Abstract
There is a significant genetic diversity of Toxoplasma gondii in Brazil. Two parasite isolates were recently obtained from chickens in Uberlândia, Minas Gerais state, Brazil, namely, TgChBrUD1 and TgChBrUD2. In this study, we investigated Calomys callosus susceptibility to these atypical T. gondii strains. Male and female animals were intraperitoneally infected with tachyzoites and monitored to evaluate body weight change, morbidity, and mortality. Immunohistochemical assay and qPCR were performed to determine the parasitism in liver, spleen, and brain. Our data showed that TgChBrUD2-infected males died earlier than TgChBrUD1-infected males and 100 % of mortality was observed after 10 and 12 days of infection, respectively. Also, TgChBrUD1-infected females died earlier than TgChBrUD1-infected males and 100 % of mortality was observed after 9 and 12 days of infection, respectively. Both strains were able to induce a decrease in body weight of males, but only the TgChBrUD1 strain induced an increase in body weight of females. TgChBrUD2-infected females had significantly higher parasite load in both liver and spleen in comparison to TgChBrUD1-infected females, but no significant difference was found between genders or strains when males were infected. There was higher parasitism in the liver than the brain from both males and females infected with either strain. In conclusion, C. callosus specimens are susceptible to both T. gondii atypical strains with differences between males and females in severity of infection. These findings open new prospects for understanding different aspects of T. gondii infection, including reinfection and vertical transmission with these atypical strains when utilizing this experimental model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The protozoan Toxoplasma gondii is an obligate intracellular parasite that infects a wide range of hosts (Cenci-Goga et al. 2011). It causes a relevant zoonotic infection, as it is primarily defined as an opportunistic pathogen in humans (Sibley 2011). Infection with Toxoplasma gondii is generally acquired by ingestion of food or water contaminated with oocysts, which are shed with cat feces or consumption of raw or undercooked meat products containing tissue cysts (Elmore et al. 2010). Infection in humans is frequently asymptomatic, but it can lead to severe disease in immunocompromised patients and congenitally infected individuals (Dubey 2010).
A recent study showed high genetic diversity of Toxoplasma gondii and the isolates collected worldwide can be roughly grouped into six clades (Su et al. 2012). The genetic differences among these genotypes at genome sequence level are generally low, but there are differences in the virulence phenotypes for mice. Type I strains are highly virulent and infection with a single parasite results in the death of mice. In contrast, types II and III are relatively avirulent, frequently resulting in chronic infections in mice (Howe and Sibley 1995). A fourth clonal lineage, referred as type 12, has recently been described in North America, where it is commonly found in wildlife. Type 12 strains in mice revealed that some of them express intermediate or high levels of acute virulence (Khan et al. 2011a). On the other hand, an entirely distinct pattern is seen in South America, where an abundance of atypical (non-clonal) strain types have been found and show markedly greater diversity and divergence within and between groups (Khan et al. 2007, 2011b; Shwab et al. 2013).
In Brazil, a higher genetic diversity was identified and the three most common genotypes were #6, #8, and #11, previously designated as type BrI, BrIII, and BrII, respectively (Pena et al. 2008; Dubey et al. 2012). Analysis of mortality rates in infected mice indicated that type BrI is highly virulent, type BrIII is nonvirulent, whereas type BrII lineages are intermediately virulent (Pena et al. 2008). Two parasite isolates were recently obtained from chickens in Uberlândia city and they were named TgChBrUD1 and TgChBrUD2. The isolate TgChBrUD1 exhibited ToxoDB polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) genotype #11 (also known as type BrII) and the TgChBrUD2 isolate exhibited ToxoDB PCR-RFLP genotype #6 (also known as type BrI Africa 1) (Salomão, personal communication). Recent studies have suggested a relationship between virulence and the genetic makeup of the different strains of Toxoplasma gondii present in the environment and in cases of human and animal diseases (Beck et al. 2009; Herrmann et al. 2012; Dubremetz and Lebrun 2012; Su et al. 2012). These phenotypic variations can be important to determine the role of Toxoplasma gondii genetic diversity in transmission between species, pathogenicity and immunological response, in addition to implications for diagnosis, treatment, and prevention of toxoplasmosis (Beck et al. 2009; Dubey et al. 2012; Su et al. 2012).
