Abstract
The control of fasciolosis, as that of other vector-borne diseases, must be related to the control of the lymnaeid snails, the intermediate hosts of the parasite. Thus, an accurate epidemiological surveillance of the transmission foci where the infected mollusks occur is essential. For this purpose, immunoassays could be a useful tool. However, information regarding specific proteins of intramolluscan larvae and previous studies concerning monoclonal antibody generation against asexual stages of trematodes are scarce. Therefore, we explored the antigenic features of intramolluscan rediae of Fasciola hepatica to evaluate three antigenic preparations in order to use the most promising one for developing specific monoclonal antibodies. Mouse antiserum was generated against each antigen for assessing the polyclonal antibody response against the crude extract of rediae and the cross-reactivity against lymnaeids. The specific C-terminal of F. hepatica cytochrome c oxidase subunit I (first antigen), selected by in silico analyses, might not be the appropriate target for immunoassay detection of infected snails, due to its low representation in the total extract of rediae. The majoritarian mixture of low-molecular-weight proteins (<30 kDa) from the rediae homogenate (second antigen) revealed a significant cross-reactivity with lymnaeids. Evidence of the existence of mimetic immunogenic epitopes in this fraction of F. hepatica rediae was achieved. High immunogenicity of the crude extract of rediae (third antigen), mainly related to parasite’s specific epitopes, was regarded. Therefore, the rediae homogenate is stated as the most promising antigen from those evaluated, for monoclonal antibody development with potentialities for detecting F. hepatica-infected snails.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fasciolosis is an important worldwide distributed disease that affects a wide range of livestock and causes economic losses of large proportions. This infection also stands among the list of reemergent diseases in humans, with estimates ranging from 2.4 to 17 million persons infected (Mas-Coma et al. 2009). The main worldwide etiological agent of this disease is the trematode Fasciola hepatica (Digenea: Fasciolidae), a parasite that requires a freshwater snail as the intermediate host, for cercariae development. The infecting form for mammals (e.g., humans, sheep and cattle) as definitive hosts is encysted metacercariae mainly attached to aquatic plants (Andrews 1999).
The transmission of F. hepatica strictly depends on the presence of the intermediate host, a freshwater snail of the Lymnaeidae family. Like other vector-borne diseases, its control must be based upon controlling the transmission foci where the infected mollusks occur (Kaplan et al. 1997; Caron et al. 2011). The epidemiological surveillance of lymnaeid populations is an effective alternative to monitor and manage endemic areas and eventually control the transmission of fasciolosis. However, the methods currently available for detecting the parasite within the snails are not sensitive and specific enough (parasitological examination) or they are too expensive (molecular biology techniques) to accomplish this goal on a larger scale (see Caron et al. (2008)). In this scenario, the development of immunoassays based on monoclonal antibody (Mab) with high sensitivity and specificity against the long-lasting intramolluscan larval stage of the parasite, the rediae, could be useful for identifying transmission foci of fasciolosis. This could allow restricting the control efforts to a particular area. Since immunoenzymatic assays (ELISAs) to detect F. hepatica infection in the definitive hosts are being currently used in both developed and developing countries (Espinoza et al. 2007; Ubeira et al. 2009; Rojas et al. 2010), a novel ELISA to be used at the epidemiological surveillance of lymnaeid snails could be easily incorporated, especially in low-income nations.
However, there are some concerns about the proper methodology for obtaining specific anti-F. hepatica rediae immunological reagents. Among these are (1) the nonexistence of immunoassays currently available to detect F. hepatica nor other helminthes in mollusks; (2) the existence of mechanisms of molecular mimicry and masking described in other digenean species, which help the parasite to evade the mollusk immunity (Yoshino and Bayne 1983; Adema and Loker 1997; Yoshino et al. 2013); and (3) limited molecular information regarding the specific proteins associated with the intramolluscan stages of Fasciola spp. Regarding this last issue, Humiczewska (1975) detected oxidase and dehydrogenase enzymatic activities in F. hepatica rediae. Specific cytochrome oxidase activity was also discovered in later rediae, ascertaining that this larval stage uses different pathways of energy release. In another study, two enzymes, Cu/Zn-superoxide dismutase (SOD) and thioredoxin (TRX) (previously characterized on the excretory-secretory antigens of F. hepatica adults), were identified on the excretory-secretory products of F. hepatica sporocysts (Gourbal et al. 2008).
Therefore, in order to develop specific anti-F. hepatica rediae Mabs for analytical purposes, we selected and evaluated three different antigenic preparations using mouse hyperimmune sera to assess the antibody response generated against each candidate. In this sense, a high recognition of the crude extract of rediae by mouse antisera together with none or reduced cross-reactivity against snails’ antigens was an issue of major importance for selecting the most promising antigenic candidate. Two rabbit anti-rediae polyclonal antibodies (Pabs) were also used to thoroughly explore some features of the antigenic composition of rediae.
