Abstract
Among the proteins involved in the invasion by merozoite, the glycosylphosphatidylinositol-anchored proteins (GPI-APs) are suggested as potential vaccine candidates because of their localization to apical organelles and the surface; these candidates are predicted to play essential roles during invasion. As a GPI-AP, Plasmodium vivax merozoite surface protein 10 (PvMSP-10) induces high antibody titers. However, such high antibody titers have shown no protective efficacy for animals challenged with P. vivax parasites in a previous study. To adequately evaluate the immunogenicity and further characterize PvMSP-10 in order to understand its vaccine potential, we assessed its immunogenicity by immunizing BALB/c mice with cell-free expressed recombinant PvMSP-10 protein. The antigenicity of MSP-10 was analyzed, and we found 42 % sensitivity and 95 % specificity using serum samples from P. vivax-infected Korean patients. The IgG1 and IgG3 were the predominant immunoreactive antibodies against PvMSP-10 in vivax patient sera, and IgG1 and IgG3 and Th1-type cytokines were predominantly secreted in PvMSP-10-immunized mice. We conclude that the immunogenicity and antigenicity of MSP-10 may serve as a potential vaccine against vivax malaria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vivax malaria threatens almost 40 % of the world’s population, particularly pregnant women and young children (Herrera et al. 2007). It is a severe disease with substantial morbidity (Genton et al. 2008; Tjitra et al. 2008; Anstey et al. 2009). The spread of the parasite’s resistance to the most common anti-malarial drugs and of mosquitoes to insecticides emphasizes the urgent need to identify and characterize potential vaccine candidates for Plasmodium vivax.
Over the past two decades, several potential vaccine candidate P. vivax merozoite surface proteins (MSPs) have been investigated, including PvMSP-1 (Soares et al. 1997; Nogueira et al. 2006; Zeyrek et al. 2008), PvMSP-3 (Bang et al. 2011; Sirima et al. 2011), PvMSP-9 (Vargas-Serrato et al. 2002; Lima-Junior et al. 2008), and PvMSP-10 (Perez-Leal et al. 2005; Giraldo et al. 2009). Previous study has suggested that human antibodies specific to PvMSP-1 correlated with protection against P. vivax malaria in a longitudinal clinical study (Nogueira et al. 2006). However, the vaccination of nonhuman primates with PvMSP-1 C- or N-terminal proteins failed or afforded only partial protection to challenge with P. vivax parasites (Collins et al. 1999; Valderrama-Aguirre et al. 2005). Such high antibody responses in PvMSP-10-immunized nonhuman primates have shown no protective efficacy when the primates were challenged with P. vivax parasites (Giraldo et al. 2009).
MSP-10 was identified in 13 Plasmodium species as a MSP containing an N-terminal signal sequence, a C-terminal with two epidermal growth factor (EGF)-like domains and glycosylphosphatidylinositol (GPI) anchor (Pacheco et al. 2012), which is similar to the intensively investigated blood-stage vivax malaria vaccine candidate, PvMSP-1. To date, the immunogenic properties and localization of PvMSP-10 have not been adequately examined. In the present study, we sought to analyze the vaccine potential of PvMSP-10 and used immunofluorescence to confirm that PvMSP-10 localized on the surface of merozoite.
Materials and methods
Human serum samples
Positive serum samples were collected from 112 patients with symptoms and positive vivax parasitemia by microscopic examination (0.113 % mean parasitemia; range 0.027–0.498 %) at local health centers and clinics in the Gangwon Province in malaria-endemic areas of the Republic of Korea (ROK). Their mean age was 25 years (range 18–52 years). The sera of healthy individuals were collected from 80 malaria-naïve people living in nonendemic areas of ROK. All subjects had no episodes in the past. Patent infection was documented by a microscopic analysis of Giemsa-stained blood thin and thick smears. This study was approved by the Institutional Review Board at Kangwon National University Hospital.
