–115, Review. German
Keita AD, Dembélé M, Kané M, Fongoro S, Traoré M, Sacko M, Diallo S, Sidibe S, Traoré HA, Doumbo O, Traoré I (2001) Ultrasonographic aspects of urinary schistosomiasis in children of the Dogon plateau and the Niger office; impact of praziquantel treatment. Bull Soc Pathol Exot 94(4):335–338
Keita AD, Sangho H, Sacko M, Diarra Z, Simaga SY, Traore I (2005) Prevalence of schistomasiasis lesions detected by ultrasonography in children in Molodo, Mali. Gastroenterol Clin Biol 29(6–7):652–655
Khalaf I, Shokeir A, Shalaby M (2012) Urologic complications of genitourinary schistosomiasis. World J Urol 30(1):31–38
Kimberly HH, Murray A, Mennicke M, Liteplo A, Lew J, Bohan JS, Tyer-Viola L, Ahn R, Burke T, Noble VE (2010) Focused maternal ultrasound by midwives in rural Zambia. Ultrasound Med Biol 36:1267–1272
King CH (2002) Ultrasound monitoring of structural urinary tract disease in Schistosoma haematobium infection. Mem Inst Oswaldo Cruz 97(suppl I):149–152
King CL, Malhotra I, Mungai P, Wamachi A, Kioko J, Muchiri E, Ouma JH (2001) Schistosoma haematobium-induced urinary tract morbidity correlates with increased tumor necrosis factor-alpha and diminished interleukin-10 production. J Infect Dis 184(9):1176–1182
King CH, Muchiri EM, Mungai P, Ouma JH, Kadzo H, Magak P, Koech DK (2002) Randomized comparison of low-dose versus standard-dose praziquantel therapy in treatment of urinary tract morbidity due to Schistosoma haematobium infection. Am J Trop Med Hyg 66(6):725–730
King CH, Blanton RE, Muchiri EM, Ouma JH, Kariuki HC, Mungai P, Magak P, Kadzo H, Ireri E, Koech DK (2004) Low heritable component of risk for infection intensity and infection-associated disease in urinary schistosomiasis among Wadigo village populations in Coast Province, Kenya. Am J Trop Med Hyg 70(1):57–62
Kirkpatrick AW (2007) Clinician-performed focused sonography for the resuscitation of trauma. Crit Care Med 35:162–172
Koukounari A, Sacko M, Keita AD, Gabrielli AF, Landouré A, Dembelé R, Clements AC, Whawell S, Donnelly CA, Fenwick A, Traoré M, Webster JP (2006) Assessment of ultrasound morbidity indicators of schistosomiasis in the context of large-scale programs illustrated with experiences from Malian children. Am J Trop Med Hyg 75(6):1042–1052
Koukounari A, Webster JP, Donnelly CA, Bray BC, Naples J, Bosompem K, Shiff C (2009) Sensitivities and specificities of diagnostic tests and infection prevalence of Schistosoma haematobium estimated from data on adults in villages northwest of Accra, Ghana. Am J Trop Med Hyg 80(3):435–441
Koukounari A, Donnelly CA, Sacko M, Keita AD, Landouré A, Dembelé R, Bosqué-Oliva E, Gabrielli AF, Gouvras A, Traoré M, Fenwick A, Webster JP (2010) The impact of single versus mixed schistosome species infections on liver, spleen and bladder morbidity within Malian children pre- and post-praziquantel treatment. BMC Infect Dis 10:227. doi:10.1186/1471-2334-10-227
Lengeler C, Kilima P, Mshinda H, Morona D, Hatz C, Tanner M (1991) Rapid, low.cost, two-step method to screen for urinary schistosomiasis at the district level: the Kilosa experience. Bull WHO 69:179–189
Lougue-Sorgho LC, Cisse R, Kagone M, Bamouni YA, Tapsoba TL, Sanou A (2002) Radiography and ultrasonography in the management of bladder tumors: 71 cases at the National Hospital Center of Yalgado Ouedraogo (Burkina Faso)]. Bull Soc Pathol Exot 95(4):244–247
Lyons B, Stothard R, Rollinson D, Khamis S, Simai KA, Hunter PR (2009) A comparison of urinary tract pathology and morbidity in adult populations from endemic and non-endemic zones for urinary schistosomiasis on Unguja Island, Zanzibar. BMC Infect Dis 9:189. doi:10.1186/1471-2334-9-189
