Abstract
The ultrastructure of the haptoral clamps of the chimaericolid monogenean Chimaericola leptogaster, a basal polyopisthocotylean from the gills of a holocephalan fish, is described. These clamps are characterized by the presence of two muscle blocks interrupted mid-anteriorly and mid-posteriorly and different kinds of hard structures: a single median and paired lateral sclerites embedded in the clamp wall; six spine-like structures directed towards the clamp lumen; and electron dense surface structures along the internal surface of the anterior clamp lips and along the luminal surface of the tegument of the clamp lumen. The lateral sclerites are situated deep within muscular tissue and are closely bounded by radial myofibrils, possessing a uniform electron dense matrix within which are hollow areas of different sizes. The median sclerite occupies an area between the clamp wall myofibrils and the luminal epithelium, is surrounded by a basement lamina and is composed of a heterogeneous matrix comprising two different morphological layers related to variations in the type and concentration of fibrils. Four of the spine-like structures are extensions of the margins of the two spindle-like muscle blocks in the clamps, i.e. the two anterior and two posterior structures, and the two others are situated at the lateral constrictions of the left and right muscle blocks. The electron dense surface structures are derivations of the clamp tegument or, to be more precise, its outer, densely fibrous region. These results are discussed in relation to the evidence that the haptoral clamps of C. leptogaster are apparently ancient origin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monogenean gill parasites of fishes belong to two subclasses and are fixed to their host mainly by means of posterior attachment organs associated with the haptor, which possesses specialized attachment structures. In members of the Monopisthocotylea, the haptor is generally simple and armed with hamuli and/or hooks and sometimes a suctorial disc, whereas in members of the Polyopisthocotylea, the haptor includes highly specialized structures, i.e. clamps or suckers, often armed with sclerotized elements (e.g. Justine et al. 2013). Light and scanning electron microscopical (SEM) characteristics of various kinds of monogenean haptoral sclerites have been much used as taxonomic criteria. However, little is known of their fine structures using transmission electron microscopy (TEM) (El-Naggar 1992, 1993), and little attention has been paid to the ultrastructure of the clamps of polyopisthocotylean monogeneans (Shaw 1979a, b, c; Ramasamy et al. 1986; Ramasamy and Bhuvaneswari 1993). In the case of the clamps of species of the family Chimaericolidae, there are detailed light microscopical descriptions given by Brinkmann (1942) and Bychowsky (1957), but no ultrastructural information is available on their morphology, with the exception of a brief mention by Pascoe (1997) of the external morphology of a clamp of Chimaericola leptogaster Leuckart, 1830 based on scanning electron microscopy. There has, however, been a study which includes aspects of the chemical composition of the attachment sclerites of this species (Lyons 1966).
C. leptogaster is a gill parasite of the rabbit fish Chimaera monstrosa, its main host, which is a member of the Holocephali, an ancient, relictual group of cartilaginous fishes, whose lineage diverged from the sharks almost 400 million years ago (Inoue et al. 2010), and which has evolved a quite unique parasite fauna. The chimaericolids are thought to have coevolved with their hosts and belong to a clade of the Polyopisthocotylea which has been considered as basal in this subclass (Boeger and Kritsky 1997; Olson and Littlewood 2002).
The aim of the present study is to examine the ultrastructure of the clamps of adult Chimaericola leptogaster from C. monstrosa. This is the second detailed ultrastructural study of this basal relict, the other being a study of the vaginae (Poddubnaya et al. 2013).
Materials and methods
Adult specimens of C. leptogaster were obtained from the gills of C. monstrosa in deep-waters, within the 500–750-m bathymetric zone of the Norwegian Sea off Tromsø, Norway. They were caught from the RV ‘Johan Ruud’ belonging to Tromsø University using a deep-water trawl. Live worms were fixed in 2.5 % glutaraldehyde in 0.1-M sodium cacodylate buffer at pH 7.4 for 20 days at 5 °C. Postfixation was completed in 1 % osmium tetroxide in the same buffer for 1 h at 5 °C. The specimens were then dehydrated in a graded series of ethanol and acetone and embedded in Araldite and Epon. Ultrathin sections, stained with uranyl acetate and lead citrate, were then viewed using a JEOL-1011 transmission electron microscope (TEM) operating at 80 kV.