The possibility that the parasite genotype influences the severity of human disease is supported by differences in pathogenicity of parasite strain in animal models (Sibley and Boothroyd 1992). A study with Toxoplasma gondii isolates from asymptomatic chickens from Brazil showed that these Brazilian isolates were more pathogenic to mice than isolates from Europe or North America, irrespective of the genotype (Dubey et al. 2006).
Calomys callosus, a rodent of the family Cricetidae widely distributed in Central Brazil, has been described as a reservoir for various infections agents (Borges et al. 1992; Favoreto-Junior et al. 1998). Adapted to laboratory studies, this species has been used as experimental model to study Toxoplasma gondii infection (Favoreto-Junior et al. 1998; Ferro et al. 1999, 2002; Franco et al. 2011). Also, our previous studies have demonstrated that C. callosus is a useful experimental model for congenital toxoplasmosis (Ferro et al. 1999, 2002; Barbosa et al. 2007; Franco et al. 2011). This rodent was shown to be highly susceptible to infection by Toxoplasma gondii RH strain with 100 % mortality after 9 days of infection (Favoreto-Junior et al. 1998). In addition, this species is able to induce brain tissue cyst formation as well as ocular lesions when infected by Toxoplasma gondii ME-49 strain (Pereira et al. 1999).
Considering that Brazilian isolates are more virulent than those from Europe or North America and that C. callosus may be considered a suitable experimental model to study different features of Toxoplasma gondii infection, the present study aimed to evaluate the influence of C. callosus gender when infected with atypical Toxoplasma gondii Brazilian isolates.
Materials and methods
Animals
Calomys callosus specimens were kept under standard conditions on a 12-h light, 12-h dark cycle in a temperature-controlled room (25 ± 2 °C) with food and water ad libitum in the Animal Experimentation Center, Federal University of Uberlândia, Brazil. Animal experiments and procedures were conducted according to institutional guidelines for ethics in animal experimentation.
Parasite strains
Tachyzoites of Toxoplasma gondii TgChBrUD1 and TgChBrUD2 strains were obtained initially from peritoneal exudates of previously infected Swiss mice and then maintained by serial passages in human fibroblast (HFF) cells. The cell culture-derived parasites were stained with 0.4 % Trypan blue and viable parasites were counted in a hemocytometric chamber for further experimental infection.
Experimental infections
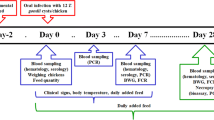
Males and females (n = 24) of C. callosus were intraperitoneally infected with 100 tachyzoites of the TgChBrUD1 or TgChBrUD2 strain. The animals were monitored to evaluate body weight change, morbidity, and mortality for 15 days. Morbidity was assessed based on the clinical parameters as previously described (Bartley et al. 2006) with modifications as follows: sleek/glossy coat, bright and active (score 0); hunched, starry stiff coat (score 1); and reluctance to move (score 2).
In a second set of experiments, animals from both genders (n = 24) were infected as described above and euthanized at 8 days post-inoculation (p.i.), when their peritoneal exudates were examined microscopically to confirm Toxoplasma gondii infection. Spleen, liver, and brain were collected for further detection of tissue parasitism.
Quantitative real-time PCR
Spleen, liver, and brain harvested from C. callosus at 8 days p.i. were frozen in liquid nitrogen. Total DNA was extracted from 20 mg of tissues using Wizard® Genomic DNA Purification Kit (Promega Co., Madison, WI, USA), according to the manufacturer’s instructions. Total DNA was quantified by UV spectrophotometry (ND1000 Spectrophotometer; NanoDrop Technologies, Wilmington, Delaware, USA). Real-time PCR was performed with a StepOnePlus® Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA) and Go Taq® quantitative real-time PCR (qPCR) Master Mix Kit (Promega Co.), according to the manufacturer’s instructions. The reaction conditions followed the protocols of Wahab et al. (2010). The primers (forward, 5′-CACAGAAGGGACAGAAGT-3′ and reverse, 5′-TCGCCTTCATCTACAGTC-3′) were used for amplification of Toxoplasma gondii as described by Homan et al. (2000), and amplify a 529-bp fragment, AF146527 [Genbank]. Positive and negative parasite controls were included in each assay. The reaction was carried out with 200 ng of DNA targets and 100-ng DNA standard curve was concomitantly performed on each reaction in a seven times dilution series.
