Abstract
Theileria equi (Laveran 1901) and Babesia caballi (Nuttall and Strickland 1910) are the causative agents of Equine Piroplasmosis (EP), a severe and problematic disease compromising international movement of horses. Infected horses usually become asymptomatic carriers and, for this reason, their movement across borders may become restricted. The aim of this study was to assess the seroprevalence of EP in Southern France and to evaluate risk factors associated with these parasites. In 2002, we performed a complement fixation test (CF) with blood samples from 443 horses stabled at 95 different farms located in the region of Camargue. Two epidemiological questionnaires have been used: one for each single horse (individual and management factors) and one for each place where horses were sampled (environment, presence of other species, etc.) to identify risk factors for seropositivity. T. equi and B. caballi had a seroprevalence of 58 % and 12.9 %, respectively. For T. equi, sex, age, activity, management, and living with or near cattle were identified as risk factors, while for B. caballi, only living in wetlands was recognized as a risk factor in the bivariate analysis. In the multivariate analysis, the best model for T. equi included as variables age, breed, and deworming, while the best model for B. caballi included the type of housing during day and the contact with cows.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Equine Piroplasmosis (EP) is the most prevalent tick-borne disease in equids (horses, mules, donkeys, zebras) in certain areas of the world and not only causes important economic losses but also leads to movement restrictions (Knowles 1996a). The disease is caused by two hemoprotozoan parasites of the phylum Apicomplexa: Babesia caballi (intraerythrocytic, Fig. 1) and Theileria equi (intraerythrocytic and intralymphocytic, Fig. 2). Both parasites are transmitted by ixodid ticks of the genera Rhipicephalus, Dermacentor, Haemaphysalis, Hyalomma, and Boophilus (Thompson 1969; Klinkmann 1981; Friedhoff 1982; Waal 1992; Walker and Keirans 2000; Bautista et al. 2001; Battsetseg et al. 2001; Jongejan and Uilenberg 2004). These two parasites have in fact very different life cycles (Mehlhorn and Schein 1984). Parasites of the Babesia species have a transovarial transmission in the vectors and then enters, as sporozoites, directly into the host red blood cells where they develop into piroplasms. T. equi, formerly called Babesia equi, was reclassified as Theileria species (Mehlhorn and Schein 1998) because of the transstadial transmission in the vector and because sporozoites do not infect red blood cells but penetrate a lymphocyte (or macrophage) where they develop into schizonts (Schein et al., 1981). The merozoites are released from the schizonts then enter the red blood cells where they grow into piroplasms.
Equine Piroplasmosis can be acute, subacute, or chronic (Rampersad et al. 2003; Uilenberg 2006). Both parasites cause severe hemolytic anemia with fever, icterus, hemoglobinuria, and edema in the distal limbs (Knowles 1996b); in case of intrauterine infection by T. equi, abortion and neonatal death can occur (Potgieter et al. 1992). In naïve horses, the clinical disease is particularly aggressive producing a mortality rate of up to 50 % (Waal 1992). Infections with B. caballi are usually less severe than those with T. equi (which is more frequently reported), but it is impossible to make the difference between the two parasites based on clinical signs only. Subacute and chronic forms are associated with less specific clinical signs including inappetence, weight loss, exercise intolerance, and depression (Ristic 1985; Waal et al. 1987; Camacho et al. 2005). Drugs used to treat EP have variable efficacy depending on the goal of treatment: attaining a complete eradication is much more difficult than just resolving clinical signs. Some horses may clear themselves spontaneously of B. caballi (Friedhoff et al. 1990; Waal 1992; Friedhoff and Soule 1996) and sterilization with treatment can be achieved (Schwint et al. 2009), while at times it is impossible to completely clear a horse from T. equi infection (Kirkham 1969; Friedhoff et al. 1990; Friedhoff and Soule 1996; Schwint et al. 2009; Grause et al. 2013).
Once recovered from an acute episode, a horse remains a carrier for up to 4 years with B. caballi and for life in the case of a T. equi infection (Waal and Heerden 1994; Bashiruddin et al. 1999; Sellon 2004), thereby serving as a source of infection for ticks. Detection of the areas where EP is endemic and of individual carriers before introduction in non-endemic countries or areas is of paramount importance and can be done using direct and indirect tests (OIE 2008). Equine Piroplasmosis is of importance for international trade, and a specific chapter in “The OIE Terrestrial code” (www.oie.com) describes the standard procedure for Babesia control in horses. In non-endemic countries like the USA, Canada, Australia, and Japan, only seronegative horses are allowed to be imported to prevent the introduction of carrier animals. For these reasons, in endemic countries, control of EP is critical for the equine industry to preserve the option of international movement of horses, because without it, horses cannot cross borders to compete in races or horse shows, be used for breeding purposes, or be sold abroad (Friedhoff et al. 1990).
