Abstract
The external morphology of adult and immature stages of mange mites of the genus Chorioptes was investigated with the aid of light and scanning electron microscopy. A molecular phylogeny of this genus was inferred based on six genes (18S, 28S rDNA, EF1-α, SRP54, HSP70, and CO1). The validity of four species (Ch. bovis, Ch. panda, Ch. texanus, and Ch. sweatmani sp. nov. described from the moose from Sweden, Finland, and Russia) was confirmed based on morphology and a Bayesian species delimitation analysis incorporating both gene tree uncertainties and incomplete lineage sorting via the coalescent process model in BPP. Sequence data for Ch. crewei and Ch. mydaus was not available but their morphology strongly suggests their validity. The six valid Chorioptes species are diagnosed using type and non-type specimens, and a key to species is provided. Ch. sweatmani differs from closely related Ch. texanus by the following features: in males, the body length, including the gnathosoma, is 380–405 μm (vs. 220–295 in Ch. texanus), the idiosoma is 3–4 times longer than setae cp (vs. 1.3-1.6 times longer), legs III are approximately three times longer than setae sRIII (vs. 1.8–2 times longer), the apical spur of tarsus III is curved (vs. straight), a spur near seta fIII base is not developed (vs. small but distinct); in females, setae h2 are 1.4–1.5 times shorter than legs IV (vs. about two times longer). Hosts and distribution records of Chorioptes species are summarized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mange mites of the genus Chorioptes (Acariformes: Psoroptidae) are permanent, highly specialized ectoparasites of various domesticated and wild artiodactyls, horses, and carnivores (ursids and badgers) (Bochkov 2010). These mites are of substantial veterinary importance causing severe economic loses (Mullen and O’Connor 2002) including decline of milk production and quality in cattle (Rehbein et al. 2005; Nong et al. 2014).
Sweatman (1957) revised species of Chorioptes and summarized the large body of known morphological and biological data. In his experiments on rearing and cross-host infestation (either directly on hosts or on their epidermal debris), he convincingly demonstrated that most of the previously known “species” of Chorioptes described from different hosts are actually not host-specific and belong to the same species, Ch. bovis (von Hering, 1845). He also recognized Ch. texanus Hirst, 1924 based on morphology, and listed several taxa of previous authors as incertae sedis.
The latest classical taxonomic treatment (Fain and Leclerc 1975) cites five Chorioptes species: Ch. bovis and Ch. texanus from various artiodactyls and the horse, Ch. crewei Lavoipierre, 1958 from a duiker in Cameroon, Ch. panda Fain and Leclerc, 1975 from several ursids, and Ch. mydaus Fain, 1975 from the Sunda stink badger. However, species delimitation in Chorioptes is complicated because of high variability of the “standard” diagnostic characters—mainly the length of the male opisthosomal setae. Zahler et al. (2001) attempted to revise this genus based on morphological and molecular data and concluded that there are only two valid species, Ch. bovis and Ch. texanus, whereas, Ch. crewei, Ch. mydaus, and Ch. panda were considered as probably invalid. Unfortunately, these authors neither reexamined the type series of Ch. panda and Ch. mydaus nor included sequences from these mites in their analyses. Later, the validity of Ch. panda was confirmed with molecular data only (Wang et al. 2012). Specimens of Ch. crewei and Ch. mydaus suitable for DNA work are still unavailable and these species are considered by some authors as questionable (Zahler et al. 2001; Hestvik et al. 2007; Suh et al. 2008). Furthermore, specimens of a putative undescribed species of Chorioptes were reported recently from the moose (Hestvik et al. 2007). Molecular (ITS-2 gene fragment) and morphometric analyses both indicated a specific status of these mites, but no formal description was made (Hestvik et al. 2007; Lusat et al. 2011). As a result, the systematics of the genus Chorioptes is unsettled, with the veterinary important species; Ch. texanus is being not clearly diagnosable due to a large number of taxa with uncertain taxonomic status.
Here, we conduct an extensive morphological analysis of Chorioptes mites; identify a novel set of diagnostic characters and use them in a new, updated key; and describe all postembryonic stages using both light and scanning electron microscopy (SEM). A new species, Ch. sweatmani sp. nov. is described from the outer ear canal of the moose in Sweden, Finland, and Russia. Our morphological study is accompanied by a phylogenetic analysis using a large set of psoroptids and outgroups (39 taxa, 6 genes) and a species delimitation analysis that accounts for both gene tree uncertainties and incomplete lineage sorting via the coalescent process model in the program BPP. In addition, host-parasite relationships of Chorioptes spp. are analyzed and all known host records are reported.
Material and methods
Collections
Mites were collected in 75 % ethanol and mounted in Hoyer’s medium. Methods of mite collection from the ears of Alces alces were described previously (Hestvik et al. 2007).
Material sources
Slide-mounted mite specimens (including type series) from the Institut Royal des Sciences Naturelles de Belgique, Brussels, Belgium (IRSNB) and the Zoological Institute of the Russian Academy of Sciences, St. Petersburg, Russia (ZISP) were examined. Additional specimens of Ch. bovis and Ch. texanus from cattle from Iceland, South Korea, and the USA were donated by colleagues and mounted by us. Specimens of Ch. sweatmani were obtained, with appropriate permits, from moose ears in Sweden and Russia collected by GH and APS from dead or freshly killed animals. Three specimens from Finland are deposited in the collection of the Institut Royal des Sciences Naturelles de Belgique, Brussels, Belgium.
Taxon sampling
DNA isolation and sequencing six genes (18S, 28S rDNA, EF1-α, SRP54, HSP70, and CO1) were sequenced. DNA extraction, rDNA secondary structure alignment, oligonucleotide primers, amplification, and sequencing of the first five genes were previously described (Klimov and O’Connor 2008; Klimov and O’Connor 2013; Knowles and Klimov 2011). Primers and protocols for amplification of CO1 are given in supplement 1. A total of 57 sequences were deposited in Genbank (KF891886-KF891942); species listing and GenBank accession numbers are given in Table 1. For several GenBank CO1 sequences, we trimmed low quality 3’ ends and corrected frameshifting errors. Our alignment matrix has a total of 10,838 nt (aligned) and 39 taxa.
Phylogenetic analyses
Models of nucleotide substitution and partition strategies were selected based on AICc in PartitionFinder ver. 1.1.1 (Lanfear et al. 2012). We explored two partition strategies (by genes and by rDNA stem and loop regions and the four coding genes) and found the following “best” partition set: rDNA stem, rDNA loop, EF1-α, SRP54 + HSP70, and CO1. We used stem and loop regions of rDNA as separate partitions because they have very different levels of saturation (Klimov and O’Connor 2008) and, therefore, may provide insights on phylogenetic signal present in the dataset. This partitioning scheme was used for RAxML and MrBayes analyses (see below), but for *BEAST we treated SRP54 and HSP70 as separate partitions since this program infers species tree based on gene trees. For all partitions but one, the general time reversible with proportion of invariable sites and gamma-distributed rate heterogeneity model (GTR + I + G) was used. For rDNA loop, the model TIM + I + G was evaluated to be the best fit, but since none of the phylogenetic programs we use here explicitly implemented this model, the nearest available model (GTR + I + G) was set for this partition.
Phylogenetic relationships were inferred in maximum likelihood and Bayesian frameworks in RAxML-HPC ver. 7.5.4 (Stamatakis et al. 2005) and MrBayes 3.2.2 (Ronquist et al. 2012) using a 52-node Mac OS X computer cluster. Four independent runs were performed for each program.
In the RAxML analyses, the model optimization precision for the final optimization of the tree topology ("-e") under GAMMAI ("-m") was set to 0.001 and a rapid bootstrap analysis (100 pseudoreplicates, "-N") followed by a search for the best-scoring ML tree was performed ("-f a"). This tree then was used to estimate the model parameters and calculate ML distances ("-f x”).
