Abstract
Microbial synthesis of nanoparticles is a green approach that interconnects nanotechnology and microbial biotechnology. Here, we synthesized the silver nanoparticles (AgNPs) using bacterial strains of Listeria monocytogenes, Bacillus subtilius and Streptomyces anulatus. We tested the efficacy of AgNPs against the larvae, pupae and adults of Anopheles stephensi and Culex quinquefasciatus. We have also investigated the antifungal activity of AgNPs against the soil keratinophilic fungus of Chrysosporium keratinophilum. The efficacy tests were then performed at different concentrations and varying numbers of hours by probit analysis. The results were obtained using a UV-visible spectrophotometer, and the images were recorded with a transmission electron microscope (TEM). The synthesized AgNPs were in varied shape and sizes. The larvae and pupae of Cx. quinquefasciatus were found highly susceptible to AgNPs synthesized using the L. monocytogenes, B. subtilius and S. anulatus than the An. stephensi, while the adults of An. stephensi were found more susceptible to the AgNPs synthesized using the L. monocytogenes, B. subtilius and S. anulatus the Cx. quinquefasciatus. Further, these nanoparticles have also been tested as antifungal activity against the entomopathogenic fungus C. keratinophilum. The higher zone of inhibition occurred at the concentration level of 50 μl. This study gives an innovative approach to develop eco-friendly AgNPs which act as an effective antifungal agent/fungicide and insecticide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mosquitoes are found across the globe, with the exception of Antarctica and Iceland. They are also a well-known vector for many diseases, including malaria, dengue fever, yellow fever and viral encephalitis. Among these, malaria, spread by the bite of a female Anopheles mosquito, and filariasis, spread by Culex mosquito are the two vector-borne diseases of the tropical region and are considered as major public health concerns.
According to WHO, there were about 219 million cases of malaria in 2010 (with an uncertainty range of 154 million to 289 million) and an estimated 660,000 deaths (with an uncertainty range of 490,000 to 836,000). Malaria mortality rate has fallen by more than 25 % globally since 2000 and by 33 % in the WHO African region. Most deaths occur among children living in Africa, where malaria claims the life of a child every minute. Country-level burden estimates available for 2010 show that an estimated 80 % of malaria deaths occur in just 14 countries and about 80 % of cases occur in 17 countries. Together, the Democratic Republic of the Congo and Nigeria account for over 40 % of the total estimated malaria deaths globally (World Health Organization 2013a).
On the other hand, nearly 1.4 billion people in 73 countries worldwide are threatened by lymphatic filariasis, commonly known as elephantiasis. Over 120 million people are currently infected, with about 40 million disfigured and incapacitated by the disease (World Health Organization 2013b). Control or eradication of the mosquito population could significantly restrict the spread of disease.
Nanoparticles attract greater attention due to their various applications in different fields. Microbes are also being used in nanotechnology for producing nanoparticles. Therefore, the present green synthesis has shown that environmentally benign and renewable sources of microbes can used as an effective reducing agent for the synthesis of silver and gold nanoparticles and mosquito control also. Biosynthesis of nanoparticles by using the bacteria Bacillus (Dhandapani and Supraja 2012; Das et al. 2013; Malarkodi et al. 2013; Priyadarshini et al. 2013; Omolbanin Shivai et al. 2013; Janardhanan et al. 2013), Streptomyces (Alani et al. 2012), Pseudomonas (Radhika Rajasree and Suma 2012; Silambarasan and Jayanthi 2013), Vibrio (Rajeshkumar et al. 2013) and Idiomarina (Seshadri et al. 2012) has been reported.
The antimicrobial activity of synthesized nanoparticles by using the Bacillus (Prakash et al. 2011; Kannan and Subblaxmi 2011; Sunkar and Valli Nachiyar 2012; Vijyaraghavan et al. 2012; El-Batal et al. 2013; Gopinath and Velusamy 2013), Streptomyces (Selvakumar et al. 2012; Subashini and Kannabiran 2013; Chauhan et al. 2013) and Lactobacillus (Salman 2013) has been investigated.