Due to global warming and ecological changes facilitating the growth of wildlife host populations (e.g., the wild boar, and deer populations increased by 10-fold within the past 30 years), permissive ticks are expanding to hitherto non-endemic countries (Sreter et al. 2005). Of the 58 millions horses bred worldwide, 90 % live in “at-risk” regions (Ristic 1988). Equine Piroplasmosis is endemic in Europe and tropical/subtropical regions (Asia, South and Central America, Africa), and the increasing movement of horses between countries contribute to the spread of the disease from endemic to non-endemic areas (Ristic 1988; Kappmeyer et al. 2012). Despite strict control protocols for the importation of horses into the USA, evidence of introduction of equids carrying piroplasms has been found by Hall and colleagues in 2013. Currently, OIE-prescribed diagnostic tests are the indirect fluorescent antibody test (IFAT), the indirect or competitive enzyme-linked immunosorbent assay (iELISA, cELISA), and the complement fixation (CF) assay (Brüning et al. 1997; Shkap et al. 1998; Ikadai et al. 2000; Hirata et al. 2002; Asgarali et al. 2007; Acici et al. 2008; Sigg et al. 2010; Mujica et al. 2011; Seo et al. 2011). Molecular techniques have been developed in recent years (Nagore et al. 2004; Criado et al. 2006; Alhassan et al. 2007; Adaszek and Winiarczyk 2008) and may contribute to improving test specificity and sensitivity. Despite the importance of EP in Southern Europe, studies on epidemiology and risk factors in France are lacking (Fritz 2010). Therefore, we conducted a serological cross-survey in an endemic area of Southern France to describe the seroprevalence and the risk factors associated with EP in the Camargue. A better understanding of the distribution and risk factors for EP can help identify at-risk populations and improve isolation and control measures when horses from these areas are moved to a disease-free zone.
Material and methods
Study design
A detailed description of the study area and selection of horse samples has already been published in Leblond et al. (2005a). Briefly, a cross-sectional study approach was employed to determine the seroprevalence and risk factors associated to T. equi and B. caballi infections in horses bred in the Camargue or living in that area for at least 1 year. Veterinarians with an equine practice in the region volunteered to participate in the study. They were asked to provide a list of their clients who owned horses that were living in the study area. Sampling of horses was performed in two steps: a random sample of stables was selected based on the list provided by the veterinarians, then the number x of horses to be selected in each stable was defined according to the total number N of horses in that stable: if N < 5, then x = N; if 5 ≤ N < 20, then x = 5; and if N ≥ 20, then x = 15.
Sample and data collection
Blood samples were collected between November 2001 and February 2002 (after a season of exposure to tick bites) by jugular venipuncture and blood collected in anticoagulant free tubes. The samples were then immediately centrifuged to harvest serum, which was subsequently stored at −18 °C until serological analysis. Data on each sampled horse (physical characteristics, use, housing, deworming program, and health status) and on the environmental conditions in which horses lived (one for each group) were collected using two questionnaires filled in at the time of blood sampling. All the locations where horses were sampled were georeferenced; they were defined as the place where horses spend most of the year, especially during the season of tick activity. Depending on the type of stable, the place was classified as a riding arena, breeding farm, or pasture. The questionnaire on the environment aimed at collecting variables indicating the presence of biotopes favorable for ticks and wild fauna. A detailed description of the questionnaires is given in Leblond et al. (2005b). An informed consent form was signed by the owner at the time of visit.
Serological test
The test chosen for detection of B. caballi and T. equi was the complement fixation (CF) assay since it was the screening test used in many EP-free countries at that time. Sera have been tested with CF using a dilution of 1:5 for both T. equi and B. caballi following the method described in the OIE Terrestrial Manual 2002. Results have been divided into six categories: negative, slightly positive (0.5), 1+, 2+, 3+, and 4+. Since CF has a good specificity (few false positives), all horses from 1+ onwards have been considered positive for further statistical analysis. Since high titers in antibodies against one of the two parasites can cause cross-reactivity, only horses with the same titers for T. equi and B. caballi were considered positive for both (i.e., a horse with 4+ for T. equi and 4+ for B. caballi was considered positive for both parasites); if one of the two titers was higher than the other, the horse was considered negative for the lower one (i.e., a horse with 4+ for T. equi and 1+ for B. caballi was considered positive only for T. equi).
Statistical analysis
To identify which variables were associated with equine seropositivity, generalized linear mixed models were used. The individual serological status was the binomial response variable. Risk factors considered included seven individual variables (breed, age, gender, activity, mosquito control, deworming, and housing) as well as the following eight environmental variables: presence of cattle, log-transformed surface of grazing area for horses, log-transformed percentage of wetlands, grassland, forests, trees, crops, and buildings in the location where horses where sampled.
All the putative risk factors were screened in univariate regression models. Variables identified as significant factors (at the level p ≤ 0.25) in univariate phase were selected for the full multivariate starting model. Stepwise selection of the final model was carried out based on the Akaike criteria (AIC) (Burnham and Anderson 2002). Clustering of horses in stables was taken into account by introducing the stable as random effect in the logistic model.
Goodness-of-fit of the final models for T. equi and B. caballi was assessed by computing the area under the curve (AUC) of the receiver operating characteristic plots.
Spatial analysis
A spatial analysis was conducted to look for clustering in the distribution of positive vs. negative stables. For this analysis, a positive stable was defined as a stable comprising at least two positive horses, and the model we used is a bivariate location point process. The Khat bivariate function was used (Leblond et al. 2005a). This function uses a distance criterion between positive and negative points to determine if a spatial structure exists or if points are randomly distributed. This method has the advantage to eliminate the population-at-risk confounding (Carpenter 2001).