Immunohistochemical assay
For immunolocalization of the parasites in spleen, liver, and brain tissues, formalin-fixed samples were dehydrated and embedded in paraffin. Sections measuring 4 μm in thickness were placed on glass slides and processed as previously described (Ferro et al. 2002). Briefly, samples were first incubated with 5 % acetic acid to block endogenous alkaline phosphatase and then with 2 % normal goat serum to block nonspecific binding sites. Next, samples were incubated at 4 °C overnight with mouse anti-Toxoplasma gondii polyclonal serum (1:100), which was produced by our laboratory by infecting Swiss mice with ME-49 strain, and then with biotinylated goat anti-mouse IgG (1:600) (Sigma-Aldrich, St. Louis, MO, USA). The reaction was amplified by avidin–biotin–alkaline phosphatase system (ABC kit, PK-4000; Vector Laboratories, Inc., Burlingame, USA) and developed with fast red-naphthol (Sigma-Aldrich). Samples were counterstained with Harris’s hematoxylin and examined under light microscopy.
Statistical analysis
The data were analyzed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA). Differences between groups were analyzed by the Mann–Whitney test. The Kruskal–Wallis test and Dunn multiple post-test were used to compare the parasitism between organs. The Kaplan–Meier analysis was applied to estimate survival rates after infection and survival curves were compared using the log-rank test. Values of P < 0.05 were considered as statistically significant.
Results
Survival rate
Mortality of TgChBrUD1-infected males was detected as early as 9 days, reaching 100 % by 12 days p.i. (Fig. 1a). When males were infected with the TgChBrUD2 strain, the mortality also occurred early (9 days p.i.), but all animals died at 10 days p.i., showing survival times significantly lower as compared to the TgChBrUD1-infected males (P = 0.0423) (Fig. 1a). On the other hand, the mortality of the infected females was detected as early as 8 days, reaching 100 % by 9 days p.i. with the TgChBrUD1 strain and 13 days p.i. with the TgChBrUD2 strain, but with no significant difference in survival curves (P = 0.1820) (Fig. 1b). Comparison between males and females infected with the TgChBrUD1 strain showed that females died earlier than males (median survival 9.0 and 10.5 days, respectively; P = 0.0038) (Fig. 1c). In contrast, TgChBrUD2-infected males and females showed similar median survival (9.5 days), with no significant difference between survival curves (P = 0.5944) (Fig. 1d).
Survival curves of C. callosus after infection with the TgChBrUD1 or TgChBrUD2 strain of T. gondii. Animals (six per group) were infected with 100 tachyzoites of the TgChBrUD1 or TgChBrUD2 strain and monitored for 15 days. a Comparison between TgChBrUD1- and TgChBrUD2-infected males; b Comparison between TgChBrUD1- and TgChBrUD2-infected females; c Comparison between TgChBrUD1-infected males and females; d Comparison between TgChBrUD2-infected males and females. The P values were determined by Log-rank test
Body weight change and morbidity
Infected males showed slight body weight changes in the initial days after infection with TgChBrUD1 or TgChBrUD2 strain, but from the sixth day p.i. onwards, TgChBrUD1-infected males started to lose weight, with significantly higher weight loss than the TgChBrUD2-infected males (Fig. 2a). In contrast, TgChBrUD1-infected females showed increasing body weight, whereas those infected with TgChBrUD2 had body weight fluctuations until the seventh day p.i. and started to lose weight onwards, with significantly lower weight loss than those infected with the TgChBrUD1 strain (Fig. 2b). Comparison between males and females showed that the TgChBrUD1 strain caused opposite effects in body weight, with weight gain in females and weight loss in males (P = 0.0003) (Fig. 2c). In contrast, the TgChBrUD2 strain caused weight loss from the eighth day p.i. onwards in both females and males, with a tendency to be higher in females in the eighth and ninth day p.i. (Fig. 2d).