Material and methods
Computational sequence and structure analysis
In order to identify promising peptides for the development of specific anti-F. hepatica rediae Mabs (non-cross-reactive with snails nor with other flukes), we compared the protein sequences of F. hepatica cytochrome c oxidase subunit I (COX I) (Q9B8Y2), SOD (Q9XY94), and TRX (Q9U1G7), with their corresponding homologues of other related digenean pathogenic parasites and of the taxon Lymnaeidae, the intermediate host species of F. hepatica. For these analyses, the following web servers were used: UniProt (www.uniprot.org) for retrieving the sequences of the target proteins; PSI-BLAST (www.ncbi.nlm.nih.gov/BLAST ) for similarity searches in the nonredundant NCBI protein database (NCBInr); M-Coffee (tcoffee.crg.cat/apps/tcoffee/do:mcoffee) for multiple sequence alignment; Jalview (www.jalview.org) for alignment editing, visualization, and analysis; and the Protein Model Portal (www.proteinmodelportal.org) for obtaining the protein homology-modeling structures when experimental structures were not available.
Peptide synthesis
The selected fragment derived from the structure analysis was synthesized at the Institute of Genetic Engineering and Biotechnology of Havana by the solid-phase method using 9-fluorenyl-methoxycarbonyl chemistry (Merrifield 1986). It was purified by reverse phase high-performance liquid chromatography to >98 % purity on an acetonitrile/H2O-trifluoracetic acid gradient and confirmed by ion-spray mass spectrometry (Micromass, UK). A cysteine residue was added to the N-terminal in order to conjugate a portion of the synthetic peptide to a carrier, bovine thyroglobulin (Pept-BTg), and used for mice immunization, as the first antigenic candidate. The nonconjugated peptide (Pept) was used for titer evaluation of mouse hyperimmune sera.
F. hepatica adults, host snails, and experimental mice and rabbits
F. hepatica adults were obtained in cattle slaughterhouses and placed in a solution of 0.85 % NaCl (saline solution) and 5 % glucose (Sigma, USA), for 5 h, and observed for egg release. Eggs were preserved at 4 °C in complete darkness, in a saline solution supplemented with gentamicin (50 μg/mL) (Sigma), until use. Populations of the Cuban intermediate hosts, Galba cubensis and Pseudosuccinea columella reared in the Laboratory of Malacology of the Institute of Tropical Medicine “Pedro Kourí”, were used in this study. Experimental BALB/c mice and chinchilla rabbits were supplied by the Centre for Laboratory Animal Production of Cuba. Animal care and maintenance were in agreement to institutional bioethical guidelines. All protocols involving experimental animals were approved by the Committee of Animal Care and Use of the institute.
Miracidia and rediae of F. hepatica: experimental infection in snails
Eggs of F. hepatica were incubated in distilled water, in total darkness, at 28 °C, during 15 days for complete maturation. At day 15, miracidia were obtained after egg hatching induced by direct light exposure. Each of snails was infected with five freshly hatched miracidia, according to the methodology described by Vázquez et al. (2013). F. hepatica rediae were obtained by dissecting experimentally infected snails at day 30 postinfection (in our conditions, cercarial shedding normally begins between 35 and 40 days postinfection in G. cubensis and lately (day 40) in P. columella). Rediae were thoroughly washed with phosphate buffered saline (pH 7.2, PBS) after collection, in order to minimize larvae contamination with snail material.
Preparation of crude extracts
Protein homogenates of F. hepatica rediae were obtained after 10 sonication cycles with 10-s intervals between each cycle, in PBS supplemented with a cocktail of protease inhibitors (Complete, Roche, USA). Crude extracts of miracidia and rediae were also obtained by the same sonication procedure in the presence of 0.01 % Triton X-100 (Sigma) as detergent, for COX solubilization (Musatov et al. 2000). Crude extracts of lymnaeid snails G. cubensis and P. columella were prepared using healthy (nonexperimentally infected) laboratory reared individuals. The mollusks were homogenized in PBS supplemented with protease inhibitors, in a Potter homogenizer. Triton X-100 was added to the homogenates of the snails used for evaluation of the serum anti-Pept. Protein quantification was performed by the bicinchoninic acid reaction (Smith et al. 1986) using bovine serum albumin (Sigma) as the standard.