For scanning electron microscopical observations, fixed worms were dehydrated in a graded ethanol series, with a final change in absolute ethanol and then critical point dried with liquid CO2. The specimens were mounted on stubs, sputter-coated with gold-palladium and examined using JEOL-JSM-6510LV scanning electron microscope operating at 15 kV.
Results
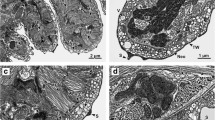
The body of C. leptogaster is divided into a broad anterior region containing the reproductive organs and a long, narrow, contractile, stalk-like region with a small haptor at the posterior end. The haptor is an attachment organ provided with eight clamps situated on short peduncles (Figs. 1 and 2). The clamps are organized in two alternating rows, with four clamps on each side of the haptoral attachment area (Figs. 1 and 2). Each clamp is a flattened bowl-shaped structure (Fig. 2). Externally and internally, the clamp walls are limited by a syncytial tegumental epithelium (Figs. 2, 3, 5 and 34) and are occupied by large areas of closely packed, radially oriented muscle bundles, which extend throughout virtually the entire clamp (Figs. 3–5 and 34). The clamp wall myofibrils are enclosed both externally and internally by basement laminae (Figs. 5–7, 8 and 34), and the clamp muscles are divided into two muscle blocks, interrupted mid-anteriorly and mid-posteriorly (Figs. 2, 6, 7 and 34). At the margins of the two muscle blocks and at their lateral constrictions, a spine-like structure is present (Figs. 4, 6, 7 and 34). At the antero-median and postero-median margins of the clamp wall myofibrils, the epithelial lining of the clamp lips is also interrupted (Figs. 6, 7 and 34). In the antero-median area, the interrupted epithelial lining of each clamp lip is covered with electron dense surface structures (Figs. 6 and 34), but along the interrupted postero-median area, they are absent (Figs. 2, 7 and 34). Curved sclerites, one median and two lateral, are embedded in the clamp wall myofibrils (Figs. 3 and 5). A cross-section through the clamp wall (Fig. 3) shows slices of the paired lateral sclerites and the median sclerite on the anterior and posterior sides of the clamp. A sagittal section through a deeper level of the clamp wall (Fig. 5) shows the mid-region of the middle sclerite as it passes between the anterior and posterior sides of the clamp.
SEM and TEM views of the clamps of Chimaericola leptogaster. 1 view of the haptor with eight clamps; 2 two flattened, bowl-shaped clamps showing their short peduncles, anterior and posterior clamp wall, two muscle blocks in the posterior wall and the interrupted area between the muscle blocks; 3 section showing slice through paired lateral sclerites and unpaired median sclerite within clamp wall myofibrils; 4 section of a muscle block, showing spine-like structures on its anterior margin (left) and at its lateral constriction (right); 5 section at a deeper clamp level, showing a median sclerite and a portion of one the lateral sclerites; 6 interrupted mid-anterior area of clamp wall, note spine-like structures at the margins of two muscle blocks and electron dense surface structures located along the internal tegument of the anterior lips; 7 section through the postero-median margins of the clamp wall myofibrils with spine-like structures at the posterior margins of two muscle blocks, note the absence of dense surface structures along internal tegumental lining of the posterior lip. Abbreviations: aw anterior wall of clamp; cl clamp; cp clamp peduncle; ds electron dense surface structures; ebl external basement lamina; ee external epithelium; h haptor; ibl internal basement lamina; ia interrupted area; ie internal epithelium; l lip; ls lateral sclerite; lu clamp lumen; mb, mb1, mb2 muscle blocks; ms middle sclerite; pw posterior wall of clamp; rm radial myofibrils of clamp wall; ss spine-like structure
Ultrastructure of the clamp wall of Chimaericola leptogaster. 8 section through the distal region of the clamp wall, note the thin layers of external and internal syncytial epithelium, the clamp wall myofibrils of the radial muscles and the surrounding external and internal basement laminae; 9 external syncytial epithelium of the clamp wall bearing microvilli; 10 internal epithelial cytoplasm of the clamp wall filled with vacuoles, note the electron dense surface structures and the electron dense masses of the internal basement lamina; 11 internal syncytial tegument showing its smooth surface, vacuoles filling the cytoplasm, thick basement lamina and dense attachment points between the myofibrils and the basement lamina; 12 structure of the internal basement lamina with a homogeneous dense matrix and small hollow areas; 13 nerve plexus between the myofibrils of the clamp wall; 14 radial muscles of the clamp wall surrounded by thick internal and thin external basement laminae; 15 section through a nerve cord in the haptor, note axons filled with different types of nerve vesicles. Abbreviations: ds electron dense surface structures; dt dense thickening apparently used for muscle attachment; ebl external basement lamina; ee external epithelium; ex exocytosis; ho hollow area within internal basement lamina; ibl internal basement lamina; ie internal epithelium; mv microvillus; np nerve plexus; rm radial muscles of clamp wall; v vacuole; vs vesicle