Cloning, expression, and purification of PvMSP-10
P. vivax genomic DNA was extracted from a P. vivax Korean isolate using a QIAamp DNA blood kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions and used for PCR the amplification of the pvmsp-10 gene. An open reading frame encoding the pvmsp-10 gene, PVX_114145 (http://www.plasmoDB.org), without signal peptide (SP) and GPI-anchor was amplified from the genomic DNA of Korean isolates. The primers used for In-Fusion cloning (Clontech, Palo Alto, CA, USA) were as follows: PvMSP-10-F (5′-gggcggatatCTCGAGGTCCACGTGAGTGCAAACG-3′) and PvMSP-10-R (3′-gcggtacccgGGATCCTTAGACGCAGAAAATCCCA-5′), as described previously (Chen et al. 2010). The vector sequences were presented in lower case letters, and the XhoI and BamHI sites were underlined. The PCR reaction mixture contained 0.5 U Platinum Taq DNA Polymerase High Fidelity (Invitrogen, Carlsbad, CA, USA), 0.2 μM of each sense and anti-sense primer, 1 μL of genomic DNA, 200 μM of deoxyribonucleotide triphosphates, and MgSO4 to a final concentration of 2.0 mM. The target DNA was amplified with an initial denaturation at 94 °C for 2 min, followed by 42 cycles at 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 1.5 min, and then a final extension at 72 °C for 10 min. The amplified DNA was cloned into the XhoI and BamHI sites of a pEU-E01-His-Tev-N2 plasmid vector (CellFree Sciences, Matsuyama, Japan), which is an expression plasmid with an N-terminal hexa-histidine (His)-tag followed by a tobacco etch virus protease cleavage site for a wheat germ cell-free system (WGCF) system (CellFree Sciences). The inserted nucleotide sequence was confirmed using an ABI PRISM 310 Genetic Analyzer and a Big Dye Terminator v. 1.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA, USA). Sequence alignments were conducted using Lasergene software (DNASTAR, Madison, WI, USA) and protein sequence alignment and analysis with the MegAlign option.
Recombinant PvMSP-10 was expressed using the WGCF system (CellFree Sciences) as previously described (Tsuboi et al. 2010). Briefly, highly purified plasmid DNA was prepared using a Maxi Plus™ Ultrapure plasmid extraction system (Viogene, Taipei, Taiwan) according to the manufacturer’s instructions. Purified plasmid DNA was eluted in 0.1× TE buffer (10 mM Tris–HCl, pH 8.0, 1 mM EDTA) and used for in vitro transcription of recombinant protein expressed using a WGCF system (CellFree Sciences). The PvMSP-10 protein was affinity-purified using a Ni-Sepharose column as described elsewhere (Arumugam et al. 2011).
SDS-PAGE and Western blot analysis
Recombinant PvMSP-10 (rPvMSP-10) was resolved using 12 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) after denaturation with β-mercaptoethanol in the sample buffer and then stained with Coomassie Brilliant Blue. For Western blot analysis to confirm the specificity of anti-PvMSP-10 antibodies in immune animals and vivax patient serum, the rPvMSP-10 was transferred electrophoretically to PVDF membranes (Millipore, Bedford, MA, USA) after the SDS-PAGE and incubated with a blocking buffer (5 % nonfat milk in phosphate-buffered saline (PBS) containing 0.2 % Tween 20; PBS/T) for 1 h at 37 °C. After blocking, anti-His antibody (1:1000), mouse immune sera (1:1000), rabbit immune sera (1:2000), or mixed patient sera (1:200) diluted in PBS/T and secondary IRDye goat anti-mouse (1:10,000 dilution), IRDye goat anti-rabbit (1:20,000 dilution), or IRDye goat anti-human (1:20,000) (LI-COR Bioscience, Lincoln, NE, USA) antibodies were used. Signals were obtained with an Odyssey Infrared Imaging System and analyzed using Odyssey software (LI-COR Bioscience).
Immunization of animals with rPvMSP-10
Female BALB/c mice (DBL Co, Seoul, ROK) were used at 6–8 weeks of age. Groups of three mice were injected intraperitoneally with about 20 μg of each of rPvMSP-10, rPvMSP1-19 diluted in PBS, or only PBS with Freund’s complete adjuvant, respectively (Sigma-Aldrich, St. Louis, MO, USA). Booster injections were given 3 and 6 weeks later using the same amount of antigen with Freund’s incomplete adjuvant (Sigma-Aldrich). Mouse blood samples were taken 2 weeks after the final booster injection. Additionally, to develop an indirect immunofluorescence assay, one Japanese white rabbit was immunized subcutaneously with 250 μg of purified rPvMSP-10 in Freund’s complete adjuvant, followed by 250 μg with Freund’s incomplete adjuvant thereafter. All immunizations were performed three times at 3-week intervals. The antisera were collected 14 days after the last immunization as previously described (Cheng et al. 2013a). All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Kangwon National University, and the experiments were conducted according to the Ethical Guidelines for Animal Experiments of Kangwon National University.