The larvicidal activities of synthesized cobalt nanoparticles (CoNPs) using biocontrol agent, Bacillus thuringiensis against malaria vector, Anopheles subpictus, and dengue vector, Aedes aegypti (Diptera: Culicidae), have been reported (Marimuthu et al. 2013). Similarly, the larvicidal activity of silver nanoparticles (AgNPs) synthesized by Bacillus thuringiensis (Bt) has been tested against Ae. aegypti (Banu et al. 2014).
In the present investigation, the AgNPs were synthesized using bacterial strains of Listeria monocytogenes, Bacillus subtilius and Streptomyces anulatus. We tested the efficacy of AgNPs against the larvae, pupae and adults of Anopheles stephensi and Culex quinquefasciatus. We have also investigated the antifungal activity of AgNPs against the soil keratinophilic fungus of Chrysosporium keratinophilum.
Materials and methods
Microbes and culture
The bacterial strains of L. monocytogenes (J0161), B. subtilius (ATCC 11774) and S. anulatus (MTCC 2528) were obtained from the Agriculture Research Services (ARS), US Department of Agriculture (USDA) and Microbial Type Culture Collection, Chandigarh, India.
The bacterial strains of L. monocytogenes, B. subtilius and S. anulatus were cultured on tryptone soy broth at 37 °C. After 24–48 h of incubation, biomass developed on the medium.
Synthesis of silver nanoparticles
After 24–48 h of incubation, culture solution was centrifuged at 7500 rpm for 15 min. Then, the supernatant was taken in a clean 250-ml conical flask and added 1 M silver nitrate into 100 ml of supernatant. Then, the mixture was incubated in the orbital shaker for the synthesis of silver nanoparticles for 24 h. The change in colour was observed visually.
Characterization of silver nanoparticles
Synthesized silver nanoparticles were confirmed by sampling the reaction mixture at regular intervals, and the absorption maxima were scanned by UV-Vis spectra, at the wavelength of 350–750 nm in a UV-3600 Shimadzu spectrophotometer at 1-nm resolution. The micrographs of silver nanoparticles were obtained by a TECHNAI 200 Kv TEM (Fei, Electron Optics) transmission electron microscope. For transmission electron microscopy analysis, samples were prepared on carbon-coated copper TEM grids.
Vector rearing
The larvae of Cx. quinquefasciatus and An. stephensi were collected from various localities including urban, rural and semi-urban regions of Agra (27°, 10′ N, 78° 05′ E), India. The larvae were reared in deionized water containing glucose and yeast powder. The colonies of Cx. quinquefasciatus and An. stephensi were maintained in the laboratory at a temperature of 25 °C with relative humidity of 75 ± 5 % and 14 h of photoperiod. The larvae of Cx. quinquefasciatus and An. stephensi were maintained in separate enamel containers as per the standard method (Gerberg et al. 1994). The pupae were collected from the culture tray and transferred to Petri dishes containing 50 ml of water. The Petri dishes were placed inside a screened cage (25 cm in length × 15 cm in width × 5 cm in depth) to retain emerging adults, for which 5 % sucrose solution in water was provided to the adults.
Bioassays, data management and statistical analysis
AgNPs synthesized by L. monocytogenes, B. subtilius and S. anulatus were tested for their killing activities against the larvae, pupae and adults of Cx. quinquefasciatus and An. stephensi and were assessed by using the standard method (World Health Organization 2005). All larvae and pupae of Cx. quinquefasciatus and An. stephensi were separated and placed in a container in microbe-free deionized water. After that, different test concentrations of silver nanoparticles in 100 ml deionized water were prepared in 250-ml beakers. Bioassays were conducted separately for each instar and pupae at five different concentrations of aqueous silver nanoparticles (2, 4, 6, 8 and 10 ppm for larvae and 20, 40, 60, 80 and 100 ppm, for pupa). To test the larvicidal and pupicidal activity of our silver nanoparticles, 20 pupae and larvae of each stage were separately exposed to 100 ml of test concentrations. Thereafter, we examined their mortality after different times of treatment during the experimental periods.