Protein electrophoresis
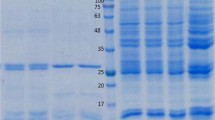
The majoritarian antigens of F. hepatica rediae were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 12 % polyacrylamide gels (Laemli 1970). The samples were diluted without any reducing reagent, and 0.1 % Coomassie Brilliant Blue R-250 (Merck, Germany) was used for staining. Homogenates of G. cubensis and P. columella were also included in the electrophoresis. Coomassie-stained gels of resolved proteins were quantified by scanning densitometry using ImageJ software 1.41. The molecular weights of the most representative proteins of rediae were estimated using a commercial standard of 78 to 12 kDa (BDH, UK).
Fractioning of the crude extract of F. hepatica rediae
Crude extract of rediae was separated into low- (<30 kDa) and high-molecular-weight fractions (>30 kDa) using an Amicon Ultra-15 (30 kDa) centrifugal device (Millipore, USA). The low-molecular-weight fraction (LMWF) was lyophilized and then diluted in saline solution for mouse immunization (second antigenic preparation). In parallel, crude extract of rediae was also used for mice immunization as the third antigenic candidate.
Mice immunization
Three groups, each with five 6–8-week-old female BALB/c mice, were immunized intraperitoneally with 40 μg of each antigen (group 1: Pept-BTg, group 2: LMWF, and group 3: crude extract of rediae) in 1:1 (v/v) emulsion of complete Freund’s adjuvant (Sigma) and saline solution. Subsequent doses with 30 μg of the antigens were administered subcutaneously, at day 15 and day 30 after initial immunization, in 1:1 (v/v) emulsion of incomplete Freund’s adjuvant (Sigma) and saline solution. The last dose of 20 μg of the antigens was administered by the subcutaneous route at day 45 postinitial immunization in 1:1 (v/v) emulsion of incomplete Freund’s adjuvant and saline solution. Before and 10 days after the last immunization, blood from each mouse was obtained. During immunization and bleeding procedures, the mice were anesthetized with thiopental (25 mg/kg, from Sigma).
Indirect immunoenzymatic assays
Mouse sera were titered against the antigenic candidate used for immunization, and each of the serum with the higher titer per immunized group was further analyzed for antibodies reacting to the crude extract of F. hepatica rediae and to the homogenates of lymnaeid snails using indirect ELISAs. Briefly, the sera titer from immunized mice from group 1 was assessed using CovaLink™ plates (NUNC) (in order to assure an accurate binding of the peptide to the plate with a proper orientation) coated with a solution containing 10 μg/mL with the Pept in PBS (100 μL/well) and incubated overnight, at 4 °C, with 10 μL of disuccinimidyl suberate (2 mg/mL) in dimethyl sulfoxide (Sigma). Instead, MaxiSorp (NUNC, Denmark) plates were coated with 100 μL/well of a solution of carbonate-bicarbonate buffer (pH 9.6) containing 10 μg/mL of (1) the LMWF or (2) the crude extract of F. hepatica rediae, for titer evaluation of mouse sera from groups 2 and 3, respectively. To assess the anti-F. hepatica rediae activity and the cross-reactivity with snails of the mouse serum with the higher titer per group of antigen, plates were sensitized with 10 μg/mL of the crude extracts of (3) F. hepatica rediae, (4) G. cubensis, and (5) P. columella diluted in carbonate-bicarbonate buffer (pH 9.6, 100 μL/well). A solution of 10 μg/mL of (6) crude extract of F. hepatica miracidia in carbonate-bicarbonate buffer (pH 9.6) was also included for the evaluation of hyperimmune serum anti-Pept-BTg. The plates were incubated at 4 °C, for 16 h, and wells were blocked to prevent nonspecific binding using 5 % bovine serum albumin (Sigma) in carbonate-bicarbonate buffer (pH 9.6), for 1 h. Sera were serially diluted in PBS, added to wells of the antigen-coated plates, and incubated for 1 h, at 37 °C. Then, a peroxidase-conjugated goat anti-mouse (Dako, Denmark) was added to the wells and incubated for 1 h. Antibody binding was detected by adding o-phenylenediamine dihydrochloride and hydrogen peroxide in phosphate-citrate buffer (pH 5.0) and by the measurement of optical density at 492 nm, after 15 min of reaction previously stopped with 12.5 % H2SO4. The plates were thoroughly washed with 0.05 % Tween 20 in PBS (PBS-T), after each incubation step.
The most promising antigenic preparation for the development of anti-F. hepatica rediae Mabs (with potential for immunoassays) was defined by balancing the anti-rediae activity achieved with none or reduced snail cross-reactivity. The serum prior to immunizations was used as a negative control for each determination at the same dilutions of the hyperimmune sera.