All analyses were performed using R software version 3.0.2.
Results
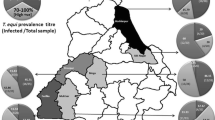
Eventually, 443 horses from 95 different farms were enrolled into the present study and tested. Among them, 257/443 were positive for T. equi and 57/443 for B. caballi, which represents a seroprevalence of 58 % and 12.9 %, respectively. Of the horses tested, 36 were positive for both parasites, 221 just for T. equi, and 21 only for B. caballi. In the Camargue, the presence of T. equi was more important than that of B. caballi (Figs. 1 and 2), but the difference in geographical location was not statistically significant.
Bivariate analysis
Statistical results (p < 0.05) of the bivariate analysis of the risk factors are reported in Table 1 for T. equi and in Table 2 for B. caballi. For T. equi, geldings among the Camargue horses living in large areas, within pastures or marshes, near or with cattle, and being used for breeding or tourism-related pleasure riding activities were at a significantly higher risk of contracting the infection than were horses younger than 4 years old, treated against mosquito bites, and being on a regular deworming regimen, which all carried a lower risk. For B. caballi, the only risk factor identified was being kept in wetlands.
Multivariate analysis
For T. equi, the full starting model included 11 variables: breed, gender, age, activity, mosquito control, deworming, housing, presence of cows, log-transformed area for horses, log-transformed percentage of wetlands, and log-transformed percentage of crops. The best model had an AIC value of 549.7 and six models had AIC values between 549.7 and 551.7. Age, breed, and deworming were included in all the selected models. Gender, activity, mosquito control, presence of cows, and wetlands were each selected once in the five other models, but these variables did not improve significantly the best model. Camargue horses and older horses were more at higher risk of contracting EP. Deworming decreased the risk of being seropositive.
For B. caballi, the full starting model included eight variables: gender, deworming, housing, activity, presence of cattle, log-transformed area for horses, log-transformed percentage of wetlands, and log-transformed percentage of crops. The best model had an AIC value of 306.8 and included the variables “housing” and “presence of cows.” Nine other models had AIC values between 306.8 and 308.8. The variable housing was included in all 10 models. Presence of cows was included four times, deworming and wetlands three times, and crops twice in the 10 selected models, but these variables did not improve significantly the best model. Living outdoor, in pasture or in marshes, was positively associated with seropositivity of horse to B. caballi. Even if not significant, the presence of cattle was associated with a higher risk of being positive to B. caballi.
The area under the ROC curve calculated for the final model was 0.78 for T. equi and 0.93 for B. caballi, indicating a reasonable ability to discriminate between the two infectious agents (Brooker et al. 2002).
Discussion
This is the first study to evaluate the prevalence and the risk factors for EP in Southern France. Since many countries worldwide have banned the entry of seropositive horses, the attempt to establish control measures against the parasites in endemic areas has become fundamental to the effort to eradicate or at least control the disease. Serological tests are widely accepted as surveillance tools because of their ease of use (Brüning et al. 1997). In 2002, standard serological tests available for EP were the complement fixation (CF) and the indirect fluorescent antibody (IFA) tests, respectively, but problems were associated with each of the these serological assays (Friedhoff 1982). The IFA test for T. equi was considered more sensitive than that of the CF test (89 % vs. 63 %), while the estimated specificity was the same (96 %) (Ogunremi et al. 2007). The IFA test for B. caballi was more sensitive than that of the CF test (92 % vs. 28 %) but less specific (95 % vs. 99 %) (Ogunremi et al. 2008). Problems associated with the IFA test included cross-reactivity, subjective judgment of the reader, and the high cost of the antigen (Bakheit et al. 2007). In this study, we decided to use the CF test because it was the test of choice to detect EP according to OIE recommendations in 2002. Moreover, as we expected high levels of prevalence for both parasites, we preferred to choose the CF test because of its higher specificity and ability to distinguish between the two parasites.
The sampling scheme we used in this study was designed to accurately represent the population of indigenous horses in the Camargue with respect to their geographical distribution in the study area. Indeed, the Camargue breed and livestock cutting horses, which are very specific to the study area, were well represented in our sample. To avoid bias arising from imported horses or maternal transfer of colostrum, horses present in the area for less than 1 year or born in 2001 were not included in the study population. However, as the database used for stable sampling accorded with those horses that were on file in veterinary practices, we cannot exclude a selection bias due to the exclusion of horses which were not patients of the veterinarians in the area. This may have influenced the results for EP prevalence in horses, but not the analysis of risk factors, provided that the selection bias was homogeneously distributed across the study area. Getting a better insight into the size and geographic distribution of the equine population should make it easier to design improved sampling schemes for future studies.
In our study, the seroprevalence of T. equi and B. caballi was 58 % and 12.9 %, respectively, and 8.1 % of the horses were positive for both parasites. Seroprevalence of T. equi was significantly higher than B. caballi, in agreement with most of the previous reports from other countries like Trinidad (Asgarali et al. 2007), Brazil (Heim et al. 2007), Greece (Kouam et al. 2010a), Switzerland (Sigg et al. 2010), Italy (Grandi et al. 2011), and Spain (Garcia-Bocanegra et al. 2013). This wide variability in seroprevalence can be explained by the fact that horses are usually able to clear themselves from B. caballi in 4 years while they commonly remain carriers of T. equi for life (Waal and Heerden 1994).