The adulticidal bioassays were carried out with laboratory-reared Cx. quinquefasciatus and An. stephensi as per standard procedures recommended by the World Health Organization with some modifications (World Health Organization 2006). The freshly emerged 3-day-old sugar-fed adults were used for the assay. The five different volumes 0.16, 0.33, 0.66, 1.33 and 2.66 μl/cm2 of aqueous silver nanoparticles of L. monocytogenes, B. subtilius and S. anulatus synthesized were sprayed in a cage (25 cm long × 15 cm wide × 5 cm deep) containing 100 mosquitoes. The exposed mosquitoes were kept under observation, and the dead mosquitoes were discarded every day. Each bioassay including the control was conducted in triplicates on different days. Similarly, the control (extract of L. monocytogenes, B. subtilius and S. anulatus without AgNO3) was run to test the natural mortality. The data on the efficacy were subjected to probit analysis (Finney 1971). The control mortality was corrected by Abbott’s formula (Abbott 1925).
Antifungal activity of AgNPs
The fungicidal activity of AgNPs synthesized by using the L. monocytogenes, B. subtilius and S. anulatus was carried out against the entomopathogenic fungus C. keratinophilum (MTCC 2828). The fungicidal activity was done by using well diffusion method. The potato dextrose agar (PDA) medium was prepared and poured into plates, and wells were made in the agar medium. Then, 24-h-old test fungicidal cultures were swabbed in PDA medium to form a confluent lawn of fungal cultures. Different concentrations (20, 30 and 50 μl) of AgNPs were loaded into the wells. Then, the plates were incubated at 25 °C for 24–84 h. After 24–48 h of inhibition, the plates were observed for zone of inhibition.
Results
UV-Vis spectroscopy and TEM analysis of synthesized AgNPs
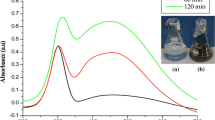
By mixing the culture broth of L. monocytogenes, B. subtilius and S. anulatus with the aqueous solution of Ag ions, the colour of culture broth of L. monocytogenes, B. subtilius and S. anulatus changed to dark brown colour after 24 h of incubation. The change in colour is a signal for the formation of silver nanoparticles. Silver nanoparticle formation was further confirmed by the UV-Vis spectroscopy. Figure 1a–c shows the UV-Vis spectra of silver nanoparticles synthesized by using the L. monocytogenes, B. subtilius and S. anulatus recorded from reaction medium before (1) and after immersion of AgNO3 (2) after 24 h. Absorption spectra of silver nanoparticles formed in the reaction medium have a broad absorption band centred at 480 nm ca.
Figure 2a–c shows the TEM micrographs of L. monocytogenes, B. subtilius and S. anulatus-synthesized silver nanoparticles. The silver nanoparticles were of varying sizes and shapes (Table 1).
Efficacy study of AgNPs synthesized by using the B. subtilius against the Cx. quinquefasciatus and An. stephensi
The different concentration doses (2, 4, 6, 8 and 10 ppm; 20, 40, 60, 80 and 100 ppm and 0.16, 0.33, 0.66, 1.33 and 2.66 μl/cm2, respectively) of silver nanoparticles synthesized by using the B. subtilius were tested against the larvae, pupae and adults of Cx. quinquefasciatus and An. stephensi. The larvae and pupae of Cx. quinquefasciatus and An. stephensi were found to be more susceptible to the synthesized silver nanoparticles than the adults. The mortality was observed after a span of different times of exposures.
The larvae of Cx. quinquefasciatus were found highly susceptible to the synthesized silver nanoparticles. The first and second instar larvae of Cx. quinquefasciatus were found highly susceptible to the synthesized silver nanoparticles and have shown 100 % mortality within 15 min. While the third and fourth larvae have shown 100 % mortality after 45 min with their confidential limits. The pupae of Cx. quinquefasciatus were found more susceptible to the synthesized silver nanoparticles than the adults. The pupae have shown 100 % mortality after 5 h, while no mortality could be observed in adults after 5 h. In the control group, no mortality could be recoded (Table 2 (a)).