Ultrastructure of the median and lateral clamp sclerites of Chimaericola leptogaster. 16 median sclerite situated between the clamp wall myofibrils and surrounded by a basement lamina showing the space between body of the sclerite and the basement lamina, note two layers of different electron density within the sclerite body; 17 lateral edge of the median sclerite showing a thin border area of high electron density, the surrounding basement lamina and a loosely packed fibrous content between the sclerite and the basement lamina; 18 nerve plexus close to the luminal side of the median sclerite; 19 median sclerite showing its position between the clamp wall muscles and the internal syncytial epithelium at a deeper level within the clamp; 20 differences in the contents of the wide outer and inner core regions of the median sclerite; 21 section of the lateral sclerite set within the clamp wall myofibrils, note that the internal basement lamina is continuous with the sclerite body; 22 internal structure of the lateral sclerite showing its dense, homogeneous contents with numerous, small, widely distributed hollow areas; 23 large hollow area within the central region of the lateral sclerite filled with mitochondria, vesicles, etc.; 24 boundary of the lateral sclerite, showing dense thickenings of the myofibril attachment zones. Abbreviations: ba dense border area of outer layer of median sclerite; bl basement lamina; dt dense thickening for muscle attachment; ibl basement lamina of internal (luminal) side of clamp; ic inner core of median sclerite; ie internal epithelium; lho large hollow area within lateral sclerite; ls lateral sclerite; lu clamp lumen; m mitochondria; mf myofibrils; np nerve plexus; ol outer layer of median sclerite; ms median sclerite; rm radial muscles of clamp wall; sho small hollow area within lateral sclerite; sp space between body of median sclerite and surrounding basement lamina
Ultrastructure of spine-like structures and electron dense surface structures of Chimaericola leptogaster. 25 the interrupted epithelium of antero-median area, the internal region of which is supplied with electron dense surface structures; 26 spine-like structure at the margins of a muscle block, note the thickened internal and external basement laminae forming its outer layer; 27 inner content of a spine-like structure; 28 an inner structure of the tip of a spine-like structure consisting a mixture of dark and more lucent material, note a long septate junction connecting the body of the structure with the surface epithelium; 29, 30 spine-like structure at the lateral constriction of a muscle block, note its outer layer formed from the inner basement lamina and the presence of a nerve plexus beneath this structure; 31 electron dense surface structure of the internal epithelium of the clamp; 32 detailed structure of electron dense surface structure; 33 electron dense surface structures along the inner clamp epithelium. Abbreviations: df thick dense fibrils within electron dense surface structure; dm dark material of tip of spine-like structure; ds electron dense surface structure; ebl external basement lamina; ee external epithelium; ibl internal basement lamina; ie internal epithelium; l lip; lm lighter material of tip of spine-like structure; mf myofibrils; np nerve plexus; rm radial muscles of clamp wall myofibrils; ss spine-like structure; sj septate junction; v vacuole
A diagrammatic representation of the location of the spine-like structures in the clamp of Chimaericola leptogaster. Abbreviations: al medio-anterior lip; aw anterior wall of clamp; ds electron dense surface structures; ebl external basement lamina; ee external epithelium; ibl internal basement lamina; ie internal epithelium; lu clamp lumen; mb1, mb2 muscle blocks; pl medio-posterior lip; pw posterior wall of clamp; ss spine-like structure
Structure of the clamp wall
Regional differences in the structure of the syncytial epithelium are apparent in different areas of the clamp wall (Figs. 9–11 and 14). The epithelium of the external region (on the outer surface of the clamp) bears microvilli and contains electron-lucent vesicles in the processes of exocytosis (Fig. 9). The epithelium of the internal wall (on the luminal surface of the clamp) exhibits variation in its surface structures (Figs. 8, 10, 11 and 14). In fact, the luminal surface may bear dense surface structures, varying shape and size, microvilli, or can even be smooth, and its syncytial cytoplasm is filled with many large vacuoles (Figs. 8, 10, 11, 14, 25, 31 and 33). The basal membrane and basement lamina of the epithelium of the outer and luminal clamp surfaces closely follow the contours of the underlying radially oriented clamp wall myofibrils, which occupy most of the clamp wall volume (Figs. 8–11 and 14). This portion of the clamp wall consists of tightly packed myofibrils and is enclosed by an internal and an external basement lamina to which they attach (Figs. 8–11 and 14). In regions of the clamp wall where lateral sclerites are present, the myofibrils are also attached to the body of the sclerites (Fig. 24). At the point of the attachment between the myofibrils and the basement lamina, or with the lateral sclerites, electron dense thickenings occur (Figs. 9, 11 and 24). The myofibrils are richly supplied with nerve elements which emanate from the haptor (Fig. 15) and extend into the clamp muscles (Fig. 13). When the basement lamina is examined in detail, it can be seen that the external region of the lamina is thinner than the internal (Figs. 8, 14 and 33), and the basement lamina is always electron dense (Figs. 8, 10, 11 and 14). The detailed structure of the basement lamina surrounding the clamp wall myofibrils has a fibrous content with numerous small, randomly distributed areas which appear to be hollow (Fig. 12).