For each MrBayes analysis, we conducted two independent runs for 20 million generations each to obtain a total of 80,002 trees discarding the first 50,000 trees as burn-in. No unrealistically long trees (Brown et al. 2010; Marshall 2010) were detected by comparison of the average post-burn in tree lengths reported by Tracer v. 1.5 (Rambaut and Drummond 2009) and the maximum likelihood tree length estimate. Convergence of model parameters and topology were assessed by the standard MrBayes convergence diagnostics (i.e., the average standard deviation of split frequencies values below 0.01 and potential scale reduction factor values approaching 1.00) and the program Are We There Yet? (AWTY) (Nylander et al. 2008). Adequacy of the posterior sample size was evaluated through autocorrelation statistics as implemented in Tracer—all effective sample size values substantially exceeded 200 (e. g., 1086.6-19800.4 for one of the runs).
Trees were visualized in FigTree 1.3.1 (Drummond and Rambaut 2007). Matrices and trees from this study are available from TreeBASE (http://www.treebase.org) accession number 15054.
From both theoretical and empirical perspectives, it is widely recognized that a gene tree may be different from the true species tree, especially in cases of closely related species (short branches on the phylogeny) or species with large population sizes (Maddison 1997; Syring et al. 2007). For single-copy genes with no horizontal gene transfer and hybridization, the incongruence is likely to be due to incomplete lineage sorting (Heled and Drummond 2010). To overcome the effect of stochastic sorting of ancestral polymorphisms and to and infer accurate phylogenies at the species level, we will use six orthologous loci and the multispecies coalescent model. Unlike concatenation analyses (e. g., in RAxML, MrBayes) where all genes are forced to share the same underlying history, species tree analytical framework models the genealogical process of each gene tree as nested within the species tree while using certain coalescence assumptions (Degnan and Rosenberg 2009). We run a multilocus, species tree analysis (Degnan and Rosenberg 2009) in *BEAST ver. 1.8.0. This program simultaneously coestimates the species trees and all gene trees, with uncertainty in gene trees incorporated through a traditional MCMC analysis (Heled and Drummond 2010). Two independent analyses were run for 700 million generations each with parameters sampled every 10,000 steps. Runs were combined using the program LogCombiner v.1.4.6 (Drummond and Rambaut 2007) and burn-in samples were discarded (10,000 out of 70,001). Convergence and adequacy of the posterior sample size were determined as above for MrBayes analyses.
Species delimitation analysis
Published molecular treatments of Chorioptes used single gene trees (ITS, 18S, CO1) to infer a phylogeny (Hestvik et al. 2007; Wang et al. 2012). Genetic distances and reciprocal monophyly from these topologies were then used to find boundaries between species with no formal species delimitation analysis. Both these procedures often require subjective decisions regarding the thresholds that demark the species boundary. Recent theoretical developments indicate that inferences relying on single locus or concatenated data cannot deal with incomplete lineage sorting and thus necessarily fail to detect recently diverged lineages (Hudson and Coyne 2002; McVay and Carstens 2013). Here, we conduct species delimitation analysis using the program BPP ver. 2.2 (Rannala and Yang 2003; Yang and Rannala 2010). This method accommodates the species phylogeny as well as lineage sorting due to ancestral polymorphism, and is considered as the most accurate among other recent species delimitation algorithms (Camargo et al. 2012; Satler et al. 2013). A gamma prior G(2, 1000), with mean 2/1000 = 0.002, is used on the population size parameters (θs). The age of the root in the species tree (τ0) is assigned the gamma prior G(2, 1000), while the other divergence time parameters are assigned the Dirichlet prior (Yang and Rannala 2010: equation 2). To evaluate the influence of the ancestral population size (θ) and root age (τ0) priors on the posterior probabilities of species models, we used two additional combinations of priors (Leache and Fujita 2010): θ ∼ G(1, 10) τ0 ∼ G(1, 10) and θ ∼ G(1, 10) τ0 ∼ G(2, 1000). The latter set of priors assumes large values for θ and small values for τ0, favoring conservative models containing fewer species (Yang and Rannala 2010). Because the automatic MCMC fine-tune method experienced difficulties in convergence and mixing when using starting trees with all or most of the nodes collapsed, we adjusted fine-tune variables for MCMC moves as described in the BPP manual. For the guide tree, we selected a subtree encompassing the canonical Psoroptidae (15 terminals in the genera Otodectes, Caparinia, Psoroptes, Chorioptes; Table 1, Fig. 1). This subtree was consistently recovered by different methods of phylogenetic inference described above. There are a total of seven internal nodes; all possible combinations of resolved or collapsed internal nodes in this subtree are 19. For each analysis, we conducted 19 independent runs using each of the 19 trees as the starting tree to confirm convergence. Inter- and intraspecific genetic distances (% mean, range) of four Chorioptes species are provided in Table 2. We explored results from the two species delimitation algorithms, with and without reversible jump (rjMCMC) each (Table 3). Because there are nuclear and mitochondrial markers in our dataset, we allowed θs to vary among loci—the heredity scalar was set to 1 (18S) and 0.25 (CO1). All analyses were run for 200,000 generations and a sampling frequency of 1; the first 20,000 MCMC samples were discarded as burn-in.
a, b. Phylogenetic trees of psoroptids and relatives (39 terminals) inferred from five nuclear loci (18S, 28S rDNA, EF1-α, SRP54, HSP70) and one mitochondrial locus (CO1) (10,838 sites). Scale bars represent expected changes per site. a Maximum likelihood tree inferred by RAxML. Bootstrap support values are shown for nodes with support higher than 50 A guide tree was derived from this phylogeny for a subset of putative species used in species delimitation analyses in BPP; seven nodes are labeled so they correspond to the seven digit numbers representing the 19 species delimitation models (see Table 3 for detail); b Maximum clade credibility tree (39 individuals, 32 species) inferred in *BEAST species tree analysis. Posterior probabilities are shown for each node
Analysis of external morphology
Drawings were made with a Leica microscope equipped with differential interference contrast (Nomarsky optics) and a camera lucida. In the description below, the idiosomal setation follows Griffiths et al. (1990) with modifications of Norton (1998) for coxal setae. The leg setation follows Grandjean (1941). Names for homologous setae used by Sweatman (1958) and Fain (1963) are provided in Table 4. All measurements are given in micrometers (μm) and were taken as follows: body length = the total length from the anterior extremity of the palps to the posterior border of the body, including the lobar membranes in males; body width = width at the level of setae cp; length of dorsal shields = maximum length, measured along the median line of the shields; and length of the posterior legs = length from the most basal point of the trochanter to the apex of the tarsus, excluding pretarsus.
Small mite structures were analyzed with a scanning electron microscope (Quanta 250). Mites were put in 96 % ethanol for 24 h, transferred to hexamethyldisilazane for 10 min, and then dried and sputtered with platinum.
Differential characters of Chorioptes species are given in Table 5. Records of all hosts are summarized in Table 6. Host systematics is given after Wilson and Reeder (2005).
The following abbreviations of institutes were used: BMNH—British Museum of Natural History, London, UK; IRSNB—Institut Royal des Sciences Naturelles de Belgique, Brussels, Belgium; OSAL—Acarology Laboratory, Ohio State University, Columbus, USA; UMMZ—Museum of Zoology, the University of Michigan, Ann Arbor, USA; ZISP—Zoological Institute of the Russian Academy of Sciences, Saint Petersburg, Russia.
Results of molecular analyses
In all our analyses (Maximum likelihood, Bayesian concatenated analyses, and *BEAST species tree analysis), mites of the subfamily Psoroptinae form a monophyletic group (100 % BS, 1.0 PP) being the sister clade to the family Pyroglyphidae (house dust mites and relatives). In all trees, inferred relationships of the four included psoroptine genera (Otodectes, Caparinia, Psoroptes, and Chorioptes) were congruent. Species level relationships for our target taxon, Chorioptes, were the same across the concatenation analyses (RAxML, MrBayes) and had a high support (BS 85–100, PP 0.75–1.00), indicating robustness of our inference under different analytical approaches (Fig. 1a, b). The new species, Chorioptes sweatmani, was placed as a sister group to Ch. texanus with substantial support (BS 99, PP 0.76). In our dataset (18S, CO1) for Chorioptes species, the average corrected genetic distances range between 5.6–14.9 %, while distances within species were between 0.2–0.5 % (Table 2). Two sister species, Ch. sweatmani and Ch. texanus had the smallest interspecific distances (5.6–6.4 % vs 9.6–14.9 % for other species). The intra- and interspecific genetic distances between the four Chorioptes species do not overlap, indicating potential absence of gene flow between these species (Table 2). Coalescent-based species delimitation analysis supports the species status of all species, including C. sweatmani sp. nov, by recovering the fully resolved guide tree in all runs employing different starting trees, species delimitation algorithms, and sets of priors for the ancestral population size (θ) and root age (τ0) (Table 3).