The larvae of An. stephensi were found susceptible to the silver nanoparticles synthesized by using B. subtilius. The first and third instar larvae of An. stephensi have shown 100 % mortality after 72 h of exposure. The second instars (lethal concentration 50 (LC50) 1, LC90 8 and LC99 10.5 ppm) have shown mortality after 72 h. The fourth instars (LC50 1, LC90 8 and LC99 10.5 ppm) have shown mortality after 72 h and were observed with their confidential limits. In the control group, no mortality could be observed. The pupae of An. stephensi have shown 100 % mortality after 24 h. The adults have shown (LC50 0.16, LC90 3 and LC99 4 μl/cm2) after 2 h and 30 min with their confidential limits (Table 2 (a)).
Efficacy study of AgNPs synthesized by using the L. monocytogenes against the Cx. quinquefasciatus and An. stephensi
The different concentration doses (2, 4, 6, 8 and 10 ppm; 20, 40, 60, 80 and 100 ppm and 0.16, 0.33, 0.66, 1.33 and 2.66 μl/cm2, respectively) of silver nanoparticles synthesized by using the L. monocytogenes were tested against the larvae, pupae and adults of Cx. quinquefasciatus and An. stephensi. The larvae and pupae of Cx. quinquefasciatus and An. stephensi were found to be more susceptible to the synthesized silver nanoparticles than the adults. The mortality was observed after a span of different times of exposures.
The larvae of Cx. quinquefasciatus were found highly susceptible to the synthesized silver nanoparticles. The first and second instar larvae of Cx. quinquefasciatus were found highly susceptible to the synthesized silver nanoparticles and have shown 100 % mortality within 24 min. For third instar larvae (LC50 2, LC90 10.5 and LC99 15.5 ppm) and fourth instar (LC50 2.66, LC90 5.33 and LC99 5.86 ppm) the susceptibility was recorded after 24 h with their confidential limits. The pupae of Cx. quinquefasciatus were found more susceptible to the synthesized silver nanoparticles than the adults. The pupae have shown 100 % mortality after 24 h, while no mortality could be observed in adults after 5 h. In the control group, no mortality could be recoded (Table 2 (b)).
The larvae of An. stephensi were found susceptible to the silver nanoparticles synthesized by using L. monocytogenes. The larvae and pupae of An. stephensi have shown 100 % mortality after 4 and 24 h. In the control group, no mortality could be observed. The adults have shown (LC50 0.08, LC90 3.99 and LC99 5 μl/cm2) after 5 h with their confidential limits (Table 2 (b)).
Efficacy study of AgNPs synthesized by using the S. anulatus against the Cx. quinquefasciatus and An. stephensi
The different concentration doses (2, 4, 6, 8 and 10 ppm; 20, 40, 60, 80 and 100 ppm and 0.16, 0.33, 0.66, 1.33 and 2.66 μl/cm2, respectively) of silver nanoparticles synthesized by using the S. anulatus were tested against the larvae, pupae and adults of Cx. quinquefasciatus and An. stephensi. The larvae and pupae of Cx. quinquefasciatus and An. stephensi were found to be more susceptible to the synthesized silver nanoparticles than the adults. The mortality was observed after a span of different times of exposures.
The larvae of Cx. quinquefasciatus were found highly susceptible to the synthesized silver nanoparticles. The larvae and pupae of Cx. quinquefasciatus have shown 100 % mortality after different hours of exposure, while no mortality could be observed in adults after 4 h. In the control group, no mortality could be recoded (Table 2 (c)).
The larvae of An. stephensi were found susceptible to the silver nanoparticles synthesized by using S. anulatus. The larvae of An. stephensi have shown 100 % mortality after 5 h of exposure. In the control group, no mortality could be observed. The pupae of An. stephensi have shown mortality (LC50 20, LC90 120 and LC99 130 ppm) after 3 h and 20 min. The adults have shown mortality (LC50 0.06, LC90 1.333 and LC99 3 μl/cm2) after 2 h and 30 min with their confidential limits (Table 2 (c)).