Malik MO, Ibrahim A (1982) Scrotal swellings in Sudanese patients: a surgical pathology study. Int Surg 67(4 Suppl):513–515
Medhat A, Zarzour A, Nafeh M et al (1997) Evaluation of an ultrasonographic score for urinary bladder morbidity in Schistosoma haematobium infection. Am J Trop Med Hyg 57:16–19
Meurs L, Mbow M, Vereecken K, Menten J, Mboup S, Polman K (2012) Bladder morbidity and hepatic fibrosis in mixed Schistosoma haematobium and S. mansoni Infections: a population-wide study in Northern Senegal. PLoS Negl Trop Dis 6(9):e1829. doi:10.1371/journal.pntd.0001829
Meurs L, Mbow M, Boon N, van den Broeck F, Vereecken K, Dièye TN, Abatih E, Huyse T, Mboup S, Polman K (2013) Micro-geographical heterogeneity in Schistosoma mansoni and S. haematobium infection and morbidity in a co-endemic community in northern Senegal. PLoS Negl Trop Dis 7(12):e2608. doi:10.1371/journal.pntd.0002608
Mostafa MH, Sheweita SA, O'Connor PJ (1999) Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev 12(1):97–111
Naples J, Isharwal S, Shiff CJ, Bosompem KM, Veltri RW (2009) Clinical utility of squamous and transitional nuclear structure alterations induced by Schistosoma haematobium in chronically infected adults with bladder damage verified by ultrasound in Ghana. Anal Quant Cytol Histol 31(3):143–152
Nayama M, Garba A, Boulama-Jackou ML, Touré A, Idi N, Garba M, Nouhou H, Decanter C (2007) Uro-genital schistosomiasis with S. haematobium and infertility in Niger. Prospective study of 109 cases. Mali Med 22(3):15–21, French
Neal PM (2004) Schistosomiasis—an unusual cause of ureteral obstruction: a case history and perspective. Clin Med Res 2(4):216–227
Niamey Working Group: Richter J, Hatz C, Campagne G, Jenkins J (2000) Ultrasound in Schistosomiasis. A practical guide to the standardized use of ultrasonography for the assessment of schistosomiasis-related morbidity. World Health Organization, World Health Organization / TDR / STR/ SCH / WHO-document: 1-51. www.who.int/tdr/publications/publications/ultrasound.htm; last accessed January 25, 2015
Njaanake KH, Simonsen PE, Vennervald BJ, Mukoko DA, Reimert CM, Gachuhi K, Jaoko WG, Estambale BB (2014) Urinary cytokines in Schistosoma haematobium-infected schoolchildren from Tana Delta District of Kenya. BMC Infect Dis 14(501) doi: 10.1186/1471-2334-14-501
Nmorsi OP, Ukwandu NC, Ogoinja S, Blackie HO, Odike MA (2007) Urinary tract pathology in Schistosoma haematobium infected rural Nigerians. Southeast Asian J Trop Med Public Health 38(1):32–37
Oranusi CK, Nwofor A, Onyiaorah IV, Ukah CO (2011) Schistosomal stricture of the ureter-diagnostic dilemma. Niger J Clin Pract 14(4):495–498
Ouma JH, King CH, Muchiri EM, Mungai P, Koech DK, Ireri E, Magak P, Kadzo H (2005) Late benefits 10-18 years after drug therapy for infection with Schistosoma haematobium in Kwale District, Coast Province, Kenya. Am J Trop Med Hyg 73(2):359–364
Poda JN, Traoré A, Sondo BK (2004) Schistosomiasis endemic in Burkina Faso. Bull Soc Pathol Exot 97(1):47–52
Poggensee G, Sikelu M, Saría M, Richter J, Krantz I, Feldmeier H (1998) Schistosomiasis of the lower reproductive tract without egg excretion in urine. Am J Trop Med Hyg 59(9):782–783
Qazi A, Stahlschmidt J, Subramaniam R (2012) Bladder morbidity and hepatic fibrosis in mixed Schistosoma haematobium and S. mansoni Infections: a population-wide study in Northern Senegal. J Pediatr Urol 8(4):438–441. doi:10.1016/j.jpurol.2011.11.008