Median sclerite
The median sclerite (Figs. 16–20) runs between the clamp wall myofibrils and the syncytial tegument, projecting beyond the general luminal surface level of the clamp wall when the clamp closes (Figs. 5, 16, 19 and 35). This sclerite is surrounded by the basement lamina along its entire length (Figs. 16, 19 and 35). Between the body of the sclerite and its surrounding basement lamina is a space which is filled with a loosely packed fibrous content and nerve elements (Figs. 16–18). Two morphologically different levels can be distinguished within the sclerite body, i.e. a wide outer layer with a dense fibrous structure, comprising a thin border area of a higher electron density and an inner core of moderate electron density (Figs. 16, 17 and 20). At high magnification, a distinct difference in the contents of the outer layer and the inner core can be seen, the former being composed of fine, well-dispersed, moderately electron dense fibrils and separate, thicker, electron dense fibrils (Fig. 20). The difference in the density between the margin and the deeper areas of the outer layer is related to the higher concentrations of thick, dense fibrils in the latter (Fig. 17). On the other hand, a different kind of fibril occurs within the inner core of the sclerite and probably accounts for its lower electron density; these fibrils are thick and form an irregular network (Fig. 20).
A diagram showing the location of the median sclerite in the deeper levels of the contracted clamp of Chimaericola leptogaster. Abbreviations: aw anterior wall of clamp; bl basement lamina around median sclerite; ds electron dense surface structures; ebl external basement lamina; ee external epithelium; ibl internal basement lamina; ie internal epithelium; lu clamp lumen; ms median sclerite; pw posterior wall of clamp
Lateral sclerites
The two lateral sclerites (Figs. 21–24) are set deep within the muscular wall (Figs. 3, 5 and 21). It is clear in the TEM micrographs that these sclerites are irregularly oval in cross-section and are continuous with the basement lamina which surrounds the muscular tissue of the clamp (Figs. 3, 5 and 21). In fact, the lateral sclerites have a similar inner structure to the basement lamina (Fig. 12) in that they are electron dense and appear to be hollow in places (Fig. 22). In sections of the sclerites, there are large hollows with loosely packed fibrils, mitochondria, muscle fibrils and numerous small hollows randomly distributed throughout sclerite length without any inclusions (Figs. 21 and 23). The sclerites are closely bounded by myofibrils of the clamp wall radial muscles, which are attached to them at points visible as electron dense thickenings (Fig. 24).
Spine-like structures and electron dense surface structures
There are six spine-like structures extending along the inner (luminal) epithelium of the clamp. Each such structure lies beneath but close to the luminal surface, projecting into the epithelial lining and is oriented towards the clamp lumen (Figs. 4, 6, 7, 26, 29 and 34). The spine-like structures are located on both the anterior and posterior sides of the clamp wall at the margins of the two muscle blocks (Figs. 6 and 7), and a single one occurs laterally on each muscle block (Figs. 4, 29 and 34). These structures themselves are extensions of the underlying muscles covered by a greatly thickened basement lamina (Figs. 26, 27, 29 and 30). The inner core of these structures, from their base to the tip, is formed by myofibrils of the underlying muscles that differ in density (Fig. 27). It should be noted that outer layer of the four spine-like structures at the margins of the muscle blocks is formed from both the external and the internal basement laminae (Figs. 6, 7 and 26). In the case of the other two, the outer layer is formed by the inner basement lamina only, and there is a nerve plexus beneath them (Figs. 29 and 30). At its tip, the body of the structure is filled with a mixture of both dark and lighter material (Fig. 28), and it is attached to the tegumental surface membrane of the luminal surface of the clamp wall via a long septate junction (Fig. 28).