Body weight change of C. callosus after infection with the TgChBrUD1 or TgChBrUD2 strain of T. gondii. Animals (six per group) were infected with 100 tachyzoites of the TgChBrUD1 or TgChBrUD2 strain and monitored for 15 days. a Comparison between TgChBrUD1-and TgChBrUD2-infected males; b Comparison between TgChBrUD1- and TgChBrUD2-infected females; c Comparison between TgChBrUD1-infected males and females; d Comparison between TgChBrUD2-infected males and females. The P values were determined by Mann-Whitney test
Males infected with TgChBrUD1 or TgChBrUD2 strain showed no change in morbidity scores in the initial days’ p.i., starting to be hunched with starry stiff coat in the sixth and seventh day p.i with the TgChBrUD1 and TgChBrUD2 strains, respectively (Fig. 3a). At 8 days p.i., morbidity scores were higher in TgChBrUD1- than TgChBrUD2-infected males (P < 0.05) (Fig. 3a). Females infected with TgChBrUD1 showed higher morbidity scores in the sixth, seventh, and eight day p.i. in comparison to females infected with TgChBrUD2 (Fig. 3b). Reluctance to move was observed 1 day before death in animals of all groups. Comparison between males and females showed that the TgChBrUD1 strain induced higher morbidity in females than males, with significant differences in the sixth, seventh, and eight day p.i (Fig. 3c). In contrast, the TgChBrUD2 strain caused higher morbidity in males than females, with significant differences in the seventh, eighth, and ninth day p.i (Fig. 3c).
Effect of T. gondii infection with the TgChBrUD1 or TgChBrUD2 strain in clinical parameters of C. callosus. Animals (six per group) were infected with 100 tachyzoites of the TgChBrUD1 or TgChBrUD2 strain and monitored for 15 days. a Comparison between TgChBrUD1- and TgChBrUD2-infected males; b Comparison between TgChBrUD1- and TgChBrUD2-infected females; c Comparison between TgChBrUD1-infected males and females; d Comparison between TgChBrUD2-infected males and females. **P < 0.01; ***P < 0.001 (Mann-Whitney test)
The mortality, body weight and morbidity data from male and female C. callosus infected with TgChBrUD1 and TgChBrUD2 strain of Toxoplasma gondii were summarized in Table 1.
Tissue parasitism
The microscopic examination revealed the presence of tachyzoites in the peritoneal fluids of all animals after 8 days of infection with either strain. Tissue parasitism was detected by qPCR in liver, spleen, and brain. The TgChBrUD2-infected females had significantly higher parasite number in liver and spleen in comparison to TgChBrUD1-infected females (Fig. 4a, b), but with no significant difference in brain tissue parasitism (Fig. 4c). Also, no significant difference in liver, spleen, and brain parasitism was observed between TgChBrUD1- and TgChBrUD2-infected males as well as between males and females infected with either strain (Fig. 4a–c). Comparison between organs showed a significantly higher tissue parasitism in the liver compared to the brain of males or females infected with the TgChBrUD1 or TgChBrUD2 strains (Fig. 5a–d). Immunohistochemical assays confirmed the presence of Toxoplasma gondii in the liver, spleen, and brain (Fig. 6a–c).
Parasite burden in the liver (a), spleen (b), and brain (c) tissues from C. callosus infected with the TgChBrUD1 or TgChBrUD2 strain of T. gondii. Animals (six per group) were infected with 100 tachyzoites of the TgChBrUD1 or TgChBrUD2 strain, euthanized at 8-day post-inoculation and infected tissues were analyzed by real-time PCR. *P < 0.05 (Mann–Whitney test)
Comparative parasite burden in the liver, spleen, and brain tissues from C. callosus infected with the TgChBrUD1 or TgChBrUD2 strain of T. gondii. Animals (six per group) were infected with 100 tachyzoites of the TgChBrUD1 or TgChBrUD2 strain, euthanized at 8-day post-inoculation and infected tissues were analyzed by real-time PCR. a TgChBrUD1-infected males; b TgChBrUD1-infected females; c TgChBrUD2-infected males; d TgChBrUD2-infected females. *P < 0.05 (Kruskal–Wallis test)
Representative photomicrographs of liver, spleen, and brain tissues from C. callosus infected with the TgChBrUD1 or TgChBrUD2 strain of T. gondii. Arrows indicate parasites inside the parasitophorous vacuoles. Animals (six per group) were infected with 100 tachyzoites of the TgChBrUD1 or TgChBrUD2 strain, euthanized at 8-day post-inoculation and infected tissues were analyzed by immunohistochemical assays. a Liver, b spleen, and c brain tissues from TgChBrUD2-infected females
Discussion
Toxoplasma gondii has a complex life cycle, involving wide range of intermediate hosts and obligate intracellular stages (Cenci-Goga et al. 2011). Within this host range, susceptibility to infection, as well as the features of acute disease is extremely variable. Differences in susceptibility to infection with Toxoplasma gondii in different hosts have been attributed to the parasite stage (tachyzoite or bradyzoite), inoculation route, host genetic background, and parasite strain (Munoz et al. 2011; Dubremetz and Lebrun 2012).
