Abstract
Epidemiological surveys have demonstrated that helminth infections are negatively related to atopic diseases, including asthma. Defining and characterising specific helminth molecules that have excellent immunomodulatory capacities as potential therapeutics for the treatment or prophylaxis of allergic manifestations are of great interest. AcCystatin, a cystatin protease inhibitor of Angiostrongylus cantonensis, is a homologue of other nematode cystatins with immunoregulatory properties. Here, we aim to determine the effects of AcCystatin on an ovalbumin/aluminium hydroxide (OVA/Al[OH]3)-induced rat model of asthma. Wistar rats were randomly divided into four groups, including a control group, an OVA/Al[OH]3-induced asthma group, a group receiving AcCystatin immunisation prior to OVA/Al[OH]3-induced asthma and a group receiving AcCystatin treatment after OVA/Al[OH]3-induced asthma. The numbers of eosinophils, basophils, neutrophils, lymphocytes and monocytes in the peripheral blood and of eosinophils in the bronchoalveolar lavage fluid (BALF) were counted for each animal. The expression levels of the cytokines interferon-γ, interleukin (IL) 4, IL-5, IL-6, IL-10, IL17A and tumour necrosis factor receptor-α in BALF, of OVA-specific immunoglobulin E in BALF and serum and of the chemokines eotaxin-1, eotaxin-2, eotaxin-3, MCP-1 and MCP-3 in lung tissue were measured. In addition, the degree of peribronchial and perivascular inflammation and the intensity of goblet cell metaplasia were qualitatively evaluated. The sensitised/challenged rats developed an extensive cell inflammatory response of the airways. AcCystatin administration significantly reduced the cellular infiltrate in the perivascular and peribronchial lung tissues and reduced both goblet mucous production and eosinophil infiltration. The rats that were treated with AcCystatin before or after sensitisation with OVA showed significant decreases in eotaxin-1, eotaxin-3 and MCP-1 expression in the lung tissue. The production of IL-4, IL-5, IL-6 and IL-17A and of OVA-specific IgE antibodies was also significantly reduced in AcCystatin-treated rats compared with untreated asthmatic rats. The AcCystatin treatment was associated with a significant increase in IL-10 levels. Our present findings provide the first demonstration that AcCystatin is an effective agent in the prevention and treatment of the airway inflammation associated with asthma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asthma, which affects more than 300 million individuals and causes approximately 250,000 annual deaths, is a serious health and socioeconomic issue across the world. This chronic inflammatory lung disease is characterised by airway eosinophil accumulation, intermittent airway obstruction, hyperresponsiveness and airway wall remodelling, in addition to mucus hyper-production in response to inhaled allergens or nonspecific stimuli (Roche et al. 1989; Kay 1991; Bousquet et al. 2000; Kudo et al. 2013). Airway remodelling is specifically characterised by structural and morphometric changes to the airway, including subepithelial fibrosis, epithelial hypertrophy, goblet cell hyperplasia and smooth muscle hypertrophy (Jeffery 1991; Aikawa et al. 1992). Eosinophils, the major effector cells in the pathogenesis of asthma, play a crucial role in the initial symptoms of asthma, including airway inflammation (Kay 1991). Asthma is classically recognised as a typical Th2 disease, in which the inflammatory process is dominated by Th2 cells that release the cytokines interleukin (IL)-4, IL-5 and IL-13. The emerging Th2 cytokines modulate airway inflammation by activating eosinophils and inducing immunoglobulin E (IgE) production (Robinson et al. 1992; Nakajima and Hirose 2010) Furthermore, chemokines have now emerged as potentially critical molecules in the pathogenesis of airway inflammation in asthma, acting as leukocyte chemoattractants, cellular activating factors and regulators of homeostatic immunity (Rothenberg et al. 1999). Oral or inhaled corticosteroids and steroids are the first choices for preventative and relief-providing asthma treatments. However, these drugs, in addition to potential resistance (Barnes 2013) and undesired systemic and local side effects, such as osteoporosis (Hurson, et al. 2007), hypertension (Goodwin et al. 2011), fluid retention (Rhen and Cidlowski 2005; Urbańska et al. 2014) and hypersensitivity (Shakouri and Bahna 2013), are not curative (Apter 2014). Thus, the development of efficient alternative agents and therapeutics for asthma is urgently needed.
Epidemiological and experimental studies have provided strong evidence that infections with parasitic worms (especially parasitic helminths) not only can downregulate parasite-specific immune responses, but also are negatively related to autoimmune and allergic inflammatory responses (Wammes et al. 2014; Garg et al. 2014). The down-modulation of allergic and inflammatory immune responses by secreted immunoregulatory factors from parasites has been regarded as a crucial strategy of worm survival, which is initiated to modulate the host immune responses directed against them (Schnoeller et al. 2008). Among the secreted immunomodulators, cysteine protease inhibitors (cystatins) from various parasitic worms have been demonstrated to be of major importance (Vray et al. 2002). Cystatins of ectoparasites exert their immunoregulatory activities by interfering with antigen processing and presentation, T cell responses and the modulation of cytokines and nitric oxide production (Zavasnik-Bergant 2008; Hartmann and Lucius 2003).
We previously described the in vitro immunoregulatory properties of the purified recombinant cystatin from Angiostrongylus cantonensis (AcCystatin; Liu et al. 2010). In this study, we describe a novel mechanism of immunomodulation in which AcCystatin prevents murine allergic airway reactivity, most likely via the following mechanisms: the suppression of the expression of the cytokines IL-4, IL-5, IL-6 and IL-17A; the reduced expression of the chemokines eotaxin-1, eotaxin-3 and MCP-1; and the decreased production of OVA-specific IgE antibodies, concomitant with the enhancement of IL-10 release.