Several studies on the seroprevalence of T. equi and B. caballi have been conducted in different countries. High infection rates were predominantly found in subtropical and tropical regions: in Brazil, between 80 % and 90 % for both parasite species have been found (Pfeifer Barbosa et al. 1995; Heuchert et al. 1999; Heim et al. 2007; Santos et al. 2011; Peckle et al. 2013; Vieira et al. 2013). In Europe, lower overall seroprevalences were found in Switzerland (7.3 %; Sigg et al. 2010) and Netherlands (4 %; Butler et al. 2012), while in Portugal and Greece, seroprevalence varied between 11 % to 17.9 % for T. equi and 2.2 to 11.9 % for B. Caballi (Kouam et al. 2010a; Ribeiro et al. 2013). In central (Hungary; Farkas et al. 2013) and Southern (Spain, Italy, and Turkey) Europe, seroprevalence was found to vary much more, ranging from 32 % to 68 % (Acici et al. 2008; Moretti et al. 2010; Camacho et al. 2005; Garcia-Bocanegra et al. 2013). Overall, these studies clearly indicate that Mediterranean countries are at much higher risk for EP than that of Northern and thus colder regions in Europe, although seroprevalence data among studies should be compared with caution due to the different diagnostic tests used and differences in sampling schemes.
A review of the risk factors for EP identified in other studies is presented in Table 3. In our study, just being a Camargue horse was recognized as being a risk factor in the case of both parasites. While a genetic predisposition has never been studied in detail in this breed, other studies seem to indicate that breed might well be a risk factor for EP. Arabs in the study of Sevinc et al. (2008) and Quarter horses and local breeds in the study by Steinman et al. in 2012 were identified as breeds being more susceptible of contracting EP. Probably, the higher prevalence found in these breeds reflects differences in management practices such as access to pasture for longer periods of time and/or living in pastures in close proximity to cows. Grazing has been reported as a risk factor in other studies without a breed predisposition (Rapoport et al. 2014; Steinman et al. 2012; Moretti et al. 2010; Ribeiro et al. 2013). In accordance with those studies, also in our investigation, horses housed on pastures or marshes during the day were at a 2.1 and 4.5 times higher risk of being seropositive for B. caballi and T. equi, respectively.
Gender was identified as a protective factor in our study with stallions being less likely positive for EP. This has also been reported in other studies (Shkap et al. 1998; Ruegg et al. 2007; Moretti et al. 2010) and is probably due to the fact that stallions are generally kept in stalls instead of being left grazing freely in pastures, in part because of their greater value and typical male behavior. In contrast, two studies, one conducted in Mongolia and the other in Turkey (cf. Table 3; Sevinc et al. 2008; Munkhjargal et al. 2013), found significantly more seropositive animals among male horses, which most likely is due to different breeding and housing conditions in these countries.
As in our study, in most reports, older age is considered a risk factor for seropositivity. This is easily explained by the longer exposure to vector ticks and to the carrier condition established in parasite-infested horses (cf. Table 3; Kouam et al. 2010a; Garcia-Bocanegra et al. 2013; Karatepe et al. 2009; Vieira et al. 2013; Munkhjargal et al. 2013; Steinman et al. 2012; Rapoport et al. 2014).
In our study, deworming and flies control measures were protective factors. Herds without a vaccination program were more seropositive also in the study of Garcia-Bocanegra et al. (2013). Deworming, vaccination, and flies control are indicators of good herd management practices and imply a reduced exposure to tick infestation (Moretti et al. 2010; Santos et al. 2011; Vieira et al. in 2013).
We also found that working or living with cows was associated with a positive serologic result as previously reported in Brazil by Heuchert et al. (1999). This is caused by the fact that tick vectors have limited abilities to disperse and their presence depends on the abundance of appropriate hosts. Other studies also showed that for Ixodes ticks, higher numbers of ticks were collected in pastures where cows were grazing as well (Léger et al. 2013).
As is clearly depicted in Figs. 3 and 4, in our study, seropositive horses for T. equi and B. caballi did not follow any geographical distribution pattern worth further statistical exploration by cluster analysis (Chadoeuf et al. 2004). This finding was quite surprising as a geographical clustering was observed when we studied the prevalence of Anaplasma phagocytophilum, another tick-borne disease, in horses in the same area (Leblond et al. 2005a). The absence of cluster observed for EP made any further studies with regard to environmental risk factors using satellite imaging, as previously done in this area for other vector-borne diseases such as West Nile virus disease (Leblond et al. 2007) useless (Carpenter 2001). The study area was probably too small (around 250 km2 = 96.5 mile2) and ecologically uniform to observe geographical variations of seroprevalence, in contrast to what was observed in other studies conducted in Greece, Spain, or Italy in which different climatic zones were studied (Kouam et al. 2010b; Garcia-Bocanegra et al. 2013; Moretti et al. 2010).