Defined areas of the luminal surface of the clamp tegument are armed with electron dense surface structures differing in shape, length and width (Figs. 5, 6, 10, 14, 25 and 33). Such structures are localized along the internal surface of the anterior lips, external to the spine-like structures (Figs. 6 and 25), and along the luminal surface of the clamp tegument in deeper regions of the clamp lumen (Figs. 5, 10, 14 and 33). Each of these dense surface structures is composed of thick, dense, randomly distributed fibrils within a fine, fibrous matrix (Fig. 32).
Discussion
The shape and number of clamps on the haptor of different polyopisthocotylean monogeneans, as well as the number, arrangements and kind of sclerites in each clamp, are the main diagnostic taxonomic characters for the group (Bychowsky 1957). This study represents the first detailed ultrastructural description of the clamps of the basal polyopisthocotylean C. leptogaster (Chimaericolidae), which possesses a haptor bearing eight well-developed clamps. Our observations show that these clamps are characterized by the presence of a muscular wall, possessing two muscle blocks interrupted mid-anteriorly and mid-posteriorly and composed of radially oriented myofibrils and different kinds of hard structures. The latter includes the following: a single median and paired lateral sclerites embedded in the clamp wall; six spine-like structures directed towards the clamp lumen; and electron dense surface structures along the internal surface of the anterior clamp lips and along the luminal surface of the tegument of the clamp lumen.
Clamp sclerites
Our ultrastructural investigations have revealed that the clamp sclerites of C. leptogaster are different in both their structure and location. The lateral sclerites are situated deep within muscular tissue of the clamp and are closely bounded by radial myofibrils. However, the median sclerite occupies an area between the clamp wall myofibrils and the luminal epithelium and is surrounded by a basement lamina, which is continuous with that delimiting the clamp wall myofibrils; between the median sclerite and surrounding basement lamina, there is a space. The study has shown that the lateral sclerites are continuous with, and have a similar internal structure to, the basement lamina. They also have in common a uniform electron dense matrix within which are hollow areas of different sizes, permitting us to consider the lateral sclerites of the C. leptogaster clamps as basement lamina derivatives. The median sclerite is composed of a heterogeneous matrix comprising two different morphological layers related to variations in the type and concentration of fibrils. It is worth noting that differences detected between the ultrastructure of the median and lateral clamp sclerites of C. leptogaster are supported by the chromatographic and biochemical observations of monogenean attachment sclerites presented by Lyons (1966), who also showed differences in the chemical composition of the median and lateral sclerites in C. leptogaster. According to Lyons (1966), most monogenean sclerites are composed of sulphur-bonded scleroproteins. However, the median clamp sclerites of chimaericolids are devoid of a sulphur-containing, protein-like material (Lyons 1966). In C. leptogaster, the median sclerite of the clamp was found to contain cystine, whereas the lateral sclerites did not. Her and our own findings, which the median and lateral sclerites of chimaericolid clamps differ considerably in many respect, are in complete agreement with the view of Bychowsky (1957) that the median clamp sclerite of chimaericolids has a different origin to the lateral sclerites and that it is probably homologous with the large, curved sclerites supporting the attachment organs of related polyopisthocotylean monogeneans, i.e. the hexabothriids and diclybothriids. It is also worth noting that the median sclerite of the chimaericolid clamp and the hexabothriid sucker bear very different relationships with the myofibrils of the clamp wall; in hexabothriids, this sclerite is situated well outside the sucker wall enmeshed in its own retractor/protractor muscle system, whereas in chimaericolids, it is embedded between the muscle blocks of the clamp wall and tends to project inwards towards the clamp cavity. This dissimilarity is probably associated with differences in the role of the median sclerite in the attachment mechanism of the two types of the attachment organ, i.e. clamps in chamaericolids and suckers in hexabothriids. According to Bychowsky (1957), the clamps of chimaericolids are given skeletal support by the sclerites, which form a framework around the clamp periphery and control its depth. Shinn et al. (1995) have indicated that a high sulphur content in gyrodactylid monopisthocotylean sclerites meant a more rigid structure; thus, the high sulphur content mentioned above in the lateral sclerites of C. leptogaster would tend to bolster Bychowsky’s (1957) hypothesis that these may be supporting structures for the clamp. On the other hand, the low sulphur content of the median sclerite suggests a more flexible structure which acts as a spring when the muscles of the clamp is relaxed, closing the mouth of the clamp onto the host’s gill tissue.