Systematics
C sweatmani sp. nov.
(Figs. 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12 and Figs. 13, 14 and 15)
?C. texanus, Sweatman 1958: 525, Fig. 1
Chorioptes bovis, Morrison et al. 2003: 498 (misidentification)
Chorioptes sp., Hestvik et al. 2007: 4, Figs 1 and 2
Chorioptes morphotype C, Lusat et al. 2011: 372, Fig. 4
Description Male (holotype, Figs. 2, 4e, 5, 6, 13c, d, 14, 15d). Body, including gnathosoma and opisthosomal lobes, 395 μm long (380–405 in 10 paratypes), 285 wide (280–295). Gnathosoma about 40 long and 70 wide. Idiosoma. Idiosoma about 310 long in midline (excluding opisthosomal lobes), about 1.1 times longer than wide. Propodonotal shield about 60 long, bearing distinctly developed median keel and alveoli of ve. Hysteronotal shield 125 long (120–125) in midline. Distance between propodonotal and hysteronotal shields about 110. Setae d1 situated on anterior margin of hysteronotal shield. Setae h2 and h3 flattened, membranous (8–9 maximum width). Aedeagus about 10 long. Diameter of adanal suckers about 25, distance between suckers about 20. Opisthosomal lobes subquadrate, their length and width subequal, 30 long (27–31) (see Figs. 5 and 12a, b for comparison with other species). Maximum distance between lobes 37 (35–37). Lengths of setae: si 22 (21–24), se 170 (170–185), c1 25 (25–27), c2 33 (30–35), cp 115 (105–120), c3 55 (50–60), d1, d2, e1, and e2 25–30, f2 260 (240–265), h2 and h3 235–260, ps1 25 (25–32), ps2 87 (83–90), ps3 about 25, 1a 40 (38–42), 3a 62 (60–67), 4a and 4b 43–50, and g 14 (12–14). Distances between setae and levels of seta bases: c1-c1 about 160, c2-c2 about 250, d1-d1 and e1-e1 about 50, d2-d2 about 135, e2-e2 about 125, setae c1 located equidistantly (about 35) between levels c2 and d1, d1-e1 about 50, e1-d2 about 10, and e1-e2 about 60. Legs. Legs III about 200 long. Tarsus III straight, about 45 long and 35 maximum wide, with curved apical spur(see Figs. 6 and 12c, d for comparison with other species). Setae fIII bifurcate, with weakly developed ventro-anterior extension (see Figs. 15d for comparison with other species). Setae eIII about 2.5 times shorter than respective tarsus; lengths of setae wIII and pretarsus are subequal. Legs IV about 60 long. Lengths of setae and solenidia: sRIII 50–70, approximately three times shorter than leg III, kTIII about 40, dIII 520–550, ω3I 25–30, ω1I, II 18–20, φI, II 40–50, φIII 45–50, φIV 32–35, and σII about 5.
Female (10 paratypes, Figs. 3 and 4a–d, Fig. 13a, b, Fig. 15a–c). Body, including gnathosoma, 400–460 μm long, 265–310 wide. Gnathosoma about 75 long and 70 wide. Idiosoma. Idiosoma about 1.3–1.5 times longer than wide. Propodonotal shield about 100 long, bearing distinctly developed median keel and patches of setal alveoli ve. Lengths of setae: si 28–30, se 170–190, c1 24–26, c2 34–37, cp 85–105, c3 48–60, d1, d2, e1, e2, and ps1 about 25, f2, h3, ps2, and ps3 15–20, h2 80–90, 1a 50–55, 3a 70–75, 4a and 4b, and g 25–28. Distances between setae and levels of seta bases: c1-c1 about 150, c2-c2 about 250, d1-d1 and e1-e1 about 50, d2-d2, e2-e2, and ps1-ps1 about 160, setae c1 located equidistantly (about 50) between levels c2 and d1, d1-e1 about 85, e1-d2 about 35, and e1-e2 about 55. Legs. Legs III about 110 long; legs IV about 125–135 long. Pretarsus IV about 35 long. Lengths of setae and solenidia: sRIII 25–40, kTIII about 50, dIII 440–550, dIV 440–5500 long, fIII 330–360, ω3I 33–35, ω1I, II 23–25, φI, II 50–55, φIII 35–37, φIV 4–5, and σII 3–4.
Larva (10 paratypes, Figs. 7 and 8). Body 290–330 μm long and 210–230 wide. Gnathosoma. Gnathosoma having structure typical for Psoroptidae with full complement of setae. Palps 2-segmented with short apical membrane. Dorsal lobes not developed, pseudorutellar membranes of subcapitulum distinctly developed, transversally striated. Palpal setae: dTi, l”, dTa, ω, ul’, and ul”; subcapitular setae: elcp and subc. Idiosoma. Propodonotal shield about 60 long, bearing median keel and pair of small unsclerotized spots (remnants of setal alveoli ve). Hysteronotal shield absent. Openings of oil glands (gl) distinct. Opisthosomal margin widely rounded. Idiosomal setae: si, se, c1, c2, cp, c3, d1, d2, e1, e2, h2, 1a, and 3a. Setae scx present. Setae si and se situated off propodonotal shield; si located close but distinctly anterior to se. Lengths of setae: se 100, situated on small sclerotized plates, cp about 40, h2 about 30, other dorsal setae short (10–12), 1a and 3a about 20 long, c3 14–16. Setae d1 situated anterior to level of d2, distance between levels of setae d1 and d2 about 40, e1 situated anterior to level of e2, distance between levels of setae e1 and e2 about 30. Apodemes Ia free. One pair of small ventro-lateral sclerites present between coxal fields II and III. Legs. Tarsi I and II bearing dorso-apical spur. Legs III with five articulated segments. Pretarsi I and II normally developed; pretarsus III absent. Setation of legs I–III: I—tarsus d, e, f, ra, wa, la, s, ba, ω1 (in apical part of tarsus, slightly shorter than respective segment), ε (button-like), tibia gT, φ (1.8–2 times longer than respective segment), genu cG, mG, σ1I (represented only by alveolar patch), femur vF, trochanter without seta; II—tarsus d, e, f, wa, s, ba, ω1 (in median part of tarsus, slightly shorter than respective segment), tibia gT, φ (1.8–2 times longer than respective segment), genu cG, mG, σII (2 long), femur vF, trochanter without seta; III—tarsus d and f (both whip-like, longer than respective leg), e, w, tibia kTIII, and φ; other segments of leg III without setae.
Male protonymph (10 paratypes, Fig. 9). Body 330–360 μm long and 220–250 wide. Propodonotal shield about 80 long. One pair of genital papillae, setae f2, h3, ps1, ps2, ps3, and g added on idiosoma. Setae f2 situated near bases of h2. Lengths of setae: se about 120, cp about 70, h3 about 40, other dorsal setae 15–20 long; 1a about 65 long, 3a about 40, c3 about 20, other ventral setae and setae f2 10–15. Distances d1-d2 and e1-e2 30 and 40, respectively. Legs IV added, with five segments, about two times shorter than legs III. Pretarsus IV absent, Setae dIV whip-like, longer than respective leg, w, r present on tarsus IV.
Male tritonymph (10 paratypes, Fig. 10). Body 400–430 μm long and 260–290 wide. Propodonotal shield about 80 long. Second pair of genital papillae, setae 4a and 4b added on idiosoma. Lengths of setae: se about 150, cp about 100, h3 about 80, other dorsal setae and c3 20–25 long, 1a and 3a 35–38, other ventral setae and f2 8–12. Solenidion ω3 added on tarsus I, setae pRI and pRII added on trochanters I and II, respectively, setae sRIII added on trochanter III, almost twice as short as respective leg. Setae eIV (very short) and fIV (subequal in length to respective leg) added on tarsus IV; kTIV and φIV added on tibia IV.