In the present study, we observed that C. callosus are susceptible to both TgChBrUD1 and TgChBrUD2 atypical strains, but significant differences in the severity of infection were observed between males and females. Our data showed that infected males and females died during the acute phase, between 9 and 13 days p.i. with either strain. Furthermore, the presence of tachyzoites in peritoneal fluids, weight loss, and several clinical signs were observed after infection. In mice, the acute phase of infection with proliferating tachyzoites lasts approximately 14 to 21 days when infected with intermediate or avirulent strains. In this case, the tachyzoites proliferation is controlled by the host immune response, and a small subpopulation that converts to bradyzoites, forming tissue cysts in the musculature and central nervous system, which results in a lifelong chronic infection (Munoz et al. 2011). This is true for C. callosus that are susceptible to infection by Toxoplasma gondii clonal RH strain, but are resistant to ME-49 clonal strain with formation of tissue cysts (Favoreto-Junior et al. 1998; Pereira et al. 1999). In addition, there are several numbers of diverse genotypes of Toxoplasma gondii that may be associated with severe infections (Beck et al. 2009). Previous studies analyzed the virulence of Toxoplasma gondii strains obtained from domestic animals in the states of Minas Gerais and São Paulo in Brazil, and showed higher prevalence of samples with high/intermediate virulence phenotype (Brandão et al. 2006; Pena et al. 2008). Recently, a study reported high genotypic diversity in parasites isolated from newborns with congenital toxoplasmosis in Brazil (Carneiro et al. 2013).
It is well known that sex steroids regulate a variety of functions such as growth, reproduction, and differentiation. More recently, evidence has accumulated that gender may also play an important role in susceptibility to infectious diseases (de Souza et al. 2001; Roberts et al. 2001; Nava-Castro et al. 2012). The ability of a parasite to differentially affect a female or a male of the same species (sexual dimorphism of an infection) can be due to the regulation of the immune response by sex hormones (Roberts et al. 1995; Walker et al. 1997; do Prado et al. 1997, 1999). Sex hormones can alter susceptibility to parasitic infection by enhancing or suppressing the immune system or by influencing the growth and development of the parasite in the host (Roberts et al. 2001). Many parasites change the concentration of steroid hormones in infected hosts, including Toxoplasma gondii (Flegr et al. 2008; Duneau and Ebert 2012). There is abundant evidence that sex steroid hormones affect the course of toxoplasmosis in humans and mice. Studies suggest that sex hormones such as estrogen can influence macrophage activity and modulate IFN-γ levels (Chao et al. 1994). A study with mice showed that infected females die significantly earlier than males, an effect associated with greater number of tachyzoites and necrosis in small intestine of females. In contrast, testosterone treatment of female mice reduced intestinal parasite numbers and pathology (Liesenfeld et al. 2001). It was also observed that a decrease occurred in testosterone levels for both male and female mice compared with uninfected animals, indicating that the infection induced a change in testosterone metabolism (Kankova et al. 2011). Interestingly, the differences according to gender are dependent on the parasites considered. Studies with C. callosus infected with Trypanosoma cruzi showed that sexual dimorphism seemed to predict the fate of infection, since males always displayed the highest percentage of positivity. These findings suggest that testosterone can influence the immune response of C. callosus, reflecting an enhanced susceptibility of males to Trypanosoma cruzi infection (Lourenço et al. 2008). Therefore, orchiectomized C. callosus displayed a lesser number of blood parasites when compared to its sham and control counterparts, indicating that steroid gonadal ablation actually influences the immune response, which led animals to become more resistant against Trypanosoma cruzi infection (Pinto et al. 2010).