Methods
Preparation of recombinant AcCystatin
Recombinant AcCystatin was obtained and purified as previously described (Liu et al. 2010), and subsequent endotoxin removal was performed using Detoxi-Gel Endotoxin Removing Columns (Thermo Scientific, USA) according to the manufacturer’s instructions, resulting in 0.05 endotoxin units per milligram protein as indicated by the Limulus Amebocyte Lysate Chromogenic Endotoxin Quantitation Kit (Thermo Scientific, USA).
Animals and experimental design
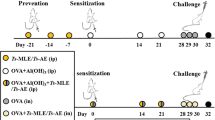
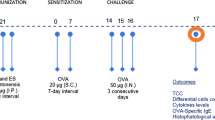
The 32 male Wistar rats used for this study were purchased from the Experimental Animal Centre of Sun Yat-sen University (SYSU). The experimental protocols were approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University. All of the animals were housed in a specific pathogen-free environment and maintained on a 12 h light–dark cycle, with water and food ad libitum at the Experimental Animal Centre of SYSU. These protocols were in accordance with the regulations of the Guide for the Care and Use of Experimental Animals of the National Institutes of Health. Eight-week-old rats were randomly divided into 4 groups (8 rats per group): controls treated with phosphate-buffered saline (PBS; group I), a group with asthma induced by ovalbumin/aluminium hydroxide (OVA/Al[OH]3) (group II), a group pre-treated with AcCystatin and with asthma induced by OVA/Al[OH]3 (group III) and a group with asthma induced by OVA/Al[OH]3 followed by treatment with AcCystatin (group IV).
Administration of OVA and aluminium hydroxide
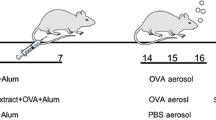
Rats in groups II, III and IV were sensitised intraperitoneally with a mixture of 10 μg of OVA (Sigma, China) in 250 μl of Al[OH]3 colloidal suspension (10 mg, Sigma, China; day 1) and were boosted subcutaneously with 10 μg of OVA emulsified in 250 μl of PBS 7 days later (day 8). On the 15th day, a single 15-min airway challenge with aerosolised 1 % OVA (w/v) in PBS was performed as described (de Oliveira et al. 2007) using an ultrasonic nebuliser (Yuyue 402A type I, Shanghai Yu Yue Medical Equipment Co., Inc., Shanghai, China) that produced a flow from 4 to 8 l/min and particle sizes from 0.5 to 5 mm. Animals in groups III and IV were intraperitoneally administered 250 μl of AcCystatin (500 μg per rat) in PBS on days 0 and 14, respectively. The rats in the control group underwent mock sensitisation with intraperitoneally administered sterile PBS and were challenged with an aerosol of sterile PBS instead of OVA. All the animals were exsanguinated by sectioning the abdominal aorta under deep chloral hydrate-induced anaesthesia (400 mg/kg, intraperitoneally) and were sacrificed by cervical dislocation 24 h after the aerosol challenge.
Bronchoalveolar lavage and cell counts
Bronchoalveolar lavage was performed 24 h after the challenge, with the rats under deep anaesthesia. The lungs from each animal were lavaged in situ with a total volume of 10 ml (2.5 ml × 4 times) ice-cold 1 × PBS. The cells in bronchoalveolar lavage fluid (BALF) were harvested by centrifugation at 3,000 rpm for 10 min at 4 °C, followed by suspension of the cell pellet in 200 μl of 1 × PBS. The cells were then smeared on a glass slide and stained with a Diff-Quick staining set (Laboklin, Germany). The eosinophil count in the BALF was determined in triplicate using standard morphologic criteria and expressed as a percentage of total cells, as described elsewhere (Chang et al. 2004).
The peripheral blood of the rats was collected by sectioning the abdominal aorta under deep chloral hydrate-induced anaesthesia. The numbers of leukocytes, including eosinophils, basophils, neutrophils, lymphocytes and monocytes, in the peripheral blood of each animal were detected using a Veterinary Multi-species Haematology System (Hemavet 950 FS, Drew Scientific Group; Li et al. 2014).
Real-time RT-PCR for the quantification of chemokine expression
The isolated lung tissues of the rats were either used immediately or snap-frozen in liquid nitrogen and stored at −80 °C until use. Total RNA was extracted from the tissue using TRIzol® reagent (Life Technologies, USA), and the concentration of purified total RNA was determined at 260 nm in a NanoDrop™ 2000/2000c Spectrophotometer (Thermo Scientific, USA). The RNA was then reverse-transcribed at 42 °C for 1 h with a RevertAidTM First Strand cDNA Synthesis Kit (Thermo Scientific, USA). The quantification of rat chemokine (eotaxin-1, eotaxin-2, eotaxin-3 and MCP-1) and β-actin (internal control) expression was determined by real-time PCR using the following primers: eotaxin-1 forward primer, 5′-GAG AAT ATC GGC ACC CAT CCC-3′, and reverse primer, 5′-AGT CCT CCT ATC ATC CTC AGT TAC C-3′; eotaxin-2 forward primer, 5′-ACC TGG ATG CCA AGA GAA AC-3′, and reverse primer, 5′-TCT GGA TGA CAA ACT TGG GA-3′; eotaxin-3 forward primer, 5′-ACG TGT GAG AGT GAA ATG CC-3′, and reverse primer, 5′-GAG CTG TCG GTG ATC TGG TA-3′; MCP-1 forward primer, 5′-GGC CTG TTG TTC ACA GTT GCT-3′, and reverse primer, 5′-TCT CAC TTG GTT CTG GTC CAG T-3′; MCP-3 forward primer, 5′-AAA GGG CAT GGA AGT CTG TG-3′, and reverse primer, 5′-ACC GTA GTC CAC CCA TTT CA-3′ and β-actin forward primer, 5′-GGC ATC CTG ACC CTG AAG TA -3′, and reverse primer, 5′-CTC TCA GCT GTG GTG GTG AA-3′. SYBR® Green I (Life Technologies, USA) was used for the detection and quantification of specific target genes and a housekeeping control (rat β-actin). This analysis was performed using the StepOne™ Real-Time PCR System (Invitrogen, USA), according to the manufacturer’s protocol. For each sample, quantitative RT-PCR was performed in triplicate with the LightCycler®480 System (Roche, Switzerland) using universal cycling conditions as follows: one 10 min cycle at 95 °C (enzyme activation), 35 cycles of 15 s at 95 °C (denaturation) and 30 s at 60 °C (annealing/extension), and one cycle from 60 to 95 °C with an increase of 0.7 °C/s for melting. The levels of chemokine expression were calculated with the corresponding normalised values of β-actin control (fold change = 2−△△CT) using the software provided with the instrument.