Antifungal activity of synthesized silver nanoparticles
Well diffusion method was used to provide the evidence for and validate the antifungal activity of synthesized silver nanoparticles against C. keratinophilum. Different concentrations (10, 30 and 50 μl) of AgNPs were used to confirm the antifungal efficiency of AgNPs. The antifungal activity of silver nanoparticles was indicated by the formation of the zone. The diameter of the inhibition zone was measured in millimetres. The maximum zone of inhibition was observed in C. keratinophilum against the AgNPs synthesized by L. monocytogenes (5, 19 and 20 mm). However, the zone of inhibition by S. anulatus (6, 9 and 11 mm) and B. subtilius (5, 5, and 11 mm) synthesized nanoparticles was observed (Fig. 3a–c). The maximum zone of inhibition occurred at 50-μl concentration of synthesized AgNPs.
Discussion
In the present investigation, the AgNPs were synthesized using bacterial strains of L. monocytogenes, B. subtilius and S. anulatus. We tested the efficacy of AgNPs against the larvae, pupae and adults of An. stephensi and Cx. quinquefasciatus. We have also investigated the antifungal activity of AgNPs against the soil keratinophilic fungus of C. keratinophilum.
The larvicidal activities of synthesized cobalt nanoparticles using the biocontrol agent, B. thuringiensis, have been investigated against the malaria vector An. subpictus and dengue vector, Ae. aegypti (Marimuthu et al. 2013). Furthermore, the larvicidal activity of silver nanoparticles synthesized by using B. thuringiensis has been revealed against the Ae. aegypti (Banu et al. 2014), whereas in the present study, we have tested the larvicidal, pupicidal and adulticidal activity of silver nanoparticles synthesized by L. monocytogenes, B. subtilius and S. anulatus against the An. stephensi and Cx. quinquefasciatus.
The efficacy of fungus-mediated silver and gold nanoparticles has been tested against the larvae of An. stephensi, Cx. quinquefasciatus and Ae. aegypti (Soni and Prakash 2012a, b, c). Furthermore, the larvicidal and pupicidal activities of silver and gold nanoparticles synthesized by fungi have also been investigated against the An. stephensi, Cx. quinquefasciatus and Ae. aegypti (Soni and Prakash 2013a, b, c). However, in the present study, the silver nanoparticles have been synthesized by L. monocytogenes, B. subtilius and S. anulatus and tested as larvicides, pupicides and adulticides against the An. stephensi and Cx. quinquefasciatus.
The antibacterial activity of silver nanoparticles synthesized by B. cereus has been evaluated against the pathogenic bacteria like Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Salmonella typhi and Klebsiella pneumoniae (Sunkar and Valli Nachiyar 2012). Antimicrobial activity of silver nanoparticles synthesized by Streptomyces rochei has been screened against common human pathogen P. aeruginosa, E. coli, K pneumoniae, Enterobactoer faecalis and S. aureus (Selvakumar et al. 2012). The antifungal effect of silver nanoparticles synthesized by B. subtilius has been screened against the Candida albicans and Aspergillus niger (Vijyaraghavan et al. 2012). The antimicrobial activity of Streptomyces sp. VITBT7 and its synthesized silver nanoparticles has been evaluated against the medically important fungal and bacterial pathogens (Subashini and Kannabiran 2013). The antifungal activity of silver nanoparticles synthesized by Bacillus sp. GP-23 has been screened towards the Fusarium oxysporum (Gopinath and Velusamy 2013), whereas in the present investigation, the antifungal activity of silver nanoparticles synthesized by L. monocytogenes, B. subtilius and S. anulatus has screened been against the entomopathogenic fungus C. keratinophilum.
Conclusion
Here, the silver nanoparticles have been synthesized by bacterial strains of L. monocytogenes, B. subtilius and S. anulatus. The synthesized silver nanoparticles have been tested as larvicides, pupicides and adulticides against the larvae, pupae and adults of An. stephensi and Cx. quinquefasciatus. The antifungal activity of synthesized silver nanoparticles has also been evaluated against the entomopathogenic fungus C. keratinophilum. The higher zone of inhibition occurred at the concentration level of 50 μl. This study gives an innovative approach to develop eco-friendly AgNPs which act as an effective antifungal agent/fungicide and insecticide. In the future, the nanoparticles could be useful in food packing, food preservation, food borne-disease, etc.