Climate and biotopes of the Mediterranean region are particularly favorable for several species of ticks, including the main vector species involved in the transmission of EP (Dermacentor, Hyalomma, and Rhipicephalus spp.). At the time of the study, in 2002, we had sparse knowledge of their prevalence and distribution in the Camargue. Further studies were conducted to investigate the prevalence and diversity of ticks in this area (Chastagner 2013). Questing ticks from horse pastures and feeding ticks from horses were collected in the spring of 2007–2008 and 2010. A total of 406 adult ticks were collected, representing six species: Rhipicephalus bursa (n = 258), Rhipicephalus sanguineus (n = 117), Rhipicephalus turanicus (n = 4), Rhipicephalus pusillus (n = 11), Dermacentor marginatus (n = 14), and Hyalomma marginatum (n = 2) (Chastagner, 2012). All these species are potential vectors for EP. Moreover, no cluster was identified when a spatial analysis was conducted for the geographical distribution of the different species. These results could explain why no geographical variation could be identified with regard to the seroprevalence of horses for EP. It is known that ticks are very sensitive to local conditions of ecological niches (Halos et al. 2010). Further studies should probably aim at looking for finer resolution to identify environmental risk factors and vectors for EP in the Camargue.
Conclusion
To our knowledge, this is the first report of a large-scale serological investigation on EP in France. We showed that the Camargue area can be considered, as much as other Mediterranean regions, as a highly endemic area for EP. Occurrence of piroplasmosis in horses is amplified by frequent movement of the animals and the high number of natural hosts and vectors favored by recent ecological and climatic changes (Porchet et al. 2007). Therefore, further studies should be conducted to identify areas at risk in France. Moreover, it is crucial to obtain more reliable and timely estimates of the numbers of cases of EP and the related economic losses. For this purpose, the ability to diagnose infected and chronic carrier horses with accuracy is critical and molecular tools have to be used for diagnosis and identification of the various strains circulating in different areas.
Nationally, there is a need for surveillance and further research on the epidemiology of EP in France since testing of horses for EP is mandatory for the international movement of horses. This applies even more so as France has one of the largest equine populations in Europe and, moreover, hosts the 2014 World Equestrian Games. For that reason too, a continuous monitoring had recently been implemented by the RESPE (French network for the surveillance of equine diseases; www.respe.net), which will help establish long-term preventative and control measures against the spreading of EP.
References
Abutarbush SM, Alqawasmeh DM, Mukbel RM, Al-Majali AM (2012) Equine Babesiosis: seroprevalence, risk factors and comparison of different diagnostic methods in Jordan. Transbound Emerg Dis 59(1):72–78
Acici M, Umur S, Guvenc T, Arslan HH, Kurt M (2008) Seroprevalence of equine babesiosis in the Black Sea region of Turkey. Parasitol Int 57(2):198–200
Adaszek L, Winiarczyk S (2008) Molecular characterization of Babesia canis canis isolates from naturally infected dogs in Poland. Vet Parasitol 152(3–4):235–241
Alhassan A, Iseki H, Kim C, Yokoyama N, Igarashi I (2007) Comparison of polymerase chain reaction methods for the detection of Theileria equi infection using whole blood compared with pre-extracted DNA samples as PCR templates. Trop Anim Health Prod 39(5):369–374
Asgarali Z, Coombs DK, Mohammed F, Campbell MD, Caesar E (2007) A serological study of Babesia caballi and Theileria equi in Thoroughbreds in Trinidad. Vet Parasitol 144(1–2):167–171
Bahrami S, Ghadrdan AR, Mirabdollahi SM, Fayed MR (2014) Diagnosis of subclinical equine theileriosis in center of Iran using parasitological and molecular methods. Trop Biomed 31(1):110–117
Bakheit MA, Seitzer U, Mbati PA, Ahmed JS (2007) Serological diagnostic tools for the major tick-borne protozoan diseases of livestock. Parassitol 49(Suppl 1):53–62
Bashiruddin J, Camma C, Rebelo E (1999) Molecular detection of Babesia equi and Babesia caballi in horse blood by PCR amplification of part of the 16S rRNA gene. Vet Parasitol 84(1–2):75–83
Battsetseg B, Mikami T, Ikadai H, Xuan X, Fujisaki K, Nagasawa H et al (2001) Detection of Babesia caballi and Babesia equi in Dermacentor nuttalli adult ticks. Int J Parasitol 31(4):384–386
Bautista JL, Ikadai H, You M, Battsetseg B, Igarashi I, Nagasawa H et al (2001) Molecular evidence of Babesia caballi (Nuttall and Strickland, 1910) parasite transmission from experimentally-infected SCID mice to the ixodid tick, Haemaphysalis longicornis (Neuman, 1901). Vet Parasitol 102(3):185–191
Brooker S, Hay SI, Bundy DAP (2002) Tools from ecology: useful for evaluating infection risk models? Trends Parasitol 18:70–74
Brüning A, Phipps P, Posnett E, Canning E (1997) Monoclonal antibodies against Babesia caballi and Babesia equi and their application in serodiagnosis. Vet Parasitol 68(1–2):11–26
Burnham KP, Anderson DR (2002) Model selection and multi-model inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Butler CM, Sloet van Oldruitenborgh-Oosterbaan MM, Stout TAE, Van Der Kolk JH, Van den Wollenberg L et al (2012) Prevalence of the causative agents of equine piroplasmosis in the South West of The Netherlands and the identification of two autochthonous clinical Theileria equi infections. Vet J 193:381–385
Camacho AT, Guitian FJ, Pallas E, Gestal JJ, Olmeda AS, Habela MA et al (2005) Theileria (Babesia) equi and Babesia caballi infections in horses in Galicia, Spain. Trop Anim Health Prod 37(4):293–302
Carpenter TE (2001) Methods to investigate spatial and temporal clustering in veterinary epidemiology. Prev Vet Med 48(4):303–320
Chadoeuf J, Leblond A, Senoussi R (2004) Using inter-event functions for bandwidth selection in intensity estimation. Environmetrics 15:513–517
Chastagner J, (2012) Phylogenetic analysis of Anaplasma phagocytophilum strains identified from ticks, Camargue, France. Master Thesis, University Claude Bernard Lyon1, 22 pp.