Development of rabbit polyclonal antibodies
Anti-F. hepatica rediae Pab was obtained by immunizing 10-week-old-female chinchilla rabbits with the crude extract of rediae. The immunization protocol involved four different doses of 350, 300, 250, and 150 μg of the antigen per rabbit. The first dose of the antigen, mixed with Freund’s complete adjuvant in 1:1 (v/v) and saline solution, was inoculated intraperitoneally. Additional doses were applied subcutaneously in Freund’s incomplete adjuvant in 1:1 (v/v) and saline solution, with 2 weeks between each immunization. Before the first dose of antigen and 10 days after the last one, sera were obtained and titered against the crude extract of rediae by the indirect ELISA described before. At this point, animals were bled and serum was precipitated with 50 % (NH4)2SO4, dialyzed against PBS, and then fractionated on a matrix of Protein A Sepharose 4 Fast Flow (Amersham Pharmacia Biotech; GE Healthcare, USA).
In order to eliminate possible cross-reactivity of the anti-F. hepatica rediae Pab with the intermediate host snails, total antigens of G. cubensis and P. columella were coupled to a matrix of ω-Aminohexyl-Sepharose® 4B (Sigma) according to the manufacturer’s guidelines. Then, the purified Pab (total Pab) was fractionated by an affinity chromatography on the matrix of ω-Aminohexyl-Sepharose 4B®-total antigens of lymnaeid snails. Cross-reactive IgG to snails’ antigens was eliminated after interacting with the matrix, and the recovered specific fraction of the anti-rediae Pab (rediae- specific Pab) was quantified and then evaluated by an indirect ELISA against the crude extract of F. hepatica rediae and the homogenates of G. cubensis and P. columella. The total Pab was also included in the ELISA, and antigens-rabbit antibodies interaction was revealed by a peroxidase-conjugated goat anti-rabbit IgG (Sigma).
Immunoblotting
Resolved proteins of F. hepatica rediae and of G. cubensis and P. columella, previously separated on a 12 % SDS-PAGE gel, were transferred onto nitrocellulose membranes. Immunodetection was carried with the total Pab and with the rediae-specific Pab. Peroxidase-conjugated goat anti-rabbit IgG, at 1:1,000 dilution, was used to detect recognized proteins.
Statistical analysis
Mean and standard deviation for triplicate values, as plotting points in graphics, and the mean of the dilution of pre-immunized mice sera plus twice the standard deviation, as analytical limit criteria for mice titer assessment, were used. Data were analyzed by Kolmogorov-Smirnov statistical test to verify normal distribution and with the Levene test to check for homogeneity of variance. One-way ANOVA and Tukey’s test were used to analyze the differences among means of serum anti-Pept reacting with the crude extracts of miracidia and redia of F. hepatica and with homogenates of snails.
Results
Selection of the antigenic candidates
Computational analysis of target proteins
In order to detect specific short regions on the F. hepatica COX I, SOD, and TRX protein sequences suitable for anti-F. hepatica rediae Mab production, we performed two separate multiple sequence alignment (MSA) of each protein with their corresponding homologous proteins in specific groups of trematodes and mollusks. Unfortunately, the number of sequences of SOD and TRX from Digenea is very small (only five for SOD and three for TRX) and there are almost no SOD and TRX sequences of snails on databases (only one for SOD and none for TRX). This limited the statistical relevance of the results obtained from the sequence analysis of these proteins. Therefore, SOD and TRX were set aside of further analyses.
The case of COX I was different as 44 sequences of Digenea and 19 sequences of Lymnaeidae were available for analysis. The similarity among the COX I sequences of flukes was high (ranging from 72 to 92 %), as expected for a well-conserved protein, although not all the sequences analyzed were fully annotated (with most fragments missing at the beginning and at the end) (see Supplementary Material 1.1 for detail). Four segments of the F. hepatica sequence with low identity percent among the flukes were identified (named B1–B4) and are shown in Fig. 1c, d.
Frontal and backward views of the comparative model of COX I. a, b Surface colored by coulombic potential. The white regions correspond to the transmembrane α-helix of the protein. c, d The four variable segments identified as specific of F. hepatica (identified on the MSA of flukes and mollusks). The figures were generated with UCSF Chimera 1.8.1
The percentage of sequence identity of COX I with the proteins from mollusks ranged from 50 to 56 %, due to the incompleteness of the snail sequences, as only the first half of the sequences (from 1 to around 224 residues) was available for comparison (except for the Galba pervia species). Therefore, we decided to take into account complete sequences of COX I from the taxonomic clade Panpulmonata, which includes snails and slugs. Those new sequences (a total of 25) were realigned to F. hepatica and G. pervia. As expected, the first half of the MSA was very similar to the one constructed with the Lymnaeidae orthologues. On the MSA of F. hepatica-mollusk the nine segments that shared a low identity percent with the rest of the proteins were identified (named A1–A9) (see Supplementary Material 1.2 for details of the MSA).