Ramarakoto CE, Leutscher PD, van Dam G, Christensen NO (2008) Ultrasonographical findings in the urogenital organs in women and men infected with Schistosoma haematobium in northern Madagascar. Trans R Soc Trop Med Hyg 102(8):767–773
Rambau PF, Chandika A, Chalya PL, Jackson K (2011) Scrotal swelling and testicular atrophy due to schistosomiasis in a 9-year-old boy: a case report. Case Rep Infect Dis 2011:787961
Red Urine Study Group (1995) Identification of high-risk communities for schistosomiasis in Africa. A multicountry study. WHO TDR document, World Health Organization Geneva, Switzerland, pp 1–83
Richter J (2000) Evolution of schistosomiasis-induced pathology after therapy and interruption of exposure to schistosomes: a review of ultrasonographic studies. Acta Trop 77:111–131
Richter J (2003) The impact of chemotherapy on the morbidity due to schistosomiasis. Acta Trop 86:161–183
Richter J, Poggensee G, Helling-Giese G, Kjetland E, Chitsulo L, Koumenda N, Gundersen S, Krantz I, Feldmeier H (1995) Transabdominal ultrasound for the diagnosis of genital schistosomiasis in women with Schistosoma haematobium infection: a preliminary report. Trans R Soc Trop Med Hyg 89:500–501
Richter J, Wagatsuma Y, Aryeetey M, Feldmeier H (1996) Sonographic screening for urinary tract abnormalities in patients with Schistosoma haematobium infection: pitfalls in examining pregnant women. Bull World Health Organ 74(2):217–221
Richter J, Stegemann U, Häussinger D (2002) Hydrocele in a young boy with Schistosoma mansoni infection. Br J Urol 89:1–2
Richter J, Hatz C, Häussinger D (2003) Ultrasound in tropical and parasitic diseases. Lancet 362:900–902
Richter J, Lux J, Reinecke P, Müller-Mattheis V (2008) Of flukes and fistulae. Lancet 371:1308
Salas-Coronas J, Vázquez-Villegas J, Villarejo-Ordóñez A, Sánchez-Sánchez JC, Espada-Chavarría J, Soriano-Pérez MJ, Cabeza-Barrera MI, Cabezas-Fernández MT (2013) Radiological findings in patients with imported schistosomiasis. Enferm Infecc Microbiol Clin 31(4):205–209
Sayed HA, Hammam O, Sabry H, Akl M, Amin T, El Lithy T, Roshdy M, El Ganzoury H (2007) Epidemiologic approach for early detection and control of renal and urinary tract diseases in rural populations. J Egypt Soc Parasitol 37(1):313–328
Schanz A, Richter J, Beyer I, Baldus SE, Hess AP, Kruessel JSJ (2010) Genital schistosomiasis as a cause of female sterility and acute abdomen. Fertil Steril 93(6):2075.e7–9
Schleenvoigt BT, Gajda M, Baier M, Groten T, Oppel-Heuchel H, Grimm MO, Pfister W, Richter J, Pletz MW (2014) Placental Schistosoma haematobium infection in a German returnee from Malawi. Infection 42(6):1061–1064
Shebel HM, Elsayes KM, Abou El Atta HM, Elguindy YM, El-Diasty TA (2012) Genitourinary schistosomiasis: life cycle and radiologic-pathologic findings. Radiographics 32(4):1031–1046
Shiff C, Veltri R, Naples J, Quartey J, Otchere J, Anyan W, Marlow C, Wiredu E, Adjei A, Brakohiapa E, Bosompem K (2006) Ultrasound verification of bladder damage is associated with known biomarkers of bladder cancer in adults chronically infected with Schistosoma haematobium in Ghana. Trans R Soc Trop Med Hyg 100:847–854
Silva IM, Thiengo R, Conceição MJ, Rey L, Pereira Filho E, Ribeiro PC (2006) Cystoscopy in the diagnosis and follow-up of urinary schistosomiasis in Brazilian soldiers returning from Mozambique, Africa. Rev Inst Med Trop Sao Paulo 48(1):39–42
Singh DD, Vogel M, Müller-Stöver I, el Scheich T, Winzer M, Göbels S, Hüttig F, Heinrich S, Mackenzie C, Jensen B, Reuter S, Häussinger D, Richter J (2011) TB or nor TB? Difficulties in the diagnosis of tuberculosis in HIV-negative immigrants to Germany. Eur J Med Res 16(9):381–384