Lyons (1966) expressed the opinion that the lateral sclerites of the clamps of the polyopisthocotylean C. leptogaster are comparable in composition to the hamuli and the median sclerite with the marginal hooks of monopisthocotylean monogeneans and considered that the lateral sclerites arose later than the median during the course of the evolution of the group. Our ultrastructural analysis of the inner structure of the median sclerites of C. leptogaster, as compared with those of the marginal hooks of the ancyrocephaline monopisthocotylean Cichlidogyrus halli typicus (see El-Naggar 1992), shows that they are both heterogeneous in their content, comprising different morphological layers; however, the detailed structure of these layers is not the same. Furthermore, the internal structure of the hamuli of C. halli typicus (see El-Naggar 1993) is also layered, i.e. it has a thin outer layer, a middle layer and a central core, whereas our results show a unique inner structure of the lateral sclerites of C. leptogaster which does not resemble that of any other studied monogenean sclerite. However, on account of the very limited data on the ultrastructure of monogenean hamuli and marginal hooks, it is not yet possible to compare in detail the ultrastructural traits of the chimaericolid polyopisthocotylean clamp sclerites with those of the hooks and hamuli of monopisthocotyleans in general.
Although few data exist on the ultrastructural characteristics of the clamp sclerites of other polyopisthocotylean monogeneans, Shaw (1979b) studied the inner structure of the clamp sclerites of the gastrocotylid Gastrocotyle trachuri and six other polyopisthocotyleans without giving any indication of the type of sclerite or their location within the clamp wall. These sclerites are composed of moderately electron dense material within which denser fibrils are embedded (Shaw 1979b). We did not observe sclerites with such a uniform morphology within the clamp wall of C. leptogaster, although a similar structure was observed in the middle layer of its median sclerite. It seems, therefore, that there is some variation in the internal structure of monogenean clamp sclerites. On the other hand, in his work on clamp development in G. trachuri, Shaw (1979c) indicated that the sclerites first appear as a collection of short, hollow tubules, the number of which increases, and areas of denser material. The sclerites become uniformly electron dense and then change in appearance in fully formed clamps, such that the developing sclerites of this species have a stage in their formation when they have an electron dense content penetrated by hollow areas and are similar in structure to the lateral sclerites of C. leptogaster.
Spine-like structures of the clamp
Our TEM investigation recorded, for the first time, the presence of six spine-like structures which are directed towards the clamp lumen of C. leptogaster. These structures are protuberances of the muscle tissue covered by a thickened basement lamina associated with the clamp wall myofibrils. More precisely, four of these spine-like structures are extensions of the margins of the two spindle-like muscle blocks in the clamps, i.e. the two anterior and two posterior structures, and the two others are situated on the lateral constrictions of the left and right muscle blocks. These structures are considered to be basement lamina derivatives. Our search for information on other monogenean species for an ultrastructural homologue for these structures in C. leptogaster was unsuccessful. As far as we can determine, these spine-like structures would appear to be unique within the Monogenea. However, it is worth noting that structures similar in morphology to those of C. leptogaster have been described as ‘spines’ on the surface of the copulatory organ of four proseriate turbellarian genera of the family Monocelididae (Martens 1984).
Electron dense surface structures
A previously unrecorded morphological feature of the C. leptogaster clamp is the presence of electron dense surface structures along the internal surface of the anterior lips and on deeper regions of the internal (luminal) surface of the clamp wall tegument. We assume that these structures are derivations of the clamp tegument, or to be more precise, its outer, densely fibrous region. The occurrence of dense surface structures on a monogenean tegument appears limited to the presence of lanceolate, electron dense surface structures which we recorded on the surface of the second region of the distal vaginal epithelium of C. leptogaster (see Poddubnaya et al. 2013). The location of these dense surface structures in C. leptogaster suggests that they have a mechanical function associated with the grip of the clamp.