In the present study, mortality of C. callosus females was earlier than males when infected with TgChBrUD1, but it was similar in males and females after TgChBrUD2 infection. However, the TgChBrUD2 infection in males caused lower survival than that induced by TgChBrUD1. These findings show that the gender and the strain type influence the infection outcome in C. callosus, with the TgChBrUD1 strain more virulent to females and the TgChBrUD2 strain more virulent to males. Moreover, males had weight loss independently of the strain type, even though it was more pronounced with the TgChBrUD1 strain. In contrast, TgChBrUD1-infected females had weight gain whereas those infected with TgChBrUD2 had weight loss. These findings show that the TgChBrUD2 strain induces weight loss in both males and females, but the TgChBrUD1 strain causes opposite effects in body weight according to the C. callosus gender. When morbidity scores were evaluated, the TgChBrUD1 strain induced higher morbidity in females than males, whereas the TgChBrUD2 strain caused higher morbidity in males than females. Also, the TgChBrUD1 strain induced higher morbidity than TgChBrUD2 in both males and females. Again, these data indicate a strong dependence of the parasite strain and the gender on the severity of infection in C. callosus, with the TgChBrUD1 strain more severe to females and the TgChBrUD2 strain more severe to males. The analysis of tissue parasitism showed higher parasite number in liver and spleen from females infected with TgChBrUD2 than TgChBrUD1, but with no significant difference between genders or strains when males were infected. These findings indicate that the tissue parasitism in the acute phase is influenced by the gender in C. callosus, notably with the TgChBrUD2 strain in females.
In conclusion, C. callosus specimens are susceptible to both atypical Toxoplasma gondii strains with significant differences between males and females in severity of infection. These findings open new perspectives to understand different aspects of Toxoplasma gondii infection in C. callosus, including reinfection and vertical transmission with these atypical strains in this experimental model.
References
Barbosa BF, Silva DA, Costa IN, Pena JD, Mineo JR, Ferro EA (2007) Susceptibility to vertical transmission of Toxoplasma gondii is temporally dependent on the preconceptional infection in Calomys callosus. Placenta 28(7):624–30
Bartley PM, Wright S, Sales J, Chianini F, Buxton D, Innes EA (2006) Long-term passage of tachyzoites in tissue culture can attenuate virulence of Neospora caninum in vivo. Parasitology 135:421–432
Beck HP, Blake D, Dardé ML, Felger I, Pedraza-Díaz S, Regidor-Cerrillo J, Gómez-Bautista M, Ortega-Mora LM, Putignani L, Shiels B, Tait A, Weir W (2009) Molecular approaches to diversity of populations of apicomplexan parasites. Int J Parasitol 39(2):175–189
Borges MM, Andrade SG, Pilatti CG, Prado JC, Kloetzel JK (1992) Macrophage activation and histopathological findings in Calomys callosus and swiss mice infected with several strains of Trypanosoma cruzi. Mem Inst Oswaldo Cruz 87(4):493–502
Brandão GP, Ferreira AM, Melo MN, Vitor RWA (2006) Characterization of Toxoplasma gondii from domestic animals from Minas Gerais. Parasite 13(2):143–149
Carneiro AC, Andrade GM, Costa JG, Pinheiro BV, Vasconcelos-Santos DV, Ferreira AM, Su C, Januário JN, Vitor RW (2013) Genetic characterization of Toxoplasma gondii revealed highly diverse genotypes for isolates from newborns with congenital toxoplasmosis in southeastern Brazil. J Clin Microbiol 51(3):901–907
Cenci-Goga BT, Rossitto PV, Sechi P, Mccrindle CM, Cullor JS (2011) Toxoplasma in animals, food, and humans: an old parasite of new concern. Foodborne Pathog Dis 8(7):751–762