The four segments identified as specific of F. hepatica on the MSA of flukes were also included (and denoted) on those identified from the MSA of mollusks (A2/B1, A4/B2, A5/B3, A9/B4) and are shown in Fig. 1c, d. The A2/B1 segment is located within a highly hydrophobic α-helix (see Fig. 1a, b for hydrophobicity analysis) and therefore is not suitable for chemical synthesis (Angeletti 1999). In designing potential antigenic epitopes, it is important to select a hydrophilic and surface oriented-segment of 12-to-16-residue length. These features are often present in the N- and C-termini of proteins that are often exposed and have a high degree of flexibility (Angeletti 1999). Therefore, we selected a sequence of 14 residues (QHNSYMNGVGRWVF) of the A9/B4 segment that is part of the C-terminal of the COX I (Fig. 1d). This sequence formed a variable and exposed segment of random conformation within a well-conserved transmembrane protein. These characteristics make the selected fragment quite suitable for chemical synthesis and further evaluation as a pertinent candidate for specific anti-F. hepatica Mab development.
Analysis of the antigenic composition of F. hepatica rediae by protein electrophoresis
The majoritarian antigenic composition of F. hepatica rediae was visualized and compared with the most representative proteins of the lymnaeid species G. cubensis and P. columella using a SDS-PAGE gel (Fig. 2). At least, 10 well-defined bands could be observed from the rediae, ranging from 93 to 12 kDa (93, 84, 71, 60, 53, 35-27, 20, 18, 16, 14, and 12 kDa), approximately. It is noteworthy that, in the case of the snails, the majoritarian proteins of the total extracts have estimated molecular weights higher than 30 kDa, while the five most representative proteins of rediae are those with lower weights, ranging from 20 to 12 kDa, which correspond approximately to the 53.5 % of the total proteins of the extract. This fact diminishes possible concerns about significant contamination of the rediae with proteins from snails during dissection procedure and also significant molecular masking mechanisms (as has been reported in other trematode-snail systems), related with the majoritarian proteins of this particular fraction of the crude extract of rediae. Moreover, the high representation of these proteins in the antigenic composition of rediae singles them out as a quite suitable mixture of antigens from the intramolluscan larvae for the development of specific anti-F. hepatica Mab. The crude extract of F. hepatica rediae was separated by ultrafiltration, and the LMWF, corresponding to the proteins below 30 kDa, was used for mouse immunization along with the other two antigenic preparations (synthetic C-terminal of the COX I (Pept-BTg) and the crude extract of rediae).
Evaluation of mice hyperimmune sera
The titer of mouse hyperimmune sera anti- each one of the three antigenic candidates was evaluated by indirect ELISAs using plates coated with each antigen. The synthetic C-terminal COX I peptide and the LMWF of the homogenate of F. hepatica rediae induced an effective stimulation of the immune system of mice with antibody titer of 1/20,000 in both cases. The higher titer (1/40,000) was achieved in mice immunized with the crude extract of F. hepatica rediae.
The mouse serum with the higher titer per group was evaluated against the crude extract of F. hepatica rediae in order to determine parasite recognition. In the same way, each serum was also evaluated against the homogenates of G. cubensis and P. columella to estimate possible cross-reactivity with the intermediate hosts. In Fig. 3, the results of the assay for the anti-Pept COX I serum diluted 1/4,000 are shown. In this case, the crude extract of F. hepatica miracidia was also included and there are significant differences between the recognition of rediae and miracidia. Even when no cross-reactivity with lymnaeid snails was attained with this serum (as was expected from the in silico analysis), the polyclonal reaction against the crude extract of rediae was discrete (Fig. 3). No significant recognition of this larval stage was achieved at serum dilution higher than 1/4,000. Therefore, COX I might not be an appropriate antigen for the development of anti-F. hepatica rediae Mabs aimed to detect the parasite within the snails due to its low representation on the total extract of this particular stage.
On the other hand, assessment of the polyclonal reaction of the anti-LMWF sera and anti-crude extract of rediae against the homogenates of F. hepatica rediae and of G. cubensis and P. columella showed considerable cross-reactivity with lymnaeids’ antigens (Fig. 4). However, the cross-reactivity of the anti-LMWF serum with snails was significantly higher in every dilution evaluated. This fact indicates that the high immunogenicity of the crude extract of rediae is mainly related to parasite’s specific epitopes and points at this antigenic preparation as the most promising for the development of specific anti-F. hepatica rediae Mabs, from those evaluated.