Cytokine and OVA-specific IgE assays
The levels of interferon-γ (IFN-γ), IL-4, IL-5, IL-6 and IL-10 in the BALF of the rats were detected using the commercially available rat enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems. The levels of tumour necrosis factor receptor-α (TNF-α) and IL-17A were measured with rat ELISA kits (eBioscience, USA).
The serum level of OVA-specific IgE was determined using a rat ELISA kit (Shanghai Enzyme-linked Biotechnology Co., Ltd, China) according to the protocol. The absorbance of the samples was measured at 450 nm using a spectrophotometer (Thermo Electron Corporation, USA).
Lung histology
Treated and control rat lungs were fixed in 10 % phosphate-buffered formaldehyde overnight and paraffin embedded, after which thin sections (2–3 μm) were cut and stained with haematoxylin-eosin or periodic acid-Schiff (PAS) reagent for mucus staining, according to standard protocols (Chen et al. 2013). Images of the stained sections were visualised with a Leica DM2500 microscope (Leica Microsystems, Canada). Lung inflammation was assessed by two independent pathologists who were blinded to the treatment groups. The degree of peribronchial and perivascular inflammation and the intensity of goblet cell metaplasia were qualitatively evaluated on a subjective scale of 0, 1, 2 and 3, respectively, corresponding to none, slight, moderate and strong inflammation or goblet cell metaplasia, as described previously (Wild et al. 2000).
Statistical analysis
The statistical analyses were performed with GraphPad Prism 5.0 (GraphPad Software, Inc., USA) using a one-way analysis of variance followed by the Tukey–Kramer test. The data were presented as the means ± standard derivation (SD). Differences among the comparisons were considered statistically significant when the P value was less than 0.05.
Results
Effects of AcCystatin administration on leukocytes and cell subpopulations
Figure 1 shows the cellular profiles of the peripheral blood. The cell numbers in the control samples ranged from 0.02 × 106 ± 0.01 × 106 (basophils) to 2.63 × 106 ± 0.48 × 106 (lymphocytes). OVA sensitisation increased the number of total leukocytes, eosinophils, neutrophils, lymphocytes and monocytes by 196, 230, 361, 189 and 287 %, respectively. Except for basophils, all the other cell numbers were significantly inhibited by the pretreatment with AcCystatin before OVA sensitisation (AcCystatin/OVA; Fig. 1), whereas no obvious changes were observed for any cell counts in rats treated with AcCystatin after OVA sensitisation (OVA/AcCystatin) compared with the counts in OVA rats (Fig. 1). AcCystatin/OVA-induced significant reductions in the numbers of leukocytes, lymphocytes and neutrophils relative to OVA/AcCystatin treatment, with no changes observed between the two groups in the numbers of other cell types (Fig. 1).
Effect of AcCystatin on inflammatory cell accumulation in the peripheral blood (PB) and the eosinophil count in the BALF. The numbers of leukocytes (a), lymphocytes (b), monocytes (c), basophils (d), eosinophils (e) and neutrophils (f) in the peripheral blood were measured using a Veterinary Multi-species Haematology System. BAL cells were smeared on a glass slide and stained using a Diff-Quick staining set and the eosinophil count in the BALF was determined in triplicate using standard morphologic criteria and was expressed as a percentage of the total number of cells. The total cell counts and the differential cell counts in the BALF and the PB were determined 24 h after the final OVA exposure. The data are presented as the means ± SD of 8 rats per group. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the Blank group. ##P < 0.01 and ###P < 0.001 compared with the OVA group. ▲P < 0.05 and ▲▲▲P < 0.001 compared with the OVA/AcCystatin group
As depicted in Fig. 1g, very few eosinophils were detected in BALF from the control animals. However, the number of eosinophils recovered from the BALF of the rats exposed to OVA was 50.1-fold higher than that recovered from the control group (P < 0.001). A significant decrease in the eosinophil number in BALF was observed in AcCystatin/OVA and OVA/AcCystatin rats compared with the OVA animals (P < 0.001 and P < 0.005, respectively), and no evident difference (P > 0.05) in the number of BALF eosinophils was found between the AcCystatin/OVA and OVA/AcCystatin groups.
Effect of AcCystatin treatment on the expression levels of chemokines in an allergic model of asthma
To assess the expression levels of the eotaxin-1, -2 and -3, and MCP-1 and -3 transcripts, real-time PCR analyses were performed using mRNA isolated from lung samples of the control and treated groups. As illustrated in Fig. 2, the mRNA expression levels of eotaxin-1, eotaxin-3, MCP-1 and MCP-3 in the lungs were strongly induced by OVA-sensitisation compared with the mRNA expression levels in control rats. These results are in agreement with the accumulation of total inflammatory cells and eosinophils in the peripheral blood and are consistent with intense lung eosinophilia in OVA-immunised rats (Fig. 1). However, the administration of AcCystatin before or after OVA sensitisation resulted in a significant decrease (P < 0.05, P < 0.01 or P < 0.001) in the transcript levels of these chemokines (except for MCP-3) compared with the expression observed in OVA-sensitised rats. No differences in the levels of eotaxin-2 were observed among the OVA/AcCystatin, AcCystatin/OVA or control groups (Fig. 2).