References
Abbott SW (1925) A method of computing the effectiveness of an insecticide. J Econom Entomol 18:265
Alani F, Moo-Young M, Anderson W (2012) Biosynthesis of silver nanoparticles by a new strain of Streptomyces sp. compared with Aspergillus fumigatus. World J Microbiol Biotechnol 28:1081–1086
Banu AN, Balasubramanian C, Moorthi PV (2014) Biosynthesis of silver nanoparticles using Bacillus thuringiensis against dengue vector, Aedes aegypti (Diptera: Culicidae). Parasitol Res 113:311–316
Chauhan R, Kumar A, Abraham J (2013) A biological approach to the synthesis of silver nanoparticles with Streptomyces sp JAR1 and its antimicrobial activity. Sci Pharm 81:607–621
Das VL, Thomas R, Vargese RT, Soniya EV, Mathew J, Radhakrishnan EK (2013) Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. 3 Biotech. doi:10.1007/s13205-013-0130-8
Dhandapani P, Supraja N (2012) Extracellular synthesis of silver nanoparticles by marine thermophilic bacteria. Int J Pharmaceut Bio Arch 3:1418–1423
El-Batal AI, Amin MA, Shehata MMK, Hallol MMA (2013) Synthesis of silver nanoparticles by Bacillus stearothermophilus using gamma radiation and their antimicrobial activity. World App Sci J 22:01–16
Finney DJ (1971) Probit analysis, 3rd edn. Cambridge University Press, Cambridge
Gerberg EJ, Barnard DR, Ward RA (1994) Manual for mosquito rearing and experimental techniques. J Am Mosq Control Assoc 5:98
Gopinath V, Velusamy P (2013) Extracellular biosynthesis of silver nanoparticles using Bacillus sp. GP-23 and evaluation of their antifungal activity towards Fusarium oxysporum. Spectrochimica Acta A Molecular and Biomolecular Spectroscopy 106:170–174
Janardhanan A, Roshmi T, Verghese RT, Soniya EV, Mathew J, Radhakrishnan EK (2013) Biosynthesis of silver nanoparticles by a Bacillus sp. of marine origin. Materials Science-Poland 31:173–179
Kannan N, Subblaxmi S (2011) Green synthesis of silver nanoparticles using Bacillus subtillius IA751 and its antimicrobial activity. Res J Nanosci Nanotech 1:87–94
Malarkodi C, Rajeshkumar S, Paulkumar K, Gnanajobitha G, Vanaja M, Annadurai G (2013) Bacterial synthesis of silver nanoparticles by using optimized biomass growth of Bacillus sp. Nanosci Nanotechnol: An Int J 3:26–32
Marimuthu S, Rahuman AA, Kirthi AV, Santhoshkumar T, Jayaseelan C, Rajakumar G (2013) Eco-friendly microbial route to synthesize cobalt nanoparticles using Bacillus thuringiensis against malaria and dengue vectors. Parasitol Res. doi:10.1007/s00436-013-3601-2
Omolbanin Shivai K, Mojtaba S, Rahim Sorouri Z, Fatemeh Khadivi D (2013) Extracellular deposition of silver nanoparticles by Bacillus megaretium. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chem 43:903–906
Prakash A, Sharma S, Ahmad N, Ghosh A, Sinha P (2011) Synthesis of AgNPs by Bacillus cereus bacteria and their antimicrobial potential. J Biomater Nanobiotech 2:156–162
Priyadarshini S, Gopinath V, Meera Priyadarshini N, MubarakAli D, Velusamy P (2013) Synthesis of anisotropic silver nanoparticles using novel strain, Bacillus flexus and its biomedical application. Colloids and Surface B Biointerface 102:232–237
Radhika Rajasree SR, Suma TY (2012) Extracellular biosynthesis of gold nanoparticles using a gram negative bacterium Pseudomonas fluorescens. Asian Pac J Trop Dis S795-S799
Rajeshkumar S, Malarkodi C, Paulkumar K, Vanaja M, Gnanajobitha G, Annadurai G (2013) Intracellular and extracellular biosynthesis of silver nanoparticles by using marine bacteria Vibrioa alginolyticus. Nanosci Nanotechnol: An Int J 3:21–25