Indirect immunofluorescence assay (IFA)
Mature intraerythrocytic stage-rich P. vivax parasites were collected from a malaria patient in Thailand. Slides smeared with parasite-infected blood were fixed with ice-cold acetone for 3 min, dried, and stored at −80 °C. Before use, the slides were thawed on blue silica gel (Samchun Chemical, Pyeongtaek, ROK) and blocked with PBS containing 5 % nonfat milk at 37 °C for 30 min. The slides were then incubated with 1:100 dilutions of primary antibodies (mouse or rabbit anti-MSP-1-19; rabbit or mouse anti-PvMSP-10) at 37 °C for 1 h (Cheng et al. 2013b). The slides were then stained with Alexa Fluor 546-conjugated goat anti-rabbit/mouse IgG or Alexa Fluor 488-conjugated goat anti-mouse/rabbit IgG secondary antibodies (Invitrogen) and the nuclear stain 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) at 37 °C for 30 min. The slides were mounted in ProLong Gold antifade reagent (Invitrogen) and fluorescence visualized under oil immersion using a confocal laser-scanning microscope (FV200; Olympus, Tokyo, Japan). Images were captured with the FV10-ASW 3.0 Viewer software (Olympus) and prepared for publication using Adobe Photoshop CS5 (Adobe Systems, San Jose, CA, USA).
Protein arrays
The preparation of amine-coated slides has been described elsewhere (Tsuboi et al. 2010). The humoral immune response in serum from 112 patients with vivax malaria and 80 unexposed individuals was evaluated using well-type amine arrays. A series of double dilutions were used to optimize the PvMSP-10 coating concentration (0.1–200 ng/μL). The purified recombinant protein was spotted onto duplicate wells of the arrays at 40 ng/μL in PBS and incubated for 1 h at 37 °C. Each well was blocked with a 1-μL blocking buffer (5 % bovine serum albumin in PBS with 0.1 % Tween 20; PBS/T) and incubated for 1 h at 37 °C. Then, the chips were probed with human malaria patient or healthy individual sera (1:10 dilution) that had been first pre-absorbed against wheat germ lysate (1:100 dilution) to block anti-wheat germ antibodies. Alexa Fluor 546 goat anti-human IgG antibody (10 ng/μL; Invitrogen) in PBS/T was used as the detection antibody and was quantified as described previously using a fluorescence scanner (ScanArray Express; PerkinElmer, Boston, MA, USA) (Chen et al. 2010). The cut-off value was equal to the mean plus two standard deviations (SD) of the mean fluorescence intensity (MFI) of the 80 negative samples.
To investigate the response of human immunoglobulin G (IgG) subclasses against PvMSP-10, twenty total IgG-positive vivax-infected patient sera from malaria-endemic areas and eight negative samples from nonendemic malaria areas of ROK were selected from above samples. As above, 1 μL of rPvMSP-10 protein (40 μg/mL) was spotted to each well and incubated for 2 h at 37 °C. After blocking, plasma was added in duplicate at previously determined dilutions. To determine IgG subclasses, secondary antibodies were added at 1:1000 using mouse monoclonal antibody to the human IgG subclass (IgG1 clone HP6096, IgG2 clone HP6002, IgG3 clone HP6047, IgG4 clone HP6025; Invitrogen). The Alexa Fluor 546 goat anti-mouse antibody (Invitrogen, 50 ng/μL) was used for the subclass assays, scanned, and analyzed as above. The cut-off value was equal to the mean plus two SD of the MFI of the eight negative samples.
Enzyme-linked immunosorbent assay (ELISA)
Ninety-six-well ELISA plates (Costar, Corning, NY, USA) were coated with 100 μL of 2.5 μg/mL PvMSP-10 in 0.05 M of NaHCO3 (pH 9.6) overnight at 4 °C and blocked with 1 % normal goat serum at 200 μL/well at 37 °C for 1 h. For standard curve construct, 100 μL of purified mouse IgG1, IgG2a, IgG2b, and IgG3 (Invitrogen) were coated onto 96-well plates at 256, 128, 64, 32, 16, 8, and 4 ng/ml, respectively, and incubated with immune mouse sera diluted 1:1,000 in PBS/T that the chips were probed with mouse sera (1:10 dilution) that had also been first pre-absorbed against wheat germ lysate (1:100 dilution) to block anti-wheat germ antibodies. HRP-conjugated anti-mouse IgG1, IgG2a, IgG2b, and IgG3 antibodies (Invitrogen) at 1:1,000, 1:1,000, 1:2,000, and 1:1,000 dilutions, respectively, were used to detect reactions. The reaction was developed by the addition of 100 μL of diluted 3,3′,5,5′-tetramethylbenzidine single solution (Invitrogen) for 15 min at 37 °C and stopped with 100 μL of 1 N HCl. The optical density (OD) of the contents of each well was determined at 450 nm. All samples were tested in duplicate, and the mean absorbance was calculated. The color intensity was measured and calculated using a log–log curve fit.