Female protonymph (10 paratypes, Fig. 11a, b). Similar to male protonymph, differing by following features. Body 410–460 μm long and 230–260 wide. Setae h2 short, 15–17 long. Posterior margin of opisthosoma bearing pair of attachment cuticular projections about 15 long and 14 wide. Distance between these projections about 15.
Female tritonymph (10 paratypes, Fig. 11c, d). Similar to male tritonymph, differing by following features. Body 420–460 μm long and 270–300 wide. Setae h2 short, 18–20 long. Posterior margin of opisthosoma bearing pair of attachment cuticular projections about 25 long and 18 wide. Distance between these projections about 20.
Remark
In the genus Chorioptes, tarsal setae eIII of females and rIV of males were overlooked by previous researchers. These setae are very short and closely adjoining to the tarsus making their detection very difficult. They are, however, well visible under a scanning electron microscope. After careful examination, we observed these setae in all species of Chorioptes under a light microscope.
The pattern of ontogenetic changes described for Ch. sweatmani here is typical for psoroptidian mites and probably is the same for all species of the genus. In Chorioptes, delays in ontogenetic setal appearance are not recorded. Setae which are absent in adults (laII, raII, rIII, sIII, and σIII) are absent also in immature stages.
Differential diagnosis
The new species is closely related to Ch. texanus. Males of both these species differ from other representatives of the genus (see Table 5 for states) by the following combination of character states. The opisthosomal lobes are subquadrate in outline and at least twice as short as tarsi III (with pretarsi), setae h2 and h3 are flattened (7–8 μm wide) and distinctly longer than ps2, tarsus III is straight, setae fIII are bifurcate, with a poorly developed apical-ventral extension.
The new species differs from Ch. texanus by the following features. In males of Ch. sweatmani sp. nov., the body length, including the gnathosoma, is 380–405 μm, the idiosoma is 1.6–1.7 times longer that setae h2 and h3, the idiosoma is 3–4 times longer than setae cp, legs III, excluding pretarsus, are approximately three times longer than setae sRIII, the apical spur of tarsus III is curved, a spur near seta fIII base is not developed; in females, setae h2 are relatively short, 1.4–1.5 times shorter than legs IV, excluding pretarsus. In males of Ch. texanus (material from US and South Korean cattle), the body length, including the gnathosoma, is 220–295, the idiosoma is 1.3–1.4 times longer than setae h2 and h3, the idiosoma is 1.3–1.6 times longer than setae cp, legs III, excluding pretarsus, are approximately 1.8–2 times longer than setae sRIII, the apical spur of tarsus III is straight, a spur near seta fIII base is small but distinct; in females, setae h2 are long, about two times longer than legs IV, excluding pretarsus.
Etymology
This species is dedicated to the Canadian parasitologist, Dr. Gordon K. Sweatman in recognition of his work on psoroptid mites.
Type material examined
Male holotype, 10 males, 10 females, 10 males tritonymph, 10 females tritonymph, 10 males protonymph, 10 females protonymph, and 20 larva paratypes (plus numerous paratype specimens preserved in 75 % ethanol) ex A. alces (outer ear canal), SWEDEN, Uppland Province, Uppsala County, Enköping Municipality, 59° 43' 9″ N, 16° 58' 10″ E, spring 2013, coll. G. Hestvik (field number V901/13); five males, five females, five male tritonymphs, five female tritonymphs, five male protonymphs, five female protonymphs, and five larva paratypes (plus numerous paratype specimens preserved in 90 % ethanol) ex A. alces (outer ear canal), SWEDEN, Uppland Province, Uppsala County, Heby Municipality, Huddungeby, 60° 2' 41″ N, 16° 58' 17″ E, spring 2013, coll. G. Hestvik (field number V1110/13).
Type deposition
Holotype (ZISP AVB T-Psor-1) and the majority of paratypes are deposited in ZISP. Other paratypes are held in the IRSNB (two males, two females, one male tritonymph, one female tritonymph, one male protonymph, one female protonymph, and two larvae); OSAL (two males, two females, one male tritonymph, one female tritonymph, one male protonymph, one female protonymph, and two larvae); UMMZ (two males, two females, one male tritonymph, one female tritonymph, one male protonymph, one female protonymph, and two larvae; several specimens preserved in 96 % ethanol for molecular work).
Additional material examined
Two males, three females, two male tritonymphs (ZISP) ex A. alces (outer ear canal), RUSSIA, Kirov Province, experimental hunting ground of the Russian Research Institute of Game Management and Fur Farming near of Polushkintcy village, 58° 35' 43″ N, 50° 42' 18″ E, 29 December 2012, coll. A.P. Saveljev. Three females (IRSNB) ex A. alces, FINLAND, no other data.
Hosts and distribution
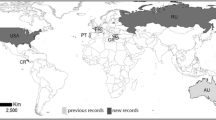
This species is known exclusively from ears of A. alces from Sweden (Morrison et al. 2003; Hestvik et al. 2007; Lusat et al. 2011), Finland, and Russia (Kirov Prov.) (Table 6). It is possible that this parasite occurs throughout its host range. It is unknown whether this species can occur on the moose body or only in the ear canals. In the former case, the reports of Ch. texanus from A. alces and other cervids from Poland (Kadulski et al. 1996) could belong to Ch. sweatmani sp. nov. Furthermore, the record of Ch. texanus from the ears of Rangifer tarandus from the Canadian Arctic (Sweatman 1958) could also belong to this species or even represent a new species (Hestvik et al. 2007; Lusat et al. 2011). Unfortunately, specimens used in these two studies are not available to us and new findings from this host are highly needed.
Microhabitat on host outer ear canal
Pathogenicity
This species is pathogenic, causing chronic skin lesions of their hosts (Hestvik et al. 2007).
Infestation rate
In Uppland (Sweden), 53 hosts were examined, 43 of them were parasitized by Ch. sweatmani sp. nov. (∼81 %) (Hestvik et al. 2007); in Kirov Prov. (Russia), 28 hosts were examined, one of them was parasitized (∼3.6 %) (our data). Most of the Swedish moose specimens were found dead in the field and had a poor nutritional state (Hestvik et al. 2007). Meantime, the Russian moose examined on chorioptic mange were killed in the process of planned shooting and were in good condition. The substantial difference in the infestation rates between the Swedish and Russian moose populations can be explained by the difference in their health condition.
Comparative material
C. bovis: Bos taurus: three males and three females (IRSNB), BELGIUM, Brussels, 5 June 1968, coll. M. Pecheur; 10 males, 10 females, five male tritonymphs, five female tritonymphs, five male protonymphs, five female protonymphs, 10 larvae (ZISP), RUSSIA, Saint Petersburg Province, state farm “Krasnij Oktabr”, 8 January 1986, coll. M. Shustrova.
Lama glama: two females (IRSNB), Belgium, [Zoo], 28 February 1959, coll. unknown; eight males and two females, same data, 2 March 1959, coll. unknown. Capricornis crispus: two males and two females (IRSNB), JAPAN, Nagano Prefecture, Shiojiri City, 36° 6' N, 137° 58' E, 22 January 1976, coll. M. Takahashi; one male and three females (IRSNB), JAPAN: Saitama Prefecture, Chichibu, 35° 59' 25'' N, 139° 4' 35'' E, 17 September 1982, coll. M. Takahashi. Equus caballus: 11 males and four females (IRSNB), BELGIUM, [Faculty of Medical Veterinary], 5 February 1968, coll. unknown; seven males and two females (IRSNB), BELGIUM, Brussels Capital Region, Uccle, 50° 48' 8'' N, 4° 20' 21'' E, 1959, coll. unknown. Rupicapra rupicapra: six males and four females (IRSNB), SWITZERLAND, Ticino, Pizzo Campo Tencia Mountain, 46° 25' 47'' N, 8° 43' 33'' E, 6 July 1960, coll. G. Bouver. Ovis aries: two males and four females (IRSNB), BELGIUM, no other data; one male, ISRAEL, 25 January 1984, no other data; 30 males, 30 females, 10 male tritonymphs, 10 female tritonymphs, 10 male protonymphs, 10 female protonymphs, and 20 larvae (ZISP), ICELAND, 13 October 1992, coll. K. Skirnisson.