Chastagner A, Bailly X, Leblond A, Pradier S, Vourche G (2013) Single genotype of Anaplasma phagocytophilum identified from ticks, Camargue, France. Emerg Infect Dis 19:825–826
Criado A, Martinez J, Buling A, Barba J, Merino S, Jefferies R et al (2006) New data on epizootiology and genetics of piroplasms based on sequences of small ribosomal subunit and cytochrome b genes. Vet Parasitol 142(3–4):238–247
Farkas R, Tanczos B, Gyurkovszky M, Foeldvari G, Solymosi N, Edelhofer R et al (2013) Serological and molecular detection of Theileria equi infection in horses in Hungary. Vet Parasitol 192(1–3):143–148
Friedhoff KT (1982) The piroplasms of equidae, significance for international commerce. Berl Munch Tierarztl Wochenschr 95:368–374
Friedhoff KT, Soule C (1996) An account on equine babesiosis. Rev Sci Tech 15:1191–1201
Friedhoff KT, Tenter AM, Muller I (1990) Haemoparasites of equines: impact on international trade of horses. Rev Sci Tech 9:1187–1194
Fritz D (2010) A PCR study of piroplasms in 166 dogs and 111 horses in France (March 2006 to March 2008). Parasitol Res 106(6):1339–1342
Garcia-Bocanegra I, Arenas-Montes A, Hernandez E, Adaszek Å, Carbonero A, Almeria S et al (2013) Seroprevalence and risk factors associated with Babesia caballi and Theileria equi infection in equids. Vet J 195(2):172–178
Grandi G, Molinari G, Tittarelli M, Sassera D, Kramer LH (2011) Prevalence of Theileria equi and Babesia caballi infection in horses from Northern Italy. Vector Borne Zoonotic Dis 11(7):955–956
Grause JF, Ueti MW, Nelson JT, Knowles DP, Kappmeyer LS, Bunn TO (2013) Efficacy of imidocarb dipropionate in eliminating Theileria equi from experimentally infected horses. Vet J 196(3):541–546
Hall CM, Busch JD, Scoles GA, Palma-Cagle KA, Ueti MW, Kappmeyer LS et al (2013) Genetic characterization of Theileria equi infecting horses in North America: evidence for a limited source of U.S. introductions. Parasit Vectors 6(1):35
Halos L, Bord S, Cotte V, Gasqui P, Abrial D, Barnouin J, Boulouis HJ, Vayssier-Taussat M, Vourche G (2010) Ecological factors characterizing the prevalence of bacterial tick-borne pathogens in Ixodes ricinus ticks in pastures and woodlands. Appl Environ Microbiol 76(13):4413–4420
Heim A, Passos LM, Ribeiro MF, Costa-Junior LM, Bastos CV, Cabral DD et al (2007) Detection and molecular characterization of Babesia caballi and Theileria equi isolates from endemic areas of Brazil. Parasitol Res 102(1):63–68
Heuchert C, Giulli D, Athaide DF Jr, Bose R, Friedhoff KT (1999) Seroepidemiologic studies on Babesia equi and Babesia caballi infections in Brazil. Vet Parasitol 85(1):1–11
Hirata H, Ikadai H, Yokoyama N, Xuan X, Fujisaki K, Suzuki N et al (2002) Cloning of a truncated Babesia equi gene encoding an 82-kilodalton protein and its potential use in an enzyme-linked immunosorbent assay. J Clin Microbiol 40(4):1470–1474
Ikadai H, Osorio CR, Xuan X, Igarashi I, Kanemaru T, Nagasawa H et al (2000) Detection of Babesia caballi infection by enzyme-linked immunosorbent assay using recombinant 48-kDa merozoite rhoptry protein. Int J Parasitol 30(5):633–635
Jongejan F, Uilenberg G (2004) The global importance of ticks. Parasitol 129(7):S3
Kappmeyer LS, Knowles DP, Gillespie JJ, Djikeng A, Caler E, Ramsay JD et al (2012) Comparative genomic analysis and phylogenetic position of Theileria equi. BMC Genomics 13(1):603
Karatepe B, Karatepe M, Cakmak A, Karaer Z, Ergun G (2009) Investigation of seroprevalence of Theileria equi and Babesia caballi in horses in Nigde province, Turkey. Trop Anim Health Prod 41(1):109–113
Kirkham WW (1969) The treatment of equine babesiosis. J Am Vet Med Assoc 155:457–460
Klinkmann G (1981) Sporozoitenstabilate von Babesia Equi aus Hyalomma Anatolicum and Rhipicephalus turanicus. PhD Thesis. West Germany: Tierarztliche Hochschule Nannover.