To evaluate PvMSP10-immunized animal serum titers, ELISA was developed as before (Cheng et al. 2013a). In brief, 100 μl of a 2.5 μg/ml PvMSP10 in 0.05 M of NaHCO3 (pH 9.6) was used overnight at 4 °C and blocked with 5 % skim milk in Tris-buffered saline (TBS) containing 0.05 % Tween 20 (TBS-T) at 200 μl/well at 37 °C for 1 h. After washing three times with TBS-T, 100 μl of two-fold serial dilutions of serum was added to each well and plates incubated at 37 °C for 1 h. The plates were washed six times, and 100 μl of peroxidase-conjugated goat anti-mouse IgG (H + L) antibody (dilution 1:20,000) (Pierce Biotechnology, Rockford, IL, USA) or peroxidase-conjugated goat anti-rabbit IgG (H + L) antibody (dilution 1:20000) (Jackson ImmunoResearch, Baltimore, MD, USA) was added to each well as a secondary antibody for 1 h at 37 °C. After color development and reaction termination, the OD value of the contents of each well was determined at 450 nm. All samples were tested in duplicate, and the mean absorbance was calculated. The ELISA titer was the dilution at which the absorbance unit was nearest to 1.0.
Splenocyte proliferation assay and cytokine production
Immunized mouse spleens were removed from mice 2 weeks after the third immunization and their spleen T cells resuspended in RPMI 1640 (Gibco™, Invitrogen, Cat. No. 23400-021) supplemented with 2 mM l-glutamine, 20 mM HEPES, 24-mM sodium bicarbonate, pH 7.4, 1 % antibiotic/anti-mycotic, and 10 % fetal bovine serum at 5 × 105 cells has been described elsewhere (Alaro et al. 2010). Briefly, aliquots containing 100 μL of cell suspension were distributed into round-bottom 96-well microculture plates (Corning), and 100 μL of MSP-10 proteins were added at final concentrations of 2.5, 5, or 10 μg/mL. Spleen T cells stimulated with 5 μg/mL of concanavalin A (Con A; Sigma-Aldrich) or with 10 μg/mL of lipopolysaccharide (LPS; Sigma-Aldrich) in medium were used as positive controls and with medium alone as negative controls. After a 72-h culture (37 °C and 5 % CO2), 100 μL of supernatant/well was collected from each well and 50 μL enhanced cell viability buffer (Daeil Lab Service, Seoul, ROK) subsequently added to the 50 μL of splenocyte suspension. Four hours later, OD was determined at 450 nm using a microplate-reading spectrophotometer.
Cytokine concentrations in culture supernatants from immunized mice were assayed using a BD CBA Flex Set kit (BD Biosciences, San Diego, CA, USA) for mouse gamma interferon (IFN-γ), tumor necrosis factor (TNF), interleukin-12p70 (IL-12p70), IL-2, IL-4, IL-5, and IL-10 (BD Biosciences). Data were acquired using a FACS Aria II cell sorter (BD Biosciences) according to the manufacturer’s instructions and analyzed using FCAP array software (Soft Flow, Kedves, Hungary).
Statistical analyses
Data were analyzed using GraphPad Prism (GraphPad Software, San Diego, CA, USA) and SigmaPlot (Systat Software Inc., San Jose, CA, USA). Student’s t tests or one-way ANOVAs were used to compare the differences between the means of each group. Values of p < 0.05 were considered statistically significant. Simple scatter regression was used for the generation of standard curves.