Salman JAS (2013) Antibacterial activity of silver nanoparticles synthesized by Lactobacillus spp. against methicillin resistant Staphylococcus aureus. Int J Adv Res 1:178–184
Selvakumar P, Viveka S, Prakash S, Jasminebeaula S, Uloganathan R (2012) Antimicrobial activity of extracellularly synthesized silver nanoparticles from marine derived Streptomyces rochei. Int J Pharmaecutical and Bio Sci 3:188–197
Seshadri S, Prakash A, Kowshik M (2012) Biosynthesis of silver nanoparticles by marine bacterium Idiomarine sp. p R58–8. Bull Mater Sci 35:1201–1205
Silambarasan S, Jayanthi A (2013) Biosynthesis of silver nanoparticles using Pseudomonas fluorescens. Res J Biotech 8:71–75
Soni N, Prakash S (2012a) Efficacy of fungus mediated silver and gold nanoparticles against Aedes aegypti larvae. Parasitol Res 110:175–184
Soni N, Prakash S (2012b) Synthesis of gold nanoparticles by the fungus Aspergillus niger and its efficacy against mosquito larvae. Reports Parasitol 2:1–7
Soni N, Prakash S (2012d) Entomopathogenic fungus generated nanoparticles for enhancement of efficacy in Culex quinquefasciatus and Anopheles stephensi. Asian Pac J Trop Dis S356-S361
Soni N, Prakash S (2013a) Possible mosquito control by silver nanoparticles synthesized by soil fungus (Aspergillus niger 2587). Adv Nanopart 2:125–132
Soni N, Prakash S (2013b) Fungus generated novel nanoparticles: a new prospective for mosquito control. Int J Recent Sci Res 4:1481–1487
Soni N, Prakash S (2013c) Microbial synthesis of spherical nanosilver and nanogold for mosquito control. Ann Microbiol. doi:10.1007/s13213-013-0749-z
Subashini J, Kannabiran K (2013) Antimicrobial activity of Streptomyces sp. VITBT7 and its synthesized silver nanoparticles against medically important fungal and bacterial pathogens. Der Pharmacia Lettre 5:192–200
Sunkar S, Valli Nachiyar C (2012) Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian Pac J Trop Biomed 2:953–959
Vijyaraghavan R, Krishna Prabha V, Rajendran S (2012) Biosynthesis of silver nanoparticles by a marine bacterium Bacillus subtilis strain and its antifungal effect. World J Sci Technol 2:01–03
World Health Organization (2005) Guidelines for laboratory and field testing of mosquito larvicides. WHO/CDS/WHOPES/GCDPP/13
World Health Organization. (2006) Vector borne diseases in India. Report of a brainstorming session, 9 November 2006
World Health Organization (2013a) Malaria. http://www.who.int/mediacentre/factsheets/fs094/en/index.html
World Health Organization (2013b) Lymphatic filariasis. http://www.who.int/mediacentre/factsheets/fs102/en/
Acknowledgments
We sincerely thank Prof P. S. Satsangi Sahab, Chairman, of the Advisory Committee on Education, Dayalbagh Educational Institute, and Prof. P. K. Kalra, Director, Dayalbagh Educational Institute for providing support and encouragements for the work. We also thank UGC, New Delhi, Major Research Project (39-599/2010) for financial support. We also thank Dr. Shashi Wadhawa for TEM (AIIMS, New Delhi).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soni, N., Prakash, S. Antimicrobial and mosquitocidal activity of microbial synthesized silver nanoparticles. Parasitol Res 114, 1023–1030 (2015). https://doi.org/10.1007/s00436-014-4268-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4268-z