C. texanus: B. taurus: three males, four females, two male tritonymphs, one female tritonymph, three male protonymphs, one female protonymph, two larvae, SOUTH KOREA, Hoseo Region, South Chungcheong Province, Cheonan, cattle farm of the National Institute of Animal Science, July 2006, coll. G.-H. Suh; three males and two females, USA, other data unknown, coll. J. Mertens.
Chorioptes mydaus: Mydaus javanensis (BMNH 82.11.9.1): holotype female, two males, one female, and male protonymph paratypes (IRSNB), MALAYSIA, North of Borneo Island, Papar Papar, November 1882, coll. A. Fain. This species was collected from alcohol preserved host and therefore an occasional museum contamination from ruminants is possible but not likely (because ruminants are large and usually not preserved in ethanol as a bulk sample).
Chorioptes panda: Ailuropoda melanoleuca: holotype male (IRSNB), 13 males, 17 females, three male tritonymphs, four female tritonymphs, three male protonymphs, six female protonymphs, and three larva paratypes (IRSNB), FRANCE, Paris Zoo, originated from China, Yunnan, Se-Tchouan, July 1974, coll. M. Leclerc. Ursus americanus: two males and two female tritonymphs (IRSNB), UK, London Zoo, 26 March 1981, coll. Laurence.
Chorioptes crewei: Holotype and paratypes were originally described from Cephalophus rufilatus in Cameroon (Lavoipierre 1958, 1959); deposited at the Liverpool School of Medicine, Liverpool, UK but, probably, lost. No other specimens were collected since the original description. For this study, character states of Ch. crewei were derived from the original description.
Key to species of the genus Chorioptes (males)
-
1.
Opisthosomal lobes subquadrate in outline, subequal or slightly elongated. Bases of setae h2 and h3 situated close to each other. Setae ps2 distinctly longer than ps1. Solenidion φIII subequal or longer than tibia III … 2
Opisthosomal lobes subtriangular in outline, 2 times longer than wide. Bases of setae h2 and h3 widely separated, situated on separate sublobes. Setae ps1 at least 3 times longer than ps2. Tibia III 2.5 times longer than solenidion φIII … Ch. crewei Lavoipierre 1958 (Fig. 12)
-
2.
Setae h2 and h3 narrowly lanceolate (7–9 μm maximum wide) or slightly flattened (3 maximum wide), subequal or longer than leg III excluding pretarsus. Setae h2 distinctly longer than ps2 and h3. Seta fIII bifurcate, with weakly developed antero-ventral extension. Tarsus III excluding pretarsus 1.5–2 times longer than seta eIII; tarsus III excluding pretarsus subequal or longer than seta wIII … 3
Leg III without pretarsus distinctly longer than setae h2 and h3, which widely lanceolate (14–18 μm maximum wide). Setae ps2 distinctly longer than h2 and h3. Seta fIII trifurcate with distinct antero-ventral extension. Setae eIII and wIII distinctly longer than tarsus III (specimens from multiple host species) or only slightly shorter than this tarsus (specimens from C. crispus) … Ch. bovis (von Hering, 1845) (Figs. 5, 6, and 15d)
-
3.
Setae d1 and e1 longer than 20 μm. Setae h2 and h3 narrowly lanceolate (7–9 μm maximum wide), longer than 160 μm. Tarsus I, including apical spur, 1.1–1.6 times longer than tibia I … 4
Setae d1 and e1 8–10 μm long. Setae h2 and h3 only slightly flattened (2–3 μm maximum wide), shorter than 140 μm. Tarsus and tibia I subequal in length …. Ch. mydaus Fain 1975 (Figs. 5 and 6)
-
4.
Tarsus III straight. Setae ps2 not thickened in comparison with ps1. Setae ps2 2.2–3 times longer than ps1. Tarsus I, including apical spur, 1.1–1.2 times longer than tibia I. Solenidion φIII maximum 1.2 times longer than respective tibia … 5
Tarsus III slightly curved. Setae ps2 slightly thickened as compared to ps1. Setae ps2 1.5–1.7 times longer than ps2. Tarsus I, including apical spur, 1.3–1.6 times longer than tibia I. Solenidion φIII 1.4–1.7 times longer than respective tibia … Ch. panda Fain and Leclerc 1975 (Figs. 5 and 6, Fig. 15d)
-
5.
Body length, including gnathosoma, 380–405 μm. Idiosoma 1.6–1.7 times longer than setae h2 and h3, body 3–4 times longer than setae cp. Legs III excluding pretarsus about three times longer than setae sRIII. Apical spur of tarsus III curved, spur near seta fIII base not developed … Ch. sweatmani sp. nov.
Body length, including gnathosoma, 220–295 μm. Idiosoma 1.3–1.4 times longer than setae h2 and h3, body 1.3–1.6 times longer than setae cp. Legs III excluding pretarsus about 1.8–2 times longer than setae sRIII. Apical spur of tarsus III straight, spur near seta fIII base small but distinct … Ch. texanus Hirst 1924 (Figs. 5, 6, and 15d)
Discussion
Different methods of species delimitations use different underlying assumptions and have different levels of accuracy. Here, we choose a Bayesian species delimitation as implemented in BPP because of its robustness and accuracy under the absence of gene flow between species (Camargo et al. 2012; Satler et al. 2013). Applying statistical methods for species delimitation brings the objectivity to this process but also have the caveat of discovering species based purely upon degree of support under a particular species delimitation model (Bauer et al. 2011), which may be wrong if its assumptions are violated by data. For example, incomplete lineage sorting is not the only source of gene tree discordance (Degnan and Rosenberg 2009; Maddison 1997) as assumed by many coalescent-based species delimitation algorithms. Horizontal gene transfer, hybridization, recombination, and gene duplication and extinction may be responsible but these are rarely checked in empirical studies. Population genetics parameters required a priori for multispecies coalescent framework are rarely known with certainty. Furthermore, misspecification of the guide tree or failure to converge may also lead to wrong estimation of species boundaries, particularly in BPP. As a result, wrong statistical inference may lead to either underestimation or overestimation of real species richness. Independent lines of evidence (morphology, morphometrics, breeding experiments) are always necessary to validate these data. Even if statistical species delimitation is accurate, a sole use of these models to propose new taxonomical names makes them unavailable under Article 13.1.1 of the International Code of Zoological Nomenclature, because it is not accompanied by a diagnosis based on intrinsic organismal properties (Bauer et al. 2011). On the other hand, species delimitation based on morphological evidence may be also error prone; it oftentimes depends on expert’s opinion, and, therefore, is subjective. Our study is a synthesis of a rigorous Bayesian analysis (using a range of population genetics parameters and careful examination of convergence of reversible jump Markov chain Monte Carlo) and extensive comparative morphological study, involving all known species of Chorioptes. As such, this approach brings objectivity to the process of species description by utilizing a robust statistical species delimitation model and providing independent validation of this model through informative morphology-based diagnostic character states.
Our morphological analysis suggests that the genus Chorioptes includes six species. Of them, four species with available DNA sequences were validated by both comparison of genetic distances and Bayesian species delimitation analyses. In particular, the corrected genetic distances of Ch. sweatmani sp. nov. were the lowest among all other Chorioptes species (5.6–6.4 % vs 9.6–14.9 %), but still much larger that the infraspecific distances in the other sequenced Chorioptes species (0.2–0.5 %) (Table 2). Similarly, published study using ITS-2 reports pairwise genetic differences between Chorioptes sweatmani sp. nov. and Ch. texanus as 9–11 % (Hestvik et al. 2007). These values were similar to those between Ch. texanus and Ch. bovis (7–11 %), which are relatively well-established species (Essig et al. 1999). For both published and our datasets, the intra- and interspecific genetic distances did not overlap, indicating potential absence of gene flow between the analyzed Chorioptes species.