Sousa-Figueiredo JC, Basáñez MG, Khamis IS, Garba A, Rollinson D, Stothard JR (2009) Measuring morbidity associated with urinary schistosomiasis: assessing levels of excreted urine albumin and urinary tract pathologies. PLoS Negl Trop Dis 3(10):e526. doi:10.1371/journal.pntd.0000526
Stothard JR, Sousa-Figueiredo JC, Simba Khamis I, Garba A, Rollinson D (2009) Urinary schistosomiasis-associated morbidity in schoolchildren detected with urine albumin-to-creatinine ratio (UACR) reagent strips. J Pediatr Urol 5(4):287–291
Strahan R, McAdam D, Schneider ME (2013) Sonographic response in the liver and urinary bladder of children 14 months after treatment for schistosomiasis. Trop Dr 43(2):71–74
Tamarozzi F, Nicoletti GJ, Neumayr A, Brunetti E (2014) Acceptance of standardized ultrasound classification, use of albendazole, and long-term follow-up in clinical management of cystic echinococcosis: a systematic review. Curr Opin Infect Dis 27(5):425–431
Tikkakoski T, Karstrup S, Lohela P, Hulkko A, Apaja-Sarkkinen M (1993) Tuberculosis of the lower genitourinary tract: ultrasonography as an aid to diagnosis and treatment. J Clin Ultrasound 21(4):269–271
Tohon ZB, Mainassara HB, Garba A, Mahamane AE, Bosqué-Oliva E, Ibrahim ML, Duchemin JB, Chanteau S, Boisier P (2008) Controlling schistosomiasis: significant decrease of anaemia prevalence one year after a single dose of praziquantel in Nigerian schoolchildren. PLoS Negl Trop Dis 2(5):e241. doi:10.1371/journal.pntd.0000241
Van der Werf MJ, de Vlas SJ (2004) Diagnosis of urinary schistosomiasis: a novel approach to compare bladder pathology measured by ultrasound and three methods for hematuria detection. Am J Trop Med Hyg 71(1):98–106
Vancauwenberghe T, Oyaert M, Termote JL, Mulkens T, Bellinck P (2013) Ureteral obstruction caused by schistosomiasis. JBR-BTR 96(5):292–294
Wagatsuma Y, Aryeetey ME, Sack DA, Morrow RH, Hatz C, Kojima S (1999) Resolution and resurgence of Schistosoma haematobium-induced pathology after community-based chemotherapy in Ghana, as detected by ultrasound. J Infect Dis 179(6):1515–1522
Wagatsuma Y, Aryeetey ME, Nkrumah FK, Sack DA, Kojima S (2003) Highly symptom-aware children were heavily infected with urinary schistosomiasis in southern Ghana. Cent Afr J Med 49(1–2):16–19
Wamachi AN, Mayadev JS, Mungai PL, Magak PL, Ouma JH, Magambo JK, Muchiri EM, Koech DK, King CH, King CL (2004) Increased ratio of tumor necrosis factor-alpha to interleukin-10 production is associated with Schistosoma haematobium-induced urinary-tract morbidity. J Infect Dis 190(11):2020–2030
Watanabe K, Muhoho ND, Mutua WR, Kiliku FM, Awazawa T, Moji K, Aoki Y (2011) Assessment of voiding function in inhabitants infected with Schistosoma haematobium. J Trop Pediatr 57(4):263–268
Wong-You-Cheong JJ, Woodward PJ, Manning MA, Davis CJ (2006) From the archives of the AFIP: inflammatory and nonneoplastic bladder masses: radiologic-pathologic correlation. Radiographics 26(6):1847–1868
Zhong X, Isharwal S, Naples JM, Shiff C, Veltri RW, Shao C, Bosompem KM, Sidransky D, Hoque MO (2013) Hypermethylation of genes detected in urine from Ghanaian adults with bladder pathology associated with Schistosoma haematobium infection. PLoS One 8(3):e59089. doi:10.1371/journal.pone.0059089
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akpata, R., Neumayr, A., Holtfreter, M.C. et al. The WHO ultrasonography protocol for assessing morbidity due to Schistosoma haematobium. Acceptance and evolution over 14 years. Systematic review. Parasitol Res 114, 1279–1289 (2015). https://doi.org/10.1007/s00436-015-4389-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4389-z