Effect of AcCystatin treatment on chemokine production in the lung tissues of OVA-sensitised asthmatic rats. The levels of chemokine expression were determined via quantitative real-time PCR using primers specific for rat eotaxin-1, eotaxin-2, eotaxin-3, MCP-1 and MCP-3. The values are presented as the means ± SD. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the Blank group. #P < 0.01, ##P < 0.01 and ###P < 0.001 compared with the OVA group
Effect of AcCystatin treatment on productions of cytokines in BALF
To more specifically address how AcCystatin affects the immune responses in a murine allergic asthma model, the production of various cytokines in the BALF was examined. Although there were no significant differences in the levels of IFN-γ, IL-10 and TNF-α between OVA-sensitised and control rats, the proinflammatory factor IL-6, Th2 cytokines IL-4 and IL-5 and Th17 cytokine IL-17A were significantly higher in the OVA-sensitised rats (P < 0.05, P < 0.01 or P < 0.001) compared with the untreated animals (Fig. 3). Significant decreases in the secretion of cytokines, including IL-4, IL-5, IL-6 and IL-17A (P < 0.05, P < 0.01 or P < 0.001), accompanied by a marked elevation of IL-10 (P < 0.05 and P < 0.001, respectively), were observed in both OVA/AcCystatin and AcCystatin/OVA groups relative to the OVA group. Moreover, IL-6, IL-10 and IL-17A were significantly downregulated in the OVA/AcCystatin group compared with the AcCystatin/OVA group. In contrast, no differences in the levels of IFN-γ, IL-4, IL-5 and TNF-α were observed between the two experimental groups (Fig. 3).
Effect of AcCystatin treatment on systemic cytokine production in the BALF of OVA-sensitised asthmatic rats. The concentrations of these cytokines were measured at 24 h after the final OVA challenge via ELISA. The values are expressed as the means ± SD. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the Blank group. #P < 0.05, ##P < 0.01 and ###P < 0.001 compared with the OVA group. ▲P < 0.05, ▲▲P < 0.01 and ▲▲▲P < 0.001 compared with the OVA/AcCystatin group
OVA-specific immunoglobulin (OVA-IgE) in sera and BALF
Antigen-specific Th2 responses are known to induce the production of the antigen-specific IgE antibody. Therefore, in the present study, we used an ELISA to evaluate the effect of AcCystatin on OVA-specific IgE release in an allergic model of asthma. As shown in Fig. 4, the levels of OVA-IgE in serum and BALF were significantly higher in OVA-sensitised rats compared with the levels observed in the control rats (P < 0.01 and P < 0.01, respectively). AcCystatin/OVA significantly suppressed OVA-induced IgE production in serum and BALF (P < 0.01 and P < 0.05, respectively), whereas a clear inhibition was only found in BALF OVA-IgE following OVA/AcCystatin administration (P < 0.05). As expected, trace OVA-IgE levels were detected in the control rats (Fig. 4).
Effect of AcCystatin treatment on lung inflammation in OVA-sensitised rats
As shown in Fig. 5, lung histological sections of rats from different groups were photographed at ×40 magnification. The lung tissue structures in the control rats were well defined, without discernible damage, oedema, or thickened bronchial mucosa. Moreover, the bronchioles and blood vessels were not infiltrated peripherally by inflammatory cells (Fig. 5d). However, H&E staining of the lung sections revealed typical pathological features of asthma in the OVA-sensitised rats. These features included internal haemorrhaging and interstitial oedema in the lung tissue and bronchial structures as well as thickened bronchial mucosa and vascular walls infiltrated by a multitude of lymphocytes, neutrophils and eosinophils (Fig. 5c). PAS staining further revealed mucus overproduction and marked goblet cell hyperplasia in the bronchial airways of the asthmatic animals (Fig. 6c). Histologic changes associated with cellular infiltration (Fig. 5a, b, H&E), goblet cell hyperplasia and mucus production (Fig. 6a, b, PAS) were strongly reduced in OVA-sensitised rats that were pre-treated or treated with AcCystatin, similar to the features observed in the control group. In addition, the scores for peribronchial and perivascular inflammation and goblet cell metaplasia were significantly higher in the asthma model than the control rats. AcCystatin pre-treatment or treatment was efficacious in decreasing these scores (Fig. 7).
Effect of AcCystatin on eosinophil infiltration into the lungs. Photomicrographs (×40) of representative sections of rat lungs from the AcCystatin/OVA group (a), the OVA/AcCystatin group (b), the OVA group (c) and the Blank group (d) stained with haematoxylin and eosin were captured to detect eosinophil infiltration
Effect of AcCystatin on goblet cell hyperplasia in the lungs. Photomicrographs (×40) of representative sections of rat lungs from the AcCystatin/OVA group (a), the OVA/AcCystatin group (b), the OVA group (c) and the Blank group (d) stained with PAS were captured to analyze goblet cell hyperplasia. The arrows indicate areas displaying goblet cell metaplasia
Effect of AcCystatin administration on peribronchial and perivascular inflammation (a) and goblet cell metaplasia (b). Two pathologists blinded to the treatment groups evaluated the degree of peribronchial and perivascular inflammation and goblet cell metaplasia on a scale of 0–3. The values are presented as the means ± SD of 8 rats per group. ∗P < 0.05 and ∗∗∗P < 0.001 compared with the Blank group. ##P < 0.01 and ###P < 0.001 compared with the OVA group
Discussion
Allergic asthma is a common chronic inflammatory airway disorder characterised by recurrent episodes of airway obstruction, and both its prevalence and worldwide burden on health-care costs have continued to increase (Martinez and Vercelli 2013). Despite a large amount of research in the past decade on the mechanisms underlying the genetics, natural history and pathogenesis of this mysterious disease, large gaps in our knowledge remain (Martinez and Vercelli 2013). In the present study, we evaluated, for the first time, the inhibitory effects of the intraperitoneal administration of AcCystatin, a cysteine protease inhibitor from A. cantonensis, on asthmatic responses using an OVA-induced murine asthma model. The OVA-challenged asthmatic rats exhibited eosinophilia, airway inflammation, mucus hypersecretion and elevated levels of IL-4, IL-5, IL-6, IL-17A, eotaxin-1, eotaxin-3, MCP-1 and MCP-3 in the BALF or lungs. In contrast, the AcCystatin-treated animals had fewer inflammatory cells, particularly eosinophils, neutrophils, monocytes and lymphocytes, in the BALF or peripheral blood. These animals also exhibited lower levels of IL-4, IL-5, IL-6, IL-17A, eotaxin-1, eotaxin-3 and MCP-1 in the BALF or lung tissue compared with the Alum/OVA-sensitised/-challenged rats. In addition, AcCystatin significantly increased the expression of IL-10 in BALF. According to the histological analysis, AcCystatin attenuated inflammatory cell infiltration into the airway and suppressed the OVA challenge-induced mucus overproduction.