Bayesian species delimitation as implemented in BPP assumes that species are populations with the same population size (θ) and divergence time (τ) parameters; however, across species, these parameters vary because of the assumed absence of gene flow between them. The discordance between gene trees are explained by incomplete ancestral sorting and modeled via the multispecies coalescent (Rannala and Yang 2003; Yang and Rannala 2010). Because population size and divergence time are unknown for Chorioptes, we conducted our species delimitation analyses using three different sets of population genetics priors (Leache and Fujita 2010). All these analyses strongly suggest that all Chorioptes species are valid (PP 0.58–0.99) (Table 3). The next favored species delimitation models involving Chorioptes had a substantially lesser support. For example, for the conservative analysis, favoring fewer species (the large population size and small root age priors: θ ∼ G(1, 10), τ0 ∼ G(2, 1000)), the next favored species delimitation model was the model joining Ch. sweatmani and Ch. texanus to a single species. The posterior probability for this model was 0.01 versus 0.58 for the “best” model treating these putative taxa as separate species (Table 3).
Published ITS-2 phylogeny of Chorioptes (Hestvik et al. 2007) differs from our inference by the position of Ch. bovis. The ITS-2 tree places this species, albeit with a low support, as a basal lineage to a clade including Ch. panda, Ch. texanus, and Ch. sweatmani. In contrast, in our phylogeny, Ch. bovis forms a monophyletic group with Ch. texanus and Ch. sweatmani (BS 85 PP 0.760 (MrBayes) PP 0.96 (*BEAST); Fig. 1a), so the entire assemblage includes parasites of domestic and wild artiodactyl hosts (except for Ch. bovis which also parasitizes horses).
Mites of the genus Chorioptes are parasites of various artiodactyls (families Bovidae, Cervidae, and Camelidae). Psoroptes, the sister group taxon of Chorioptes, includes parasites of bovid artiodactyls, suggesting that Artiodactyla was the ancestral hosts for Chorioptes. E. caballus is the only known perissodactyl host of these mites, although some other species of Equus were successfully infected in the lab (Table 6). Parasitism of Chorioptes spp. on carnivores is probably secondary. These mites are known from a few wild Asian carnivores (Ailuropoda, Ursus, and Mydaus). Chorioptes spp. from carnivores are morphologically close to mites of the Ch. texanus + Ch. sweatmani clade and probably diverged from the common ancestor inhabiting ruminants. It is interesting that Chorioptes spp., being widely distributed on ruminants, are absent on their main predators of the families Canidae and Felidae but parasitize some ursids and badgers. It could be hypothesized that parasitic mites of the genus Otodectes (Psoroptidae) living in ear auricles of these carnivores are better competitors and can prevent colonization of their hosts by Chorioptes.
Among Chorioptes spp. associated with herbivorous hosts, Ch. bovis was recorded from Artiodactyla (12 species) and E. caballus, Ch. texanus is known from artiodactyls (6 species), whereas Ch. crewei and Ch. sweatmani were recorded from a single artiodactyl host species each (Table 6). The wide host ranges and worldwide distributions of the former two species can be explained by their associations with domesticated hosts. When spreading into new areas with domesticated hosts, these mites could also attack wild ruminants. It is also possible that ancestrally Chorioptes were not strictly host-specific because many different artiodactyl species can graze together (e. g., in African savannas) offering the opportunity for cross-species infestation. This hypothetic scenario can be seen in the sarcoptic mange mite, Sarcoptes scabei (Acariformes: Sarcoptidae). This species switched from humans (principal host) to domesticated animals and from them to wild hosts (Fain 1968). In Australia, these shifts occurred contemporary—S. scabei co-dispersed to this continent along with humans or domesticated animals and then shifted to the wild wombats (Skerrat et al. 2002).
Although both Ch. bovis and Ch. texanus were recorded from multiple host species, only Ch. bovis is known from horses. At the same time, Ch. texanus, parasitizing domesticated ruminants in different parts of the world, nevertheless, had never been recorded from equids. It is possible, but unlikely due to significant morphological differences, that some mites from horses were mistakenly identified as Ch. bovis. It is possible that either Ch. bovis secondarily transferred to horses from domesticated ruminants (hypothesis I) or was ancestrally associated with equids (hypothesis II). Ch. bovis strongly differs morphologically from all other species of the genus (Table 5) and it may be the earliest branch of the genus, as suggested by ITS-2 data (Hestvik et al. 2007). However, the latter hypothesis (II) is less likely because our molecular inference suggests that Ch. bovis is a monophyletic group with other atriodactyl-inhabiting species (Fig 1a, b).
References
Ahmad M, Ahmad S, Ali FA (1993) Efficacy of diazinon against mange in sheep. Punjab Univ J Zool 8:41–44
Alogninouwa T, Parent R (1986) Traitement par l’ivermectine d’une gale mixte (Sarcoptes scabiei et Chorioptes caprae) chez la ch’evre au Senegal. Observations cliniques. Bull Mens Soc Vet Prat France 70:399–403
Bauer AM et al (2011) Availability of new Bayesian-delimited gecko names and the importance of character-based species descriptions. P Roy Soc B-Biol Sci 278:490–492. doi:10.1098/Rspb.2010.1330
Bochkov AV (2010) A review of mammal associated Psoroptidia (Acariformes: Astigmata). Acarina 18:99–260
Brown JM, Hedtke SM, Lemmon AR, Lemmon EM (2010) When trees grow too long: investigating the causes of highly inaccurate Bayesian branch-length estimates. Syst Biol 59(2):145–161. doi:10.1093/Sysbio/Syp081
Camargo A, Morando M, Avila LJ, Sites JW Jr (2012) Species delimitation with ABC and other coalescent-based methods: a test of accuracy with simulations and an empirical example with lizards of the Liolaemus darwinii complex (Squamata: Liolaemidae). Evol Int J Organ Evol 66:2834–2849. doi:10.1111/j.1558-5646.2012.01640.x
Castro BS de, Hipolito M Jr, Jonke LAC, Silva WT (1978) Sarna corioptica (Chorioptes bovis, Gerlach, 1857) em bovinos no municipio de Pirassununga, Sao Paulo. Biologico (Sao Paulo) 44:165–168
Cave TW (1909) The foot-scab mite of sheep (Symbiotes communis var. ovis, Railliet). Cop Pathol Therap 22:50–52
Chen C-C, Pei KJ-C (2007) Case report: the first report of mange infestation in wild Formosan serows (Capricornis swinhoei) in Taiwan. Taiwan Vet J 33:181–185
Cremers HJW (1985a) Chorioptes bovis (Acarina: Psoroptidae) in some camelids from Dutch zoos. Vet Quart 7:198–199
Cremers HJW (1985b) The incidence of Chorioptes bovis (Acari: Psoroptidae) on the feet horses, sheep and goats in the Netherlands. Quart 7:283–289
D’Alterio GL, Callaghan C, Just C, Manner-Smith A, Foster AP, Knowles TG (2005) Prevalence of Chorioptes sp mite infestation in alpaca (Lama pacos) in the south-west of England: implications for skin health. Small Rumin Res 57:221–228. doi:10.1016/j.smallrumres.2004.07.001
Dalapati MR, Bhowmik MK (1996) Clinico-haematological, biochemical and pathological changes of psoroptic and chorioptic mange in goats. Ind Vet J 73:728–733