Conclusions and comments
In terms of the phylogeny of the monogenean subclass Polyopisthocotylea, the Chimaericolidae is an important family. A number of workers have considered the chimaericolids to be close to the base of the polyopisthocotylean lineage of the Monogenea (Bychowsky 1957; Llewellyn 1965; Boeger and Kritsky 1997, 2001; Jovelin and Justine 2001). Bychowsky (1957) believed that chimaericolid clamps are the most primitive of all the polyopisthocotyleans. Evidence obtained from the present ultrastructural study of the haptoral clamps of the chimaericolid C. leptogaster from a relictual holocephalan fish confirms their simple and apparently ancient nature. Our investigation revealed that the clamp complex of C. leptogaster is different from that of other polyopisthocotyleans, possessing an apparently unique form of skeletal support. First, differences in the location, ultrastructure and chemical composition (see Lyons 1966), in addition to the apparently different origins of the paired lateral and single median sclerites, likely have considerable importance in relation to analyses of the phylogeny of polyopisthocotylean attachment organs. Second, the lateral sclerites of the studied chimaericolids are situated within the muscular tissue of the clamp and are closely bounded by the radial myofibrils attached to them. These sclerites are derivatives of the basement lamina surrounding the clamp musculature, with which they share a similar internal homogeneous structure sprinkled with apparently hollow areas. According to Lyons (1966), the lateral sclerites of C. leptogaster contain sulphur-bonded scleroproteins which, following comments of Shinn et al. (1995), suggests that they are rigid and act as structural supports for the clamp (Bychowsky 1957). Third, the heterogeneous body of the median sclerite, which occupies both muscular and tegumental areas of the clamp wall, surrounded by a basement lamina situated at some distance from the body of the sclerite. This median sclerite in C. leptogaster contains the amino acid cystine (Lyons 1966), which is present within keratin, suggesting that it has a flexible structure (Shinn et al. 1995). It may therefore act as a spring, which closes the clamp on the gill tissue when the muscle blocks relax. In this context, it is worth noting that Lyons (1966) also found cystine present within body spines of the gyrocotylidean cestode species Gyrocotyle urna and G. fimbriata, as well as in their larval hooks, suggesting the possibility of the coevolution of chimaericolids and gyrocotylids within their holocephalan hosts and a possible sister relationship between these two neodermatan groups. She also suggested a relationship between the median sclerite of chimaericolid, hexabothriid and diclybothriid polyopisthocotyleans, all of which occur on ancient fish groups with ancient lineages. Fourth, the apparently unique structure of six previously unrecorded spine-like structures in the clamps of C. leptogaster appear to resemble structures also seen in proseriate turbellarians and may represent another piece of evidence suggesting an ancient origin for chimaericolids. Last, an additional type of clamp armament occurs in the form of electron dense tegumental surface structures, the occurrence of which appears rare among monogenean species.
Studies on the ultrastructure of monogenean haptors are few and very scattered. Future investigations of a large number of both monopisthocotylean and polyopisthocotylean adult haptors and their sclerites are needed in order to elucidate possible evolutionary steps in the formation of the different types of the sclerites found in these groups, especially in the more basal taxa. As indicated by this and our previous study of C. leptogaster (see Poddubnaya et al. 2013), there are ultrastructural characters which can be used to support hypotheses on an ancient origin for the Chimaericolidae.