Thus, in order to continue exploring the potentialities of the crude extract of rediae as the proper candidate for Mab development (aimed for immunoanalytical purposes) and the cross-reactivity with snails’ antigens attained with both sera, we developed some proofs of concept with an anti-crude extract of F. hepatica rediae Pab developed in rabbits.
Proofs of concept
Chinchilla rabbits were immunized with the crude extract of F. hepatica rediae, and high titer of antibody was achieved (1/128,000). The IgG fraction of the serum was purified, and then, the significant cross-reactivity of the Pab with the antigens of lymnaeid snails was totally eliminated by an affinity chromatography on a matrix of ω-Aminohexyl-Sepharose®-homogenates of G. cubensis and P. columella (Fig. 5). This emphasizes the fact that a specific response of anti-F. hepatica rediae antibodies (non-cross-reactive with snails) can be developed using the crude extract of rediae as an antigenic preparation. Further on, the immunoblotting analysis of the homogenates of rediae and of lymnaeids revealed that the majority of the cross-reactive epitopes identified by the total Pab are indeed proteins below 30 kDa of the crude extract of the parasite (Fig. 6). These low-molecular-weight proteins ultimately form the so-called LMWF used here for mouse immunization, which also induced a polyclonal response in mice highly cross-reactive with snails.
Immunoblotting of resolved proteins of rediae and lymnaeid snails, immunodetected with the specific Pab (tracks 2–4) and the total Pab (tracks 5–7). Track 1, molecular weight standard from 78 to 12.3 kDa; tracks 2 and 5, crude extract of F. hepatica rediae; tracks 3 and 6, crude extract of G. cubensis; and tracks 4 and 7, crude extract of P. columella
Discussion
F. hepatica is a parasite with a complex life cycle characterized by the development of free-living larvae (miracidia and cercariae) and of several stages of the trematode inside two different hosts (mollusks and mammals) (Andrews 1999). These morphological and behavioral modifications occur together with metabolic changes linked to the up- or downregulation of a number of biomolecules (Robinson et al. 2009). Molecular information regarding the interaction of F. hepatica-lymnaeid snails could be fundamental for a better understanding of this system. Nevertheless, this subject has not been deeply explored in this particular model.
Here, the electrophoretic bands of molecular weight of 93, 53, and 27 kDa of the crude extract of F. hepatica rediae are consistent with the estimated molecular weight of three of the electrophoretic bands found in miracidia by Hernández et al. (2002), possibly due to the same protein expression in both stages. However, the electrophoretic pattern achieved with the homogenate of rediae showed a more complex antigenic composition than the one reported for miracidia of only six bands (Hernández et al. 2002). The existence of majoritarian proteins below 20 kDa on the crude extract of F. hepatica rediae contrasts with the absence of low-molecular-weight electrophoretic bands in the extract of miracidia analyzed by Hernández et al. (2002), suggesting a significant antigenic variation between the two larval stages. This antigenic variation could be related to the lower recognition of the crude extract of rediae by the anti-Pept serum compared to the polyclonal reaction assessed against the homogenate of miracidia. Even when in later aerobic metabolism of F. hepatica rediae is possible (Humiczewska 1975), oxygen availability for the parasite larvae inside the snails is lower compared to the natural environment where the free-living miracidia occurs. This might affect the level of expression of aerobic proteins like the COX I between these stages. In fact, differential expression of COX complex among different developmental stages of species from other phyla has been reported (Liénard et al. 2006). On the other hand, transitions in energy metabolism have been described and characterized on the fully aerobic functioning of F. hepatica juvenile to the almost completely anaerobic metabolism of the adult parasites (extensively reviewed by Tielens (1999)). The results already discussed could be an interesting starting point to further explore the regulation of the energy pathways in miracidia-sporocyst-rediae switching larvae of F. hepatica.
A complex fraction of low-molecular-weight proteins is an important component of F. hepatica sporocyst, particularly of the excretory-secretory antigens (Gourbal et al. 2008), as it turned out to be for the crude extract of rediae. Gourbal et al. (2008) identified the antioxidative enzymes SOD and TRX as molecular weight dots of 16 and 12 kDa, respectively, on a bidimensional electrophoresis. Regarding the important function of these proteins in the oxidative stress that occurs during the immune response of the snail Biomphalaria glabrata against Schistosoma mansoni sporocyst (Mourão et al. 2009) and in mammalian immunity against F. hepatica adults (Salazar-Calderón et al. 2001; Piedrafita et al. 2007), it is reasonable to infer that SOD and TRX could also be part of the antigenic composition of rediae (electrophoretic bands of 16 and 12 kDa identified in this study). Unfortunately, the scarce number of sequences of SOD and TRX of flukes and mollusks on databases limited the statistical relevance of the results from the in silico analyses.