Knowles DP (1996a) Equine Babesiosis (piroplasmosis): a problem in the international movement of horses. Br Vet J 152(2):123–126
Knowles DP (1996b) Control of Babesia equi parasitemia. Parasitol Today 12(5):195–198
Kouam MK, Kantzoura V, Gajadhar AA, Theis JH, Papadopoulos E, Theodoropoulos G (2010a) Seroprevalence of equine piroplasms and host-related factors associated with infection in Greece. Vet Parasitol 169(3–4):273–278
Kouam MK, Masuoka PM, Kantzoura V, Theodoropoulos G (2010b) Geographic distribution modeling and spatial cluster analysis for equine piroplasms in Greece. Infect Genet Evol 10(7):1013–1018
Leblond A, Pradier S, Pittel PH, Fortier G, Boireau P et al (2005a) Enquete epidemiologique sur l'Anaplasmose Equine dans le Sud de la France. Rev Sci Tech 4:899–908
Leblond A, Zientara S, Chadouef J, Comby N, Heng MA et al (2005b) Prevalence de l'infection par le West Nile Virus chez le cheval en Camargue. Med Vet Entomol 156:77–84
Leblond A, Sandoz A, Lefebvre G, Zeller H et al (2007) Remote sensing-based identification of environmental risk factors associated with West Nile Virus circulation in Camargue, France. Prev Vet Med 79:20–31
Léger E, Vourche G, Vial L, Chevillon C, Mccoy KD (2013) Changing distributions of ticks: causes and consequences. Exp Appl Acarol 59(1–2):219–244
Mehlhorn H, Schein E (1984) The piroplasms: life cycle and sexual stages. Adv Parasitol 23:37–103
Mehlhorn H, Schein E (1998) Redescription of Babesia equi Laveran, 1901 as Theileria equi Mehlhorn, Schein 1998. Parasitol Res 84:467–475
Moretti A, Tampieri MP, Gabrielli S, Moretta I, Torina A, Scoccia E et al (2010) Prevalence and diagnosis of Babesia and Theileria infections in horses in Italy: a preliminary study. Vet J 184(3):346–350
Mujica FF, Perrone T, Forlano M, Coronado A, Melendez RD, Barrios N et al (2011) Serological prevalence of Babesia caballi and Theileria equi in horses of Lara State, Venezuela. Vet Parasitol 178(1–2):180–183
Munkhjargal T, Yokoyama N, Igarashi I, Terkawi MA, Byambaa B, Bayarsaikhan D et al (2013) Prevalence and genetic diversity of equine piroplasms in Tov province, Mongolia. Infect Genet Evol 16:178–185
Nagore D, Garcia-Sanmartin J, Garcia-Perez AL, Juste RA, Hurtado A (2004) Detection and identification of equine Theileria and Babesia species by reverse line blotting: epidemiological survey and phylogenetic analysis. Vet Parasitol 123(1–2):41–54
Ogunremi O, Georgiadis MP, Halbert G, Benjamin J, Pfister K, Lopez-Rebollar L (2007) Validation of the indirect fluorescent antibody and the complement fixation tests for the diagnosis of Theileria equi. Vet Parasitol 148(2):102–108
Ogunremi O, Halbert G, Mainar-Jaime R, Benjamin J, Pfister K, Lopez-Rebollar L, Georgiadis MP (2008) Accuracy of an indirect fluorescent-antibody test and of a complement-fixation test for the diagnosis of Babesia caballi in field samples from horses. Prev Vet Med 83(1):41–51
OIE (2008) Manual of diagnostic tests and vaccines for terrestrial animals. OIE (World Organization for Animal Health), from http://www.oie.int/Eng/Normes/Mmanual/2008/pdf/2.05.08_EQUINE_PIROPLASMOSIS.pdf
Peckle M, Pires MS, Santos TM, Roier EC, Silva CB, Vilela JA et al (2013) Molecular epidemiology of Theileria equi in horses and their association with possible tick vectors in the state of Rio de Janeiro, Brazil. Parasitol Res 112(5):2017–2025
Pfeifer Barbosa I, Bose R, Peymann B, Friedhoff KT (1995) Epidemiological aspects of equine babesioses in a herd of horses in Brazil. Vet Parasitol 58(1–2):1–8
Porchet MJ, Sager H, Muggli L, Oppliger A, Müller N, Frey C et al (2007) A descriptive epidemiological study on canine babesiosis in the Lake Geneva region. Schweiz Arch Tierheilkd 149:457–465
Potgieter FT, Waal DT, Posnett ES (1992) Transmission and diagnosis of equine babesiosis in South Africa. Mem Inst Oswaldo Cruz 87:139–142
Rampersad J, Cesar E, Campbell M, Samlal L, Ammons D (2003) A field evaluation of PCR for the routine detection of Babesia equi in horses. Vet Parasitol 114:81–87
Rapoport A, Aharonson-Raz K, Berlin D, Tal S, Gottlieb Y, Klement E et al (2014) Molecular characterization of the Babesia caballi rap-1 gene and epidemiological survey in horses in Israel. Infect Genet Evol 23:115–120