Asthma is a complex condition with multiple aetiologies, and its development is considered to be an intricate interaction between genetic and environmental factors, although which environmental factors are responsible is not clear (Sears 1997; Tattersfield et al.; 2002). Increasing numbers of investigations have demonstrated that chronic helminth infections are significantly associated with a reduced prevalence of inflammatory disorders, including allergic asthma, among populations in helminth-endemic areas (Leonardi-Bee et al. 2006; Amoah et al. 2012). This protective effect may be greatest in individuals who are sensitised to allergens (van den Biggelaar et al. 2000) and is likely driven by end-organ suppression of Th2-mediated immunity by mechanisms that evolved in the parasite to protect it against host immunity (Scrivener et al. 2001; Tattersfield et al. 2002). The immunological mechanisms of the helminth-mediated modulation of allergies mainly consist of (1) the enhancement or suppression of allergic inflammation directed against the parasite (Hoerauf et al. 2005), (2) immunological cross-reactivity between helminth allergens and aeroallergens (Arruda and Santos 2005) and (3) impacts on allergic inflammation directed against aeroallergens through bystander effects in tissues such as the lungs (Araujo et al. 2004; Cooper et al. 2006; Turner et al. 2008; Cooper 2009). Helminth-induced mechanisms not only regulate host immunity to the worms, resulting in a mutually beneficial environment for survival of both the parasite and host, but also may control the development of allergic diseases. Defining and characterising specific helminth molecules that have excellent immunomodulatory capacities as targets for therapeutic application in the treatment or prophylaxis of allergic manifestations are of great interest (Elliott and Weinstock 2009; Smits et al. 2010).
Cystatins, natural tight-binding reversible inhibitors of cysteine proteases, are present in mammals and parasites, and increasing data have proven that parasite cystatins belong to a new category of immunomodulatory molecules (Vray et al. 2002; Brid et al. 2009). In the immune system, cystatins interfere with the processes of antigen processing and presentation by inhibiting the activities of cysteine proteases, including cathepsins B, L and S. These are the key enzymes for antigen processing and presentation in antigen-presenting cells and inhibition of their function results in a reduction in T cell responses (Zavasnik-Bergant 2008). Parasite cystatins also increase nitric oxide synthesis by interferon gamma-activated murine macrophages, demonstrating proinflammatory properties under certain circumstances (Klotz et al. 2011). Cystatins from parasites, particularly nematodes, are characterised by the stimulation of IL-10 production and the modulation of cytokine responses. These effects contribute to the induction of an anti-inflammatory environment and a significant suppression of cellular proliferation (Hartmann and Lucius 2003).
Th2 cytokines, including IL-4 and IL-5, play key roles in the initiation and progression of allergic asthma and are highly associated with airway inflammation and mucus production (Brusselle et al. 1994; Lee et al. 2011). IL-4 enhances tissue homing of inflammatory effector cells (Busse and Rosenwasser 2003; Romagnani 2004), promotes immunoglobulin class switching to IgG1 and IgE and positively regulates mast cell and eosinophil growth and differentiation, further promoting Th2 differentiation (Hsieh et al. 1992; Rincon et al. 1997). IL-5 is closely associated with airway eosinophilia and hyper-reactivity; moreover, it is critical for the differentiation, maturation and survival of eosinophils, resulting in an overproduction of IgE and mucus (Zhou et al. 2011). Taken together, these previous results provide strong evidence for a crucial role of Th2 cytokines in the pathogenesis of allergic asthma. Therefore, a decrease in Th2 cytokine secretion could exhibit therapeutic benefits to asthmatic patients and animal models (Lee et al. 2011; Yuk et al. 2011). In contrast, the AcCystatin-treated rats exhibited reduced Th2 cytokine expression, with a reduction in IL-4 and IL-5 levels. In addition, these animals exhibited decreased inflammatory cell infiltration into the airway and a suppression of mucus release compared with the OVA-sensitised/challenged animals. These results are consistent with the results of the histological analyses. Based on our observations, AcCystatin appears to effectively ameliorate the asthmatic response by downregulating Th2 cytokines.
IL-10 was reported to redirect pathologic allergic responses by a broad range of suppressive mechanisms. This anti-inflammatory cytokine downregulates Th2 responses (Barnes 2001) and modulates pulmonary inflammation and asthma by inhibiting allergen-specific IgE production and inducing non-inflammatory antibody isotypes (Borish et al. 1996; Larché et al. 2006; Taylor et al. 2006). IL-10 is also reported to be a multi-potent immunosuppressive cytokine, suppressing proinflammatory cytokines, such as TNF-α, IFN-γ and IL-6. Such cytokines are released by resident cells and inflammatory cells, such as endothelial cells, monocytes/macrophages, lymphocytes and mast cells (Moore et al. 2001; Asadullah et al. 2003). Other studies have also shown the underproduction of IL-10 by alveolar macrophages and in the sputum of patients with asthma (Borish et al. 1996), but the IL-10 levels in allergic patients undergoing successful immunotherapy are increased (Akdis 2006). Moreover, IL-10 secreted by T regulatory cells prevents allergic inflammation in healthy individuals and provides long-term relief from symptoms in allergic inflammation (Fu et al. 2006; Urry et al. 2006). Our data indicate a significant elevation of IL-10 production in the OVA-induced murine asthma model treated with AcCystatin, suggesting an essential role of IL-10 in AcCystatin-regulated airway inflammation.