The existence of a polyclonal reaction against snails’ antigens developed in mice and rabbits immunized with the crude extract of F. hepatica rediae, and especially in mice immunized with the LMWF, supports the idea of possible mechanisms of host immune evasion through parasite molecular masking and/or mimicry. These evasion mechanisms have been reported for other trematode-snail systems (Yoshino and Bayne 1983; Adema and Loker 1997; Yoshino et al. 2013), but none previously studied on the F. hepatica-lymnaeid model. The immunoblotting assay with the total anti-rediae Pab showed a marked recognition of the antigens from both parasite and mollusks, while the specific Pab reacted only with the proteins of the rediae of molecular weights higher than 30 kDa. This constitutes the first evidence of the possible existence of immunogenic mimetic epitopes in intramolluscan stages of F. hepatica and suggests that the marked cross-reactivity with snails achieved with mouse anti-LMWF serum was due to a molecular mimicry phenomenon. The high representation of this particular low-molecular-weight fraction on the crude extract of F. hepatica rediae (and the nonexistence of these electrophoretic bands reported in miracidia), as well as the evidence of signifying mimicry between antigens of parasite and snails, leads us to think of important roles for these proteins during the intramolluscan development of the parasite.
Parasites like F. hepatica are masters of a variety of strategies for survival, and new preliminary insights into the molecular features of F. hepatica rediae have been discussed in order to extend the knowledge about parasite-snail interaction. Antigenic variation among different stages allows the parasite to overcome an amazing diversity of hostile environments from free-living nonfeeding larvae to snails and mammalian hosts, ensured by accurate regulation routes. Also, parasite evasion mechanisms of host immunity like molecular mimicry elude the self-nonself-recognition, thus compromising the effective activation of defense strategies. Further studies are required to achieve a more comprehensive overview of the development of intramolluscan stages of F. hepatica.
In terms of Mab generation for detecting ongoing F. hepatica infection in snails, the redia rises as the most promising larval stage not only because it is the long-lasting intramolluscan larva (Rondelaud et al. 2009) but also for the discussed evidences of antigenic variations between different stages of the parasite that occurred within the snails. Moreover, the existence of specific immunodominant epitopes in the crude extract of this stage, as was assessed with the specific rabbit anti-rediae Pab, strongly supports this view. These results provide a feasible initiation point for future investigations into immunoassay development aimed at the epidemiological surveillance of snail populations of intermediate hosts of F. hepatica.
References
Adema CM, Loker ES (1997) Specificity and immunobiology of larval digenean-snail associations. In: Fried B, Graczyk TK (eds) Advances in trematode biology. CRC, Boca Raton, USA, pp 229–263
Andrews SJ (1999) The life cycle of Fasciola hepatica. In: Dalton JP (ed) Fasciolosis. CAB International, Wallingford, UK, pp 1–30
Angeletti RH (1999) Design of useful peptide antigens. J Biomol Tech 10(1):2–10
Caron Y, Rondelaud D, Rosson B (2008) The detection and quantification of a digenean infection in the snail host with emphasis in Fasciola sp. Parasitol Res 103:735–744
Caron Y, Righi S, Lempereur L, Saegerman C, Losson B (2011) An optimized DNA extraction and multiplex PCR for the detection of Fasciola sp. in lymnaeid snails. Vet Parasitol 178(1–2):93–99
Espinoza JR, Maco V, Marcos L, Saez S, Neyra V, Terashima A, Salmavides F, Gotuzzo E, Chavarry E, Huaman MC, Bargues MD, Valero MA, Mas-Coma S (2007) Evaluation of Fas2-ELISA for the serological detection of Fasciola hepatica infection in humans. Am J Trop Med Hyg 76(5):977–982
Gourbal BEF, Guillou F, Mitta G, Sibille P, Thèron A, Pointier JP, Coustau C (2008) Excretory–secretory products of larval Fasciola hepatica investigated using a two-dimensional proteomic approach. Mol Biochem Parasitol 161:63–66
Hernández H, Marcet R, Martínes AR, Gutiérrez A, Sánchez J, Sarracent J (2002) Obtención y caracterización de anticuerpos monoclonales contra antígenos de miracidios de Fasciola hepatica. J Med Appl Malacol 11:35–40
Humiczewska M (1975) Oxidative enzymes in the development of Fasciola hepatica L. IV. The activity of oxidases and dehydrogenases in redia. Folia Histochem Cytochem (Krakow) 13(3–4):161–174