Results
Synthesis of recombinant PvMSP-10 protein using a wheat germ cell-free system
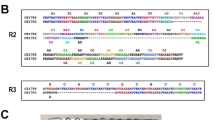
The complete PvMSP-10 protein sequence in the Sal-1 strain consists of 479 residues, similar to the lengths found in other species. PvMSP-10 contains one signal peptide (amino acid [aa] 1–23), two EGF domains (aa 370–454), and a GPI anchor (aa 455–479) (Fig. 1a). Full-length PvMSP-10 (ΔSP/GPI) with a His-tag was designed and expressed using the WGCF. A single strong target band around 65 kDa indicated that the recombinant protein resolved in a 12 % SDS-polyacrylamide gel (Fig. 1b, arrow), slightly higher than the (about 52 kDa) molecular weight predicted for PvMSP-10. This slight increase in molecular weight has also been observed in PvMSP-10 expressed previously (Giraldo et al. 2009). The purified soluble PvMSP-10 concentration was 450 μg/mL.
Schematic of the recombinant PvMSP-10 protein designed for expression. a The PvMSP-10 protein comprises 479 amino acids, with a calculated molecular mass of 52.3 kDa. Indicated are the signal peptide (amino acid [aa] positions 1–23), two EGF domains (aa 370–410 and 416–454), and the GPI-anchor domain (aa 456–479). b Purified PvMSP-10 (approximately 64 kDa) was resolved by 12.5 % SDS-PAGE. M protein marker, T total translation mix, S supernatant, P purification, Ft flow-through, E elution fraction. c Western blot analysis of PvMSP-10 using anti-His antibody (lane a), mouse immune serum (lane b), rabbit immune serum (lane c), and mixed vivax patient sera (lane d). Arrowheads indicate bands specific to each recombinant protein
Western blot analysis
To determine the recombinant protein purity, the specificity of the animal anti-serum and the immunogenic proportion, anti-His antibody, anti-PvMSP-10 mouse immune sera, anti-PvMSP-10 rabbit sera, and vivax patient pooled sera were used, respectively, as primary antibodies for Western blot analysis (Fig. 1c). We purified recombinant PvMSP-10 protein, which was detected by anti-His antibody (Fig. 1c, line a). Highly purified recombinant PvMSP-10 protein was used to immunize mice and rabbits to produce polyclonal antibodies. These polyclonal antibodies were specific for PvMSP-10 (Fig. 1c, lines b and c). Vivax patient sera specifically reacted with this recombinant PvMSP-10 (Fig. 1c, line d), suggesting that the PvMSP-10 prepared using the WGCF system retained native epitopes. Preimmune animal sera and noninfected human sera were also examined, but no band was detected (data not shown).
Humoral immune response analysis
To evaluate the humoral immune response to PvMSP-10, a protein array of purified recombinant PvMSP-10 protein was used to screen the presence of antibodies in human sera. Antibody responses against recombinant PvMSP-10 in serum samples from 112 patients infected with P. vivax and 80 healthy individuals were determined. The sera from individuals exposed to P. vivax showed significantly higher MFI than that from malaria-naïve subjects (Fig. 2a, p < 0.0001). Prevalence of anti-PvMSP-10 antibodies showed that the sensitivity was 42 % (95 % confidence interval [CI], 33.2–51.2 %) and specificity was 95 % (95 % CI, 87.8–98.0 %) (Table 1).
Total IgG and IgG subclass responses to PvMSP-10 as determined using protein microarrays and ELISA. a PvMSP-10 was probed with the sera of 112 malaria patients and 80 healthy individuals from the ROK. There were significant differences in the total prevalence of anti-PvMSP-10 IgG between vivax patients and healthy individuals (p < 0.0001). Bar indicates the cut-off value defined as two standard deviations (SDs) above the mean fluorescence intensity of healthy individuals, and dashed line indicates the mean value. b Immunoreactivity of IgG subclass antibodies against PvMSP-10 with the sera of 20 patient samples selected from total IgG-positive samples. c IgG subclass levels in immune mouse samples and their proportions. IgG subclass responses after the final immunizations were analyzed by ELISA for IgG1, IgG2a, IgG2b, and IgG3, respectively. Error bars indicate the mean ± SDs. p values were calculated using Student’s t tests. Single asterisk, p < 0.05; double asterisks, p < 0.01; triple asterisks, p < 0.0001
IgG subclass distribution among 20 malaria-infected individuals from the 47 total IgG-positive patient samples was tested. Though the intensity of IgG subclass against PvMSP10 varied, the pattern of antibody subclass reactivity to PvMSP10 was cytophilic antibodies IgG1 and IgG3 as dominant (prevalence of IgG1 and IgG3, ≥80 %). Substantial levels of IgG2 antibodies were