The production of IL-17A increases in the bronchoalveolar lavage fluid of asthmatic patients, with a positive correlation with disease severity and airway reactivity (Molet et al. 2001; Al-Ramli et al. 2009). IL-17A mediates neutrophil infiltration into the airway (Newcomb et al. 2013), increases Th2-mediated airway reactivity and airway inflammation (Wakashin et al. 2008; Barlow et al. 2011) and increases mucous cell metaplasia in airway epithelial cells (Hashimoto et al. 2005; Newcomb et al. 2013). Notably, these effects are inhibited by the regulatory cytokine IL-10 through the negative regulation of IL-17A expression in Th17 cells (Newcomb et al. 2012). Our report shows that treatment with AcCystatin during sensitisation with a model allergen, including treatment before the challenge, leads to a strong reduction in IL-17A levels and a significant increase in IL-10 levels, suggesting that AcCystatin attenuates asthmatic inflammation by direct or IL-10-dependent interference with IL-17A production.
Eosinophils and neutrophils have been proven to be involved in the development and severity of asthma by directly damaging lung tissue due to the toxic products in their granules (Taube et al. 2004; Kita 2013) Therefore, chemokines, released by airway epithelial cells and dendritic cells upon allergen contact, are at a critical interface of the asthmatic immune response with respect to the recruitment and accumulation of eosinophils and neutrophils in the airway undergoing an allergic reaction (Folkerts and Nijkamp 1998; Lukacs et al. 1999). The data from our animal study demonstrate that the administration of AcCystatin in OVA-sensitised and challenged rats can decrease airway eosinophil and neutrophil numbers, and these results are consistent with the reductions in the levels of the chemoattractants eotaxin-1, eotaxin-3, MCP-1 and IL-5.
A recent study demonstrated that parasite cystatins exhibit immunomodulatory effects on the immunocytes of mice via the suppression of Th2-related inflammation and the ensuing asthmatic disease in a murine model of OVA-induced allergic airway responsiveness. Specifically, these results were found to be mediated by an induction of IL-10-producing macrophages (Schnoeller et al. 2008). These previous findings are supported by the results of our study. In summary, the present results suggest that AcCystatin, an immunoregulatory molecule from A. cantonensis, attenuates the asthmatic response induced by OVA challenge, resulting in a clear increase in the anti-inflammatory cytokine IL-10 levels, a suppression of Th2-mediated cytokine and IL-17A release and a decrease in chemokine expression. These findings indicate that AcCystatin could be a candidate for a novel type of therapeutic for the prevention and treatment of allergic asthma.
References
Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T (1992) Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest 101(4):916–921
Akdis CA (2006) Allergy and hypersensitivity: mechanisms of allergic disease. Curr Opin Immunol 18(6):718–726
Al-Ramli W, Préfontaine D, Chouiali F, Martin JG, Olivenstein R, Lemière C (2009) Hamid Q (2009) T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol 123(5):1185–1187
Amoah AS, Forson AG, Boakye DA (2012) A review of epidemiological studies of asthma in Ghana. Ghana Med J 46(2 Suppl):23–28
Apter AJ (2014) Advances in adult asthma diagnosis and treatment in 2013. J Allergy Clin Immunol 133(1):49–56
Araujo MI, Hoppe B, Medeiros M Jr, Alcântara L, Almeida MC, Schriefer A, Oliveira RR, Kruschewsky R, Figueiredo JP, Cruz AA, Carvalho EM (2004) Impaired T helper 2 response to aeroallergen in helminth-infected patients with asthma. J Infect Dis 190(10):1797–1803
Arruda LK, Santos AB (2005) Immunologic responses to common antigens in helminthic infections and allergic disease. Curr Opin Allergy Clin Immunol 5(5):399–402
Asadullah K, Sterry W, Volk HD (2003) Interleukin-10 therapy: review of a new approach. Pharmacol Rev 55(2):241–269
Barlow JL, Flynn RJ, Ballantyne SJ, McKenzie AN (2011) Reciprocal expression of IL-25 and IL-17A is important for allergic airways hyperreactivity. Clin Exp Allergy 41(10):1447–1455
Barnes PJ (2001) Cytokine-directed therapies for asthma. J Allergy Clin Immunol 2001 108(2 Suppl):S72–S76
Barnes PJ (2013) Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol 131(3):636–645
Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S (1996) Interleukin-10 regulation in normal subjects and patients with asthma. J Allergy Clin Immunol 97(6):1288–1296
Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM (2000) Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 161(5):1720–1745
Brid PI, Trapani JA, Villadangos JA (2009) Endolysosomal proteases and their inhibitors in immunity. Nat Rev Immunol 9(12):871–882
Brusselle GG, Kips JC, Tavernier JH, van der Heyden JG, Cuvelier CA, Pauwels RA, Bluethmann H (1994) Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy 24(1):73–80
Busse WW1, Rosenwasser LJ (2003) Mechanisms of asthma. J Allergy Clin Immunol 111(3 Suppl):S799–S804
Chang EE, Chung LY, Yen CM (2004) Kinetics of change in the eotaxin concentration in serum and cerebrospinal fluid of mice infected with Angiostrongylus cantonensis. Parasitol Res 92(2):137–141