References
Boeger WA, Kritsky DC (1997) Coevolution of the Monogenoidea (Platyhelminthes) based on a revised hypothesis of parasite phylogeny. Int J Parasitol 27:1495–14511. doi:10.1016/S0020-7519(97)00140-9
Boeger WA, Kritsky DC (2001) Phylogenetic relationships of the Monogenoidea. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor & Francis, London, pp 92–102
Brinkmann A Jr (1942) On “Öctobothrium” leptogaster F.S. Leuckart. Göteborgs Kungl. Vetenskaps och Vitterhets Samhälles Handlingar. Ser B 2:3–29
Bychowsky BE (1957) Monogenetic trematodes, their systematics and phylogeny. Izdatel’stvo Academiya Nauk SSSR, Moscow, Russia. In Russian
El-Naggar MM (1992) Ultrastructural observations on the marginal hooklets of the monogenean gill parasite Cichlidogyrus halli typicus. Int J Parasitol 22:613–619. doi:10.1016/0020-7519(92)90009-A
El-Naggar MM (1993) Ultrastructural observations on the hamuli of the monogenean gill parasite Cichlidogyrus halli typicus. Int J Parasitol 23:745–748. doi:10.1016/0020-7519(93)90070-F
Inoue JG, Miya M, Lam K, Tay BH, Danks JA, Bell J, Walker TI, Venkatesh B (2010) Evolutionary origin and phylogeny of the modern holocephalans (Chondrichthyes: Chimaeriformes): a mitogenomic perspective. Mol Biol Evol 27:2576–2586. doi:10.1093/molbev/msq147
Jovelin R, Justine J-L (2001) Phylogenetic relationships within the polyopisthocotylean monogeneans (Platyhelminthes) inferred from partial 28S rDNA sequences. Int J Parasitol 31:393–401. doi:10.1016/S0020-7519(01)00114-X
Justine J-L, Rahmouni C, Gey D, Schoelinck C, Hoberg EP (2013) The monogenean which lost its clamps. PLoS One 8(11):e79155
Llewellyn J (1965) The evolution of parasitic Platyhelminths. In: Taylor A (ed) Third symposium of the British society for parasitology. Blackwell Scientific Publications, Oxford, pp 47–78
Lyons KM (1966) The chemical nature and evolutionary significance of monogenean attachment sclerites. Parasitology 56:63–100
Martens EE (1984) Ultrastructure of the spines in the copulatory organ of some Monocelididae (Turbellaria, Proseriata). Zoomorphology 104:261–265. doi:10.1007/BF00312007
Olson PD, Littlewood DTJ (2002) Phylogenetics of the Monogenea—evidence from a medley of molecules. Int J Parasitol 32:233–244
Pascoe PL (1997) Monogenean parasites of deep-sea fishes from the rockall trough (N.E. Atlantic) including a new species. J Marine Biol Assoc UK 67:603–622. doi:10.1017/S0025315400027326
Poddubnaya LG, Hemmingsen W, Gibson DI (2013) Ultrastructural characteristics of the vaginae of the basal monogenean Chimaericola leptogaster (Leuckart, 1830). Parasitol Res 112:1169–1177. doi:10.1007/s00436-013-3596-8
Ramasamy P, Bhuvaneswari R (1993) The ultrastructure of the tegument and clamp attachment organ of Gotocotyla bivaginalis (Monogenea, Polyopisthocotylea). Int J Parasitol 23:213–220. doi:10.1016/0020-7519(93)90143-M
Ramasamy P, Hanna REB, Threadgold T (1986) The surface topography and ultrastructure of the tegument and haptor of Price multae (Monogenea). Int J Parasitol 16:581–589. doi:10.1016/0020-7519(86)90024-X
Shaw MK (1979a) The ultrastructure of the clamp wall of the monogenean gill parasite Gastrocotyle trachuri. Parasitol Res 58:243–258. doi:10.1007/BF00933931
Shaw MK (1979b) The ultrastructure of the clamp sclerites in Gastrocotyle trachuri and other clamp-bearing monogeneans. Parasitol Res 59:43–51. doi:10.1007/BF00927845
Shaw MK (1979c) The development of the clamp attachment organs of the monogenean Gastrocotyle trachuri. Parasitol Res 59:277–294. doi:10.1007/BF00927522
Shinn AP, Gibson DI, Sommerville C (1995) A study of the composition of the sclerites of Gyrodactylus Normann, 1932 (Monogenea) using X-ray elemental analysis. Int J Parasitol 25:797–805. doi:10.1016/0020-7519(95)00008-P
Acknowledgments
Special thanks are due to Prof. Odd Halvorsen, Natural History Museum, Oslo University, Norway, for his kind assistance with finding a source of chimaeras. The authors would like to thank the staff of the RV ‘Johan Ruud’, belonging to Tromsø University, for their invaluable help with the fishing. We are also grateful to the staff of the Centre of Electron Microscopy of the I.D. Papanin Institute of the Biology for Inland Waters, RAS, for technical assistance. The present study was supported by the Russian Foundation for Fundamental Research project no. 12-04-00149a (to LGP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poddubnaya, L.G., Hemmingsen, W. & Gibson, D.I. Clamp ultrastructure of the basal monogenean Chimaericola leptogaster (Leuckart, 1830) (Polyopisthocotylea: Chimaericolidae). Parasitol Res 113, 4023–4032 (2014). https://doi.org/10.1007/s00436-014-4070-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4070-y