Chao TC, Van Alten PJ, Walter RJ (1994) Steroid sex hormones and macrophage function: modulation of reactive oxygen intermediates and nitrite release. Am J Reprod Immunol 32:43–52
de Souza EM, Rivera MT, Araújo-Jorge TC, de Castro SL (2001) Modulation induced by estradiol in the acute phase of Trypanosoma cruzi infection in mice. Parasitol Res 87(7):513–520
do Prado JC Jr, Leal MP, Anselmo-Franci JA, de Andrade júniur HF, Kloetzel JK (1997) Influence of female gonadal hormones on the parasitemia of female Calomys callosus infected with the “Y” strain of Trypanosoma cruzi. Parasitol Res 84(2):100–105
do Prado JC Jr, Levy AM, Leal MP, Bernard E, Kloetzel JK (1999) Influence of male gonadal hormones on the parasitemia and humoral response of male Calomys callosus infected with the Y strain of Trypanosoma cruzi. Parasitol Res 85(10):826–829
Dubey JP (2010) Toxoplasmosis of animals and humans. Boca Raton, Florida
Dubey JP, Gennari SM, Labruna MB, Camargo LMA, Vianna MCB, Marcet PL, Lehmann T (2006) Characterization of Toxoplasma gondii isolates in free-range chickens from Amazon, Brazil. J Parasitol 92(1):36–40
Dubey JP, Lago EG, Gennari SM, Su C, Jones JL (2012) Toxoplasmosis in humans and animals in Brazil: high prevalence, high burden of disease, and epidemiology. Parasitology 139(11):1375–1424
Dubremetz JF, Lebrun M (2012) Virulence factors of Toxoplasma gondii. Microbes Infect 14(15):1403–1410
Duneau D, Ebert D (2012) Host sexual dimorphism and parasite adaptation. PLoS Biol 10(2):e1001271
Elmore SA, Jones JL, Conrad PA, Patton S, Lindsay DS, Dubey JP (2010) Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol 26(4):190–196
Favoreto-Junior S, Ferro EAV, Clemente D, Silva DAO, Mineo JR (1998) Experimental infection of Calomys callosus (Rodentia, Cricetidae) by Toxoplasma gondii. Mem Inst Oswaldo Cruz 93(1):103–107
Ferro EAV, Bevilacqua E, Favoreto-Junior S, Silva DAO, Mortara RA, Mineo JR (1999) Calomys callosus (Rodentia: Cricetidae) trophoblast cells as host cells to Toxoplasma gondii in early pregnancy. Parasitol Res 85:647–654
Ferro EAV, Silva DAO, Bevilacqua E, Mineo JR (2002) Effect of Toxoplasma gondii infection kinetics on trophoblast cell population in Calomys callosus, a model of congenital toxoplasmosis. Infect Immun 70:7089–7094
Flegr J, Lindová J, Kodym P (2008) Sex-dependent toxoplasmosis-associated differences in testosterone concentration in humans. Parasitology 135(4):427–431
Franco PS, Silva DA, Costa IN, Gomes AO, Silva AL, Pena JD, Mineo JR, Ferro EA (2011) Evaluation of vertical transmission of Toxoplasma gondii in Calomys callosus model after reinfection with heterologous and virulent strain. Placenta 32:116–120
Herrmann DC, Bärwald A, Maksimov A, Pantchev N, Vrhovec MG, Conraths FJ, Schares G (2012) Toxoplasma gondii sexual cross in a single naturally infected feline host: generation of highly mouse-virulent and avirulent clones, genotypically different from clonal types I, II and III. Vet Res 43:39
Homan WL, Vercammen M, De Braekeleer J, Verschueren H (2000) Identification of a 200- to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int J Parasitol 30(1):69–75
Howe DK, Sibley LD (1995) Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis 172(6):1561–1566
Kankova S, Kodym P, Flegr J (2011) Direct evidence of Toxoplasma-induced changes in serum testosterone in mice. Exp Parasitol 128(3):181–183
Khan A, Fux B, Su C, Dubey JP, Darde ML, Ajioka JW, Rosenthal BM, Sibley LD (2007) Recent transcontinental sweep of Toxoplasma gondii driven by a single monomorphic chromosome. Proc Natl Acad Sci U S A 104(37):14872–14877