Kaplan RM, Dame JB, Reddy GR, Courtney CH (1997) The prevalence of Fasciola hepatica in its snail intermediate host determined by DNA probe assay. Int J Parasitol 27(12):1585–1593
Laemli U (1970) Clevage of structural proteins during the assembling of the head of bacteriophage T4. Nature 227:680–685
Liénard MA, Lassance JM, Paulmier I, Picimbon JF, Löfstedt C (2006) Differential expression of cytochrome c oxidase subunit III gene in castes of the termite Reticulitermes santonensis. J Insect Physiol 52(6):551–557
Mas-Coma S, Bargues MD, Valero MA (2009) Chapter 2. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv Parasitol 69:41–146
Merrifield B (1986) Solid phase synthesis. Science 232:341–347
Mourão MM, Dinguirard N, Franco GR, Yoshino TP (2009) Role of the endogenous antioxidant system in the protection of Schistosoma mansoni primary sporocysts against exogenous oxidative stress. PLoS Negl Trop Dis 3(11):e550. doi:10.1371/journal.pntd.0000550
Musatov A, Ortega-Lopez J, Robinson NC (2000) Detergent-solubilized bovine cytochrome c oxidase: dimerization depends on the amphiphilic environment. Biochemistry 39(42):12996–13004
Piedrafita D, Estuningsih E, Pleasance J, Prowse R, Raadsma HW, Meeusen EN, Spithill TW (2007) Peritoneal lavage cells of Indonesian thin-tail sheep mediate antibody-dependent superoxide radical cytotoxicity in vitro against newly excysted juvenile Fasciola gigantica but not juvenile Fasciola hepatica. Infect Immun 75(4):1954–1963
Robinson MW, Menon R, Donnelly SM, Dalton JP, Ranganathan S (2009) An integrated transcriptomics and proteomics analysis of the secretome of the helminth pathogen Fasciola hepatica: proteins associated with invasion and infection of the mammalian host. Mol Cell Proteomics 8(8):1891–1907
Rojas L, Vázquez AA, Domenech I, Robertson L (2010) Fascioliasis: can Cuba conquer this emerging parasitosis? Trends Parasitol 26(1):26–34
Rondelaud D, Belfaiza M, Vignoles P, Moncef M, Dreyfuss G (2009) Redial generations of Fasciola hepatica: a review. J Helminthol 83:245–254
Salazar-Calderón, Martín-Alonso JM, Ruiz de Eguino AD, Parra F (2001) Heterologous expression and functional characterization of thioredoxin from Fasciola hepatica. Parasitol Res 87(5):390–395
Smith PK, Krohn RI, Hermanson GT (1986) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Tielens AGM (1999) Metabolism. In: Dalton JP (ed) Fasciolosis. CAB International, UK, pp 277–306
Ubeira FM, Muiño L, Valero MA, Periago MV, Pérez-Crespo I, Mezo M, González-Warleta M, Romarís F, Paniagua E, Cortizo S, Llovo J, Más-Coma S (2009) MM3-ELISA detection of Fasciola hepatica coproantigens in preserved human stool samples. Am J Trop Med Hyg 81:156–162
Vázquez AA, Sánchez J, Pointier JP, Théron A, Hurtrez-Boussès S (2013) Fasciola hepatica in Cuba: compatibility of different isolates with two intermediate intermediate hosts, Galba cubensis and Pseudosuccinea columella. J Helminthol. doi:10.1017/S0022149X13000382
Yoshino TP, Bayne CJ (1983) Mimicry of snail host antigens by miracidia and primary sporocysts of Schistosoma mansoni. Parasite Immunol 5:317–328
Yoshino TP, Wu XJ, Gonzalez LA, Hokke CH (2013) Circulating Biomphalaria glabrata hemocyte subpopulations possess shared schistosome glycans and receptors capable of binding larval glycoconjugates. Exp Parasitol 133(1):28–36
Acknowledgments
The authors would like to thank Dr. Osvaldo Reyes from the Havana Center of Genetic Engineering and Biotechnology for the assistance on the peptide synthesis and conjugation. We would also like to thank an anonymous reviewer for the proper comments and suggestions. In addition, we would like to express our thanks to the editor who smoothed the English.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1.1
Multiple sequence alignment of COX I of F. hepatica with their corresponding homologous proteins from flukes (Digenea) (PNG 957 kb)
Supplementary Material 1.2
Multiple sequence alignment of COX I of F. hepatica with their corresponding homologous proteins from mollusks (Panpulmonata) (PNG 600 kb)
Rights and permissions
About this article
Cite this article
Alba, A., Hernández, H.M., Marcet, R. et al. Exploring the antigenic features of Fasciola hepatica rediae (Trematoda: Digenea) through the evaluation of different antigenic candidates for further monoclonal antibody generation. Parasitol Res 113, 3185–3193 (2014). https://doi.org/10.1007/s00436-014-3981-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3981-y