Degnan JH, Rosenberg NA (2009) Gene tree discordance, phylogenetic inference and the multispecies coalescent. Trends Ecol Evol 24(6):332–340. doi:10.1016/J.Tree.2009.01.009
Delafond O, Bourguignon H (1857–1858) Recherches sur les animalcules de la gale de l'homme et des animaux et la transmission de la gale des animaux a l'homme. Bull Acad Med (Paris) 23:110–126
Domrow R (1992) Acari Astigmata (excluding feather mites) parasitic on Australian vertebrates: an annotated checklist, keys and bibliography. Inv Tax 6:1459–1606. doi:10.1071/IT9921459
Dorny P, Van Wyngaarden T, Vercruysse J, Symoens C, Jalia A (1994) Survey on the importance of mange in the aetiology of skin lesions in goats in Peninsular Malaysia. Trop Anim Health Prod 26:81–86
Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214
Essig A, Rinder H, Gothe R, Zahler M (1999) Genetic differentiation of mites of the genus Chorioptes (Acari: Psoroptidae). Exp Appl Acarol 23:309–318. doi:10.1023/A:1006131402463
Faccini JLH, Massard CL (1976) O genero Chorioptes Gervais, 1895, parasita de ruminantes no Brasil (Psoroptidae, Acarina). Revista Bras Biol 36:871–872
Fain A (1963) Les acariens produceurs de gale chez les lemuriens et les singes aves une etude des Psoroptidae (Sarcoptiformes). Inst R Sci Nat Bel:1–125
Fain A (1968) Etude de la variabilite de Sarcoptes scabiei avec une revision des Sarcoptidae. Acta Zool Pathol Antverp 47:1–196
Fain A (1975) Nouveaux taxa dans les Psoroptinae hypothese sur l’origine de ce groupe (Acarina, Sarcoptiformes, Psoroptidae). Acta Zool Pathol Antverp 61:57–84
Fain A, Leclerc M (1975) Sur un cas de gale chez le panda geant produit par une nouvelle espece du genre Chorioptes (Acarina: Psoroptidae). Acarologia 17:177–182
Foster A, Jackson A, D'fAlterio GL (2007) Skin diseases of South American camelids. Practice 29:216–223. doi:10.1136/inpract.29.4.216
Grandjean F (1941) La comparee des pattes chez oribates (1re série). Bull Soc Zool France 66:33–50
Griffiths DA, Atyeo WT, Norton RA, Lynch CA (1990) The idiosomal chaetotaxy of astigmatid mites. J Zool (London) 220:1–32
Heath ACG (1979a) Ectoparasites of feral sheep on Campbell Island. N Z J Zool 6:141–144
Heath ACG (1979b) Arthropod parasites of goats. N Z J Zool 6:655
Heath ACG (1983) Parasites of the feral sheep of Pitt Island, Chatham group. N Z J Zool 10:365–370
Heath ACG, Bishop DM, Tenquist JD (1989) Observations on the potential for natural transfer of Psoroptes cuniculi and Chorioptes bovis (Acari: Psoroptidae) between goats and sheep. N Z Vet J 37:56–58
Heled J, Drummond AJ (2010) Bayesian inference of species trees from multilocus data. Mol Biol Evol 27(3):570–580. doi:10.1093/Molbev/Msp274
Helson GAH (1956) Some arthropods affecting man and livestock in New Zealand. N Z Vet 4:11–18
Hestvik G, Zahler-Rinder M, Gavier-Widen D, Lindberg R, Mattsson R, Morrison D, Bornstein S (2007) A previously unidentified Chorioptes species infesting outer ear canals of moose (Alces alces): characterization of the mite and the pathology of infestation. Acta Vet Scand 49:21–30. doi:10.1186/1751-0147-49-21
Hirst S (1924) On a new mite of the genus Chorioptes parasitic on goats in the United States. Ann Mag Nat Hist 13:538
Hudson RR, Coyne JA (2002) Mathematical consequences of the genealogical species concept. Evol Int J Organ Evol 56:1557–1565
Izdebska JN (2006) Skin mites (Acari: Demodecidae, Psoroptidae, and Sarcoptidae) of the European bison, Bison bonasus. Biol Let 43:169–174
Kadulski S (1996a) Ectoparasites of Cervidae in north-east Poland. Acta Parasitol 41:204–210
Kadulski S (1996b) Further studies on parasitic arthropods of elk Alces alces from Poland. Wiad Parazitol 42:349–355
Kadulski S, Izdebska JN, Konczyk M (1996) Parasitic arthropods of Bison bonasus from Bialowieza primaeval forest. Wiad Parazitol 42:255–260
Kamyszeh F, Wertejuk M (1983) A comparative study of ectoparasite infection of sheep in the Wielkopolska and Szczecin regions during the years 1971–1980. Wiad Parazytol 29:335–341
Kamyszek F (1986) Observations on occurrence and control of chorioptic scab in sheep in Wielkopolska region. Prace Komisji Nauk Rolniczych i Komisji Nauk Lesnych 59:49–55
Kemper HE, Roberts IH, Peterson HO (1952) Tests with benzene hexachloride on chorioptic scab mites. Sheep Goat Raiser 32:56–58
Kennedy JM, Kralka RA (1986) A survey of ectoparasites of cattle in Central Alberta, November 1984–July 1985. Can Vet J 11:459–460
Klimov PB, O’Connor BM (2008) Origin and higher-level relationships of psoroptidian mites (Acari: Astigmata: Psoroptidia): evidence from three nuclear genes. Mol Phyl Evol 47:1135–1156. doi:10.1016/j.ympev.2007.12.025
Klimov PB, O’Connor BM (2013) Is permanent parasitism reversible?—Critical evidence from early evolution of house dust mites. Syst Biol 62:411–423. doi:10.1093/sysbio/syt008
Knowles L, Klimov PB (2011) Estimating phylogenetic relationships despite discordant gene trees across loci: the species tree of a diverse species group of feather mites (Acari: Proctophyllodidae). Parasitol 138:1750–1759
Kollbrunner M, Luginbuhl A, Pfister K (2010) Epidemiological aspects of Chorioptes mange in dairy cows in Switzerland: a field study. Schweiz Arch Tierheilkd 152:231–236. doi:10.1024/0036-7281/a000054
Lanfear R, Calcott B, Ho SY, Guindon S (2012) Partitionfinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol 29(6):1695–1701. doi:10.1093/molbev/mss020
Lavoipierre MMJ (1958) A preliminary description of a new species of Chorioptes from an African antelope. Ann Trop Med Parasitol 52:3
Lavoipierre MMJ (1959) A description of the male and female of Chorioptes crewei Lavoipierre, 1958 (Acarina: Psoroptidae), together with some remarks on the family Psoroptidae and a key to the genera contained in the family. Acarologia 1:354–364
Leache AD, Fujita MK (2010) Bayesian species delimitation in West African forest geckos (Hemidactylus fasciatus). P Roy Soc B-Biol Sci 277:3071–3077. doi:10.1098/Rspb.2010.0662
Lusat J, Morgan ER, Wall R (2009) Mange in alpacas, llamas and goats in the U.K.: incidence and risk. Vet Parasitol 163:179–184. doi:10.1016/j.vetpar.2009.04.007
Lusat J, Bornstein S, Wall R (2011) Chorioptes mites: re-evaluation of species integrity. Med Vet Entomol 25:370–376. doi:10.1111/j.1365-2915.2011.00951.x
Maddison WP (1997) Gene trees in species trees. Syst Biol 46(3):523–536
Marshall DC (2010) Cryptic failure of partitioned Bayesian phylogenetic analyses: lost in the land of long trees. Syst Biol 59:108–117. doi:10.1093/Sysbio/Syp080
McKenna CT, Pulsford MF (1947) A note on the occurrence of Chorioptes communis var. ovis on sheep in South Australia. Austral Vet J 23:146–147. doi:10.1111/j.1751-0813.1947.tb14731.x
McVay JD, Carstens BC (2013) Phylogenetic model choice: justifying a species tree or concatenation analysis. J Phyl Evol Biol 1:1–8
Megnin JP (1872) Memoire sur un nouvel acarien psorique du genre Symbiote. J Anat Physiol 8:337–358
Mollereau MA (1889) Gale symbiotique de la chevre. Bull Soc Centr Med Vet 43:156–157
Morrison DA, Ljunggren EA, Mattsson JG (2003) The origin of Sarcoptes scabiei in wombats. Parasitol Res 91:497–499. doi:10.1007/s00436-003-0987-2
Mullen GR, O’Connor BM (2002) Mites (Acari). In: Mullen GR, Durden LA (eds) Medical and veterinary entomology. Academic Press/Elsevier Science, San Diego, pp 449–516
Nagata TA, Roncali R, Mishiba T, Matuoka K, Yamada K, Ura S (1995) Efficacy of ivermectin pour-on formulation against naturally infected mange mites (Chorioptes spp.) in cattle, Japan. Bull Animal Hyg 41:27–33