Khan A, Dubey JP, Su C, Ajioka JW, Rosenthal BM, Sibley LD (2011a) Genetic analyses of atypical Toxoplasma gondii strains reveal a fourth clonal lineage in North America. Int J Parasitol 41(6):645–655
Khan A, Miller N, Roos DS, Dubey JP, Ajzenberg D, Dardé ML, Ajioka JW, Rosenthal B, Sibley LD (2011b) A monomorphic haplotype of chromosome Ia is associated with widespread success in clonal and nonclonal populations of Toxoplasma gondii. mBio 2(6):e00228–11
Liesenfeld O, Nguyen TA, Pharke C, Suzuki Y (2001) Importance of gender and sex hormones in regulation of susceptibility of the small intestine to peroral infection with Toxoplasma gondii tissue cysts. J Parasitol 87(6):1491–1493
Lourenço AM, Levy AM, Caetano LC, Carraro Abrahão AA, Prado JC Jr (2008) Influence sexual dimorphism on the persistence of blood parasites in infected Calomys callosus. Res Vet Sci 85(3):515–521
Munoz M, Liesenfeld O, Heimesaat MM (2011) Immunology of Toxoplasma gondii. Immunol Rev 240(1):269–285
Nava-Castro K, Hernández-Bello R, Muñiz-Hernández S, Camacho-Arroyo I, Morales-Montor J (2012) Sex steroids, immune system, and parasitic infections: facts and hypotheses. Ann N Y Acad Sci 1262:16–26
Pena HFJ, Gennari SM, Dubey JP, Su C (2008) Population structure and mouse-virulence of Toxoplasma gondii in Brazil. Int J Parasitol 38(5):561–569
Pereira MF, Silva DAO, Ferro EAV, Jr M (1999) Acquired and congenital ocular toxoplasmosis experimentally induced in Calomys callosus (Rodentia, Cricetidae). Mem Inst Oswaldo Cruz 94(1):103–114
Pinto AC, Caetano LC, Levy AM, Fernandes RD, Santos CD, do Prad JC Jr (2010) Experimental Chagas’ disease in orchiectomized Calomys callosus infected with the CM strain of Trypanosoma cruzi. Exp Parasitol 124(2):147–152
Roberts CW, Cruickshank SM, Alexander J (1995) Sex-determined resistance to Toxoplasma gondii is associated with temporal differences in cytokine production. Infect Immun 63(7):2549–2555
Roberts CW, Walker W, Alexander J (2001) Sex-associated hormones and immunity to protozoan parasites. Clin Microbiol Rev 14(3):476–488
Shwab EK, Zhu XQ, Majumdar D, Pena HFJ, Gennari SM, Dubey JP, Su C (2013) Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology. doi:10.1017/S0031182013001844
Sibley LD (2011) Invasion and intracellular survival by protozoan parasites. Immunol Rev 240(1):72–91
Sibley LD, Boothroyd JC (1992) Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature 359(6390):82–85
Su C, Khan A, Zhou P, Majumdar D, Ajzenberg D, Darde ML, Zhu XQ, Ajioka JW, Rosenthal BM, Dubey JP, Sibley LD (2012) Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proc Natl Acad Sci U S A 109(15):5844–5849
Wahab T, Edvinsson B, Palm D, Lindh J (2010) Comparison of the AF146527 and B1 repeated elements, two real-time PCR targets used for detection of Toxoplasma gondii. J Clin Microbiol 48(2):591–592
Walker W, Roberts CW, Ferguson DJ, Jebbari H, Alexander J (1997) Innate immunity to Toxoplasma gondii is influenced by gender and is associated with differences in interleukin-12 and gamma interferon production. Infect Immun 65(3):1119–1121
Acknowledgments
This work was supported by Brazilian Research Funding Agencies (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa de Minas Gerai (FAPEMIG) and Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq)).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Franco, P.S., Ribeiro, M., Lopes-Maria, J.B. et al. Experimental infection of Calomys callosus with atypical strains of Toxoplasma gondii shows gender differences in severity of infection. Parasitol Res 113, 2655–2664 (2014). https://doi.org/10.1007/s00436-014-3920-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3920-y