Chen W, Sivaprasad U, Gibson AM, Ericksen MB, Cunningham CM, Bass SA, Kinker KG, Finkelman FD, Wills-Karp M, Khurana Hershey GK (2013) IL-13 receptor α2 contributes to development of experimental allergic asthma. J Allergy Clin Immunol 132(4):951–958
Cooper PJ (2009) Interactions between helminth parasites and allergy. Curr Opin Allergy Clin Immunol 9(1):29–37
Cooper PJ, Barreto M, Rodrigues LC (2006) Human allergy and intestinal helminth infections: a review of the literature and discussion of a conceptual model to investigate the possible causal association. Br Med Bull 79–80(1):203–218
de Oliveira AP, Domingos HV, Cavriani G, Damazo AS, Dos Santos Franco AL, Oliani SM, Oliveira-Filho RM, Vargaftig BB, de Lima WT (2007) Cellular recruitment and cytokine generation in a rat model of allergic lung inflammation are differentially modulated by progesterone and estradiol. Am J Physiol Cell Physiol 293(3):C1120–1128
Elliott DE, Weinstock JV (2009) Helminthic therapy: using worms to treat immune-mediated disease. Adv Exp Med Biol 666:157–166
Folkerts G, Nijkamp FP (1998) Airway epithelium more than just a barrier. Trends Pharmacol Sci 19(8):334–341
Fu CL, Ye YL, Lee YL, Chiang BL (2006) Effects of overexpression of IL-10, IL-12, TGF-β and IL-4 on allergen induced change in bronchial responsiveness. Respir Res 7:72–85
Garg SK1, Croft AM, Bager P (2014) Helminth therapy (worms) for induction of remission in inflammatory bowel disease. Cochrane Database Syst Rev 1:CD009400
Goodwin JE, Zhang J, Gonzalez D, Albinsson S, Geller DS (2011) Knockout of the vascular endothelial glucocorticoid receptor abrogates dexamethasone-induced hypertension. J Hypertens 29(7):1347–1356
Hartmann S, Lucius R (2003) Modulation of host immune responses by nematode cystatins. Int J Parasitol 33(11):1291–1302
Hashimoto K1, Durbin JE, Zhou W, Collins RD, Ho SB, Kolls JK, Dubin PJ, Sheller JR, Goleniewska K, O’Neal JF, Olson SJ, Mitchell D, Graham BS, Peebles RS Jr (2005) Respiratory syncytial virus infection in the absence of STAT 1 results in airway dysfunction, airway mucus, and augmented IL-17 levels. J Allergy Clin Immunol 116(3):550–557
Hoerauf A, Satoguina J, Saeftel M, Specht S (2005) Immunomodulation by filarial nematodes. Parasite Immunol 27(10–11):417–429
Hsieh CS, Heimberger AB, Gold JS, O’Garra A, Murphy KM (1992) Differential regulation of T helper phenotype development by interleukins 4 and 10 in an αβ T-cell-receptor transgenic system. Proc Natl Acad Sci U S A 89(13):6065–6069
Hurson CJ, Butler JS, Keating DT, Murray DW, Sadlier DM, O’Byrne JM, Doran PP (2007) Gene expression analysis in human osteoblasts exposed to dexamethasone identifies altered developmental pathway as putative drivers of osteoporosis. BMC Musculoskelet Disord 8:12
Jeffery PK (1991) Morphology of the airway wall in asthma and in chronic obstructive pulmonary disease. Am Rev Respir Dis 143(5 Pt 1):1152–1158
Kay AB (1991) Asthma and inflammation. J Allergy Clin Immunol 87(5):893–910
Kita H (2013) Eosinophils: multifunctional and distinctive properties. Int Arch Allergy Immunol 161(Suppl 2):3–9
Klotz C, Ziegler T, Daniłowicz-Luebert E, Hartmann S (2011) Cystatins of parasitic organisms. Adv Exp Med Biol 712:208–221
Kudo M, Ishigatsubo Y, Aoki I (2013) Pathology of asthma. Front Microbiol 4:263
Larché M, Akdis CA, Valenta VR (2006) Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol 6(10):761–771
Lee MY, Lee JA, Seo CS, Ha H, Lee NH, Shin HK (2011) Protective effects of Mentha haplocalyx ethanol extract (MH) in a mouse model of allergic asthma. Phytother Res 25(6):863–869
Leonardi-Bee J, Pritchard D, Britton J (2006) Asthma and current intestinal parasite infection: systematic review and Meta-analysis. Am J Respir Crit Care Med 174(5):514–523
Li S, Yang F, Ji P, Zeng X, Wu X, Wei J, Ouyang L, Liang J, Zheng H, Wu Z, Lv Z (2014) Eosinophil chemotactic chemokine profilings of the brain from permissive and non-permissive hosts infected with Angiostrongylus cantonenis. Parasitol Res 113(2):517–525
Liu YH, Han YP, Li ZY, Wei J, He HJ, Xu CZ, Zheng HQ, Zhan XM, Wu ZD, Lv ZY (2010) Molecular cloning and characterization of cystatin, a cysteine protease inhibitor, from Angiostrongylus cantonensis. Parasitol Res 107(4):915–922
Lukacs NW1, Oliveira SH, Hogaboam CM (1999) Chemokines in asthma: redundancy of functions or a co-ordinated effort? J Clin Invest 104(8):995–999
Martinez FD, Vercelli D (2013) Asthma. Lancet 382(9901):1360–1372
Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, Olivenstein R, Elias J, Chakir J (2001) IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol 108(3):430–438
Moore KW, de Waal MR, Coffman RL, O’Garra A (2001) Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19:683–765
Nakajima H, Hirose K (2010) Role of IL-23 and Th17 cells in airway Inflammation in asthma. Immune Netw 10(1):1–4
Newcomb DC, Peebles RS Jr (2013) Th17-mediated inflammation in asthma. Curr Opin Immunol 25(6):755–760
Newcomb DC, Boswell MG, Huckabee MM, Goleniewska K, Dulek DE, Reiss S, Lukacs NW, Kolls JK, Peebles RS Jr (2012) IL-13 regulates Th17 secretion of IL-17A in an IL-10-dependent manner. J Immunol 188(3):1027–1035