Ribeiro AJ, Cardoso L, Maia JM, Coutinho T, Cotovio M (2013) Prevalence of Theileria equi, Babesia caballi, and Anaplasma phagocytophilum in horses from the north of Portugal. Parasitol Res 112(7):2611–2617
Ristic G (1985) Equine Babesiosis and trypanosomiasis. Symposium on Equine Haematology, Proc Int
Ristic M (1988) Babesiosis of domestic animals and man. CRC Press, Boca Raton
Rosales R, Rangel-Rivas A, Escalona A, Jordan LS, Gonzatti MI, Aso PM et al (2013) Detection of Theileria equi and Babesia caballi infections in Venezuelan horses using competitive-inhibition ELISA and PCR. Vet Parasitol 196(1–2):37–43
Ruegg SÂR, Torgerson P, Deplazes P, Mathis A (2007) Age-dependent dynamics of Theileria equi and Babesia caballi infections in southwest Mongolia based on IFAT and/or PCR prevalence data from domestic horses and ticks. Parasitol 134(07):939
Santos TM, Machado RZ, Baldani CD, Almeida FQ, Moraes LM, Vilela JA et al (2011) Factors associated to Theileria equi in equids of two microregions from Rio de Janeiro, Brazil. Rev Bras Parasitol Vet 20(3):235–241
Schein E, Rehbein G, Voigt WP, Zweygarth E (1981) Babesia equi (Laveran, 1901). 1. Development in horses and in lymphocyte culture Tropenmed. Parasitol 32:223–237
Schwint ON, Ueti MW, Palmer GH, Kappmeyer LS, Hines MT, Cordes RT et al (2009) Imidocarb dipropionate clears persistent Babesia caballi infection with elimination of transmission potential. Antimicrob Agents Chemother 53(10):4327–4332
Sellon DC (2004) Disorders of the hematopoietic system. In: Reed SM, Bayly WM, Sellon DC (eds) Equine internal medicine, 1st edn. Saunders, Philadelphia p, p 735
Seo M, Kwon YS, Yun S, Kwak D, Park S, Jeong K et al (2011) Seroprevalence of equine piroplasms in the Republic of Korea. Vet Parasitol 179(1–3):224–226
Sevinc F, Maden M, Kumas C, Sevinc M, Ekici OD (2008) A comparative study on the prevalence of Theileria equi and Babesia caballi infections in horse sub-populations in Turkey. Vet Parasitol 156(3–4):173–177
Shkap V, Cohen I, Leibovitz B, Pipano E, Avni G, Shofer S et al (1998) Seroprevalence of Babesia equi among horses in Israel using competitive inhibition ELISA and IFA assays. Vet Parasitol 76(4):251–259
Sigg L, Gerber V, Gottstein B, Doherr MG, Frey CF (2010) Seroprevalence of Babesia caballi and Theileria equi in the Swiss horse population. Parasitol Int 59(3):313–317
Sreter T, Szell Z, Varga I (2005) Spatial distribution of Dermacentor reticulatus and Ixodes ricinus in Hungary: evidence for change? Vet Parasitol 128(3–4):347–351
Steinman A, Zimmerman T, Klement E, Lensky IM, Berlin D, Gottlieb Y et al (2012) Demographic and environmental risk factors for infection by Theileria equi in 590 horses in Israel. Vet Parasitol 187(3–4):558–562
Thompson PH (1969) Ticks as vectors of Equine Piroplasmosis. J Am Vet Med Assoc 155:454–745
Uilenberg G (2006) Babesia—a historical overview. Vet Parasitol 138(1–2):3–10
Vieira RF, Biondo AW, Nascimento DD, Vieira TS, Finger MA, Sicupira PM et al (2013) Seroepidemiological survey of Theileria equi and Babesia caballi in horses from a rural and from urban areas of Parama State, southern Brazil. Ticks Tick Borne Dis 4(6):537–541
Waal DD (1992) Equine piroplasmosis: a review. Br Vet J 148(1):6–14
Waal DT, Heerden JV (1994) Equine Piroplasmosis. In: Coetzer JA (ed) Infectious diseases of livestock with special reference to Southern Africa. Oxford University Press, Cape Town
Waal DD, Heerden JV, Potgieter FT (1987) An investigation into the clinical pathological changes and serological response in horse experimentally infected with Babesia equi and Babesia caballi. Onderstepoort J Vet Res 54:561–587
Walker JB, Keirans JE (2000) The genus Rhipicephalus (Acardi, Ixodidae): a guide to the brown ticks of the world. Cambridge University Press, Cambridge
Acknowledgments
Many thanks to doctor Pascal Boireau, head of the Animal Health Laboratory—ANSES for the blood analysis and to Benoit Rannou, clinical pathologist at VetAgro-Sup.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guidi, E., Pradier, S., Lebert, I. et al. Piroplasmosis in an endemic area: analysis of the risk factors and their implications in the control of Theileriosis and Babesiosis in horses. Parasitol Res 114, 71–83 (2015). https://doi.org/10.1007/s00436-014-4161-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4161-9