Neog R, Borkakoty MR, Lahkar BC (1992) Mange mite infestation in goats in Assam. Ind Vet J 69:891–893
Nicol G (1946) Parasites of the horse. J Dep Agr Vict 44:53–56
Nong X, Li S-H, Wang J-H, Xie Y, Chen F-Z, Liu T-F, He R, Gu X-B, Peng X-R, Yang G-Y (2014) Acaricidal activity of petroleum ether extracts from Eupatorium adenophorum against the ectoparasitic cattle mite, Chorioptes texanus. Parasitol Res 113:1201–1207. doi:10.1007/s00436-014-3758-3
Norton R (1998) Morphological evidence for the evolutionary origin of Astigmata (Acari: Acariformes). Exp Appl Acarol 22:559–594
Nylander JA, Wilgenbusch JC, Warren DL, Swofford DL (2008) AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24:581–583. doi:10.1093/bioinformatics/btm388
Oba MSP, Ogassawara S, Costa AJS (1977) Ocorrencia de Chorioptes bovis Gerlach, 1857 (Acari: Psoroptoidea, Psoroptidae) em bovinos no municipo de Sao Carlos, Sao Paulo. Arquivos Inst biol S Paulo 44:95–97
Oudemans AC (1926) Chorioptes caprae (Del. & Bourg. 1858). Tijdschr Entomol 69:1–18
Palimpestov MA (1947) Peculiarities in the development of psoroptid mange mites. Vet 24:6–9 (In Russian)
Perris EE (1995) Parasitic dermatoses that cause pruritus in horses. Vet Clin North Am Equine Pract 11:11–28
Rambaut A, Drummond AJ (2009) Tracer v1.5. Available from http://tree.bio.ed.ac.uk/software/tracer/
Ramirez MA, Amao de MacGregor M, Matute QR (1964) Cattle mange: first report of Chorioptes bovis in Peru. Anais dent Lima 2:68–76
Rannala B, Yang Z (2003) Bayes estimation of species divergence times and ancestral population sizes using DNA sequences from multiple loci. Genetics 164:1645–1656
Rehbein S, Winter R, Visser M, Maciel AE, Marley SE (2005) Chorioptic mange in dairy cattle: treatment with eprinomectin pour-on. Parasitol Res 98:21–25. doi:10.1007/s00436-005-0005-y
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61(3):539–542. doi:10.1093/sysbio/sys029
Rose AL (1940) Leg mange or "Itchy Heel" of horses. Description of the disease and methods of treating it. Agr Gaz N S W 51:491–496
Satler JD, Carstens BC, Hedin M (2013) Multilocus species delimitation in a complex of morphologically conserved trapdoor spiders (Mygalomorphae, Antrodiaetidae, Aliatypus). Syst Biol 62:805–823. doi:10.1093/sysbio/syt041
Schmaschke R, Eulenberger K, Notzold G (1995) Generalisierte Chorioptes raude beim Dallschaf (Ovis dalli dalli). Erkrank Zoot 37:423–428
Shibata A, Yachimori S, Morita T, Kanda E, Ike K, Imai S (2003) Chorioptic mange in a wild Japanese serow. J Wild Dis 39:437–440. doi:10.7589/0090-3558-39.2.437
Skerrat LF, Campbell NJH, Murrell A, Walton S, Kemp D, Barker SC (2002) The mitochondrial 12S gene is a suitable marker of populations of Sarcoptes scabiei from wombats, dogs and humans in Australia. Parasitol Res 88:376–379. doi:10.1111/j.1751-0813.2005.tb11585.x
Stamatakis A, Ludwig T, Meier H (2005) RAxML-III: a fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics 21(4):456–463. doi:10.1093/Bioinformatics/Bti191
Suh G-H, Hur T-Y, Lim S, Shin S-M, Kwon J, Cho S-H, Lee C-Y, Shin SS (2008) The first outbreak of Chorioptes texanus (Acari: Psoroptidae) infestation in a cattle farm in Korea. Korean J Parasit 46:273–278. doi:10.3347/kjp.2008.46.4.273
Sweatman GK (1957) Life history, non-specificity and revision of the genus Chorioptes, a parasitic mite of herbivores. Canad J Zool 35:641–689
Sweatman GK (1958) Redescription of Chorioptes texanus, a parasitic mite from the ears of reindeer in the Canadian Arctic. Canad J Zool 36:525–528
Syring J, Farrell K, Businsky R, Cronn R, Liston A (2007) Widespread genealogical nonmonophyly in species of Pinus subgenus Strobus. Syst Biol 56(2):163–181. doi:10.1080/10635150701258787
Szczurek B, Kadulski S (2004) Ectoparasites on fallow deer, Dama dama (L.) in Pomerania, Poland. Acta Parasitol 49:80–86
Takahashi M, Nogami S, Misumi H, Matsumoto M, Takahama M, Uchikawa K (2001) Mixed infestation of sarcoptic and chorioptic mange mites in Japanese serow, Capricornis crispus Temminck, 1845 in Japan, with a description of Chorioptes japonensis sp. nov. (Acari: Psoroptidia). Med Entomol Zool 52:297–306
Tavasoli M, Rahbari S, Zarei S (1998) Chorioptes bovis mites in cows: a case report from Iran. J Fac Vet Med Univ Tehran 53:50–51
Turk FA (1946) Studies of Acari. V.—notes on and descriptions of new and little-known British Acari. Ann Mag Nat Hist 12:785–820
Wang S, Gu X, Fua Y, Lai S, Wang S, Peng X, Yang G (2012) Molecular taxonomic relationships of Psoroptes and Chorioptes mites from China based on COI and 18S rDNA gene sequences. Vet Parasitol 184:392–397. doi:10.1016/j.vetpar.2011.09.011
Whitten LK (1962) Parasitic mites of domestic animals in New Zealand. N Z Entomol 3:9–12
Wilson DE, Reeder DM (2005) Mammal species of the world: a taxonomic and geographic reference, 3rd edn. Johns Hopkins University Press, Baltimore
Yang ZH, Rannala B (2010) Bayesian species delimitation using multilocus sequence data. Proc Natl Acad Sci U S A 107:9264–9269. doi:10.1073/Pnas.0913022107
Yeruham I, Hadani A, Sklar A, Monbaz A (1981) The occurrence of chorioptic mange in dairy cattle in Israel. Ref Veter 38:176–179
Yeruham I, Rosen S, Hadani A (1999a) Chorioptic mange (Acarina: Psoroptidae) in domestic and wild ruminants in Israel. Exp Appl Acarol 23:861–869. doi:10.1023/A:1006217016688
Yeruham I, Rosen S, Hadani A, Braverman Y (1999b) Arthropod parasites of Nubian ibexes (Capra ibex nubiana) and gazelles (Gazella gazella) in Israel. Vet Parasitol 83:167–173. doi:10.1016/S0304-4017(99)00073-4
Zahler M, Hendrix WML, Essig A, Rinder H, Gothe R (2001) Taxonomic reconsideration of the genus Chorioptes Gervais and van Beneden, 1859 (Acari: Psoroptidae). Exp Appl Acarol 25:517–523. doi:10.1023/A:1011857802317
Zurn FA (1847) Raudemilben im Ohr der Hunde und bei Kaninchen. Woch Tierh Viehzucht 18:277–283
Acknowledgments
We thank Drs Sung-Shik Shin (Chonnam National University, South Korea), Karl Skirnisson (Institute for Experimental Pathology, Keldur, University of Iceland), and James Mertens (National Veterinary Services Laboratories, US) for specimens. Dr. Anne Baker kindly confirmed the presence of the C. texanus holotype in the collection of BMNH. We are grateful to Alexey Mirolubov (ZISP) for his help with the scanning electron microscope. The molecular work of this study was conducted in the Genomic Diversity Laboratory of the University of Michigan Museum of Zoology. This research was supported by a grant from the Belgian Federal Science Policy cofinanced by the Marie Curie actions of the European Commission, by the Russian Foundation for Basic Research (Grant No 13-04-00608a) to AVB, the US National Science Foundation (NSF DEB-0613769) to Barry OConnor, and by the University of Michigan Museum of Zoology research incentive fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Andre V. Bochkov and Pavel B. Klimov have equal contribution.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 79 kb)
Rights and permissions
About this article
Cite this article
Bochkov, A.V., Klimov, P.B., Hestvik, G. et al. Integrated Bayesian species delimitation and morphological diagnostics of chorioptic mange mites (Acariformes: Psoroptidae: Chorioptes). Parasitol Res 113, 2603–2627 (2014). https://doi.org/10.1007/s00436-014-3914-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3914-9