Newcomb DC, Boswell MG, Sherrill TP, Polosukhin VV, Boyd KL, Goleniewska K, Brody SL, Kolls JK, Adler KB, Peebles RS Jr (2013) IL-17A induces STAT6-independent airway mucous cell metaplasia. Am J Respir Cell Mol Biol 48(6):711–716
Rhen T, Cidlowski JA (2005) Antiinflammatory action of glucocorticoids-new mechanisms for old drugs. N Engl J Med 353(16):1711–1723
Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA (1997) Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med 185(3):461–469
Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB (1992) Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 326(5):298–304
Roche WR, Beasley R, Williams JH, Holgate ST (1989) Subepithelial fibrosis in the bronchi of asthmatics. Lancet 1(8637):520–524
Romagnani S (2004) Immunologic influences on allergy and the TH1/TH2 balance. J Allergy Clin Immunol 113(3):395–400
Rothenberg ME, Zimmermann N, Mishra A, Brandt E, Birkenberger LA, Hogan SP, Foster PS (1999) Chemokines and chemokine receptors: their role in allergic airway disease. J Clin Immunol 19(5):250–265
Schnoeller C, Rausch S, Pillai S, Avagyan A, Wittig BM, Loddenkemper C, Hamann A, Hamelmann E, Lucius R, Hartmann S (2008) A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J Immunol 180(6):4265–4272
Scrivener S, Yemaneberhan H, Zebenigus M, Tilahun D, Girma S, Ali S, McElroy P, Custovic A, Woodcock A, Pritchard D, Venn A, Britton J (2001) Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: a nested case–control study. Lancet 358(9292):1493–1499
Sears MR (1997) Epidemiology of childhood asthma. Lancet 350(9083):1015–1020
Shakouri AA, Bahna SL (2013) Hypersensitivity to antihistamines. Allergy Asthma Proc 34(6):488–496
Smits HH, Everts B, Hartgers FC, Yazdanbakhsh M (2010) Chronic helminth infections protect against allergic diseases by active regulatory processes. Curr Allergy Asthma Rep 10(1):3–12
Tattersfield AE, Knox AJ, Britton JR, Hall IP (2002) Asthma. Lancet 360(9342):1313–1322
Taube C, Dakhama A, Gelfand EW (2004) Insights into the pathogenesis of asthma utilizing murine models. Int Arch Allergy Immunol 135(2):173–186
Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA (2006) Mechanisms of immune suppression by interleukin-10 and transforming growth factor-β: the role of T regulatory cells. Immunology 117(4):433–442
Turner JD, Jackson JA, Faulkner H, Behnke J, Else KJ, Kamgno J, Boussinesq M, Bradley JE (2008) Intensity of intestinal infection with multiple worm species is related to regulatory cytokine output and immune hyporesponsiveness. J Infect Dis 197(8):1204–1212
Urbańska J, Karewicz A2, Nowakowska M (2014) Polymeric delivery systems for dexamethasone. Life Sci 96(1–2):1–6
Urry Z, Xystrakis E, Hawrylowicz CM (2006) Interleukin-10-secreting regulatory T cells in allergy and asthma. Curr Allergy Asthma Rep 6(5):363–371
van den Biggelaar AH, van Ree R, Rodrigues LC, Lell B, Deelder AM, Kremsner PG, Yazdanbakhsh M (2000) Decreased atopy in children infected with Schistosoma haematobium a role for parasite-induced interleukin-10. Lancet 356(9243):1723–1727
Vray B, Hartmann S, Hoebeke J (2002) Immunomodulatory properties of cystatins. Cell Mol Life Sci 59(9):1503–1512
Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, Saito Y, Hatano M, Tokuhisa T, Iwakura Y, Puccetti P, Iwamoto I, Nakajima H (2008) IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med 178(10):1023–1032
Wammes LJ, Mpairwe H, Elliott AM, Yazdanbakhsh M (2014) Helminth therapy or elimination: epidemiological, immunological, and clinical considerations. Lancet Infect Dis S1473–3099(14):70771–70776
Wild JS, Sigounas A, Sur N, Siddiqui MS, Alam R, Kurimoto M, Sur S (2000) IFN-gamma-inducing factor (IL-18) increases allergic sensitization, serum IgE, Th2 cytokines, and airway eosinophilia in a mouse model of allergic asthma. J Immunol 164(5):2701–2710
Yuk JE, Lee MY, Kwon OK, Cai XF, Jang HY, Oh SR, Lee HK, Ahn KS (2011) Effects of astilbic acid on airway hyperresponsiveness and inflammation in a mouse model of allergic asthma. Int Immunopharmacol 11(2):266–273
Zavasnik-Bergant T (2008) Cystatin protease inhibitors and immune functions. Front Biosci 13:4625–4637
Zhou DY, Du Q, Li RR, Huang M, Zhang Q, Wei GZ (2011) Grape seed proanthocyanidin extract attenuates airway inflammation and hyperresponsiveness in a murine model of asthma by downregulating inducible nitric oxide synthase. Planta Med 77(14):1575–1581
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (grant no. 2010CB530004), the National Natural Science Foundation of China (grant no. 81371836, 30771888, 81271855 and 30800966), the 111 Project (grant no. B12003) and the Research Fund for Students of Sun Yat-sen University (2012, 2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Ji Pengyu, Hu Huiling, Yang Xiangyun and Wei Xiaoxia are joint first authors.
Rights and permissions
About this article
Cite this article
Ji, P., Hu, H., Yang, X. et al. AcCystatin, an immunoregulatory molecule from Angiostrongylus cantonensis, ameliorates the asthmatic response in an aluminium hydroxide/ovalbumin-induced rat model of asthma. Parasitol Res 114, 613–624 (2015). https://doi.org/10.1007/s00436-014-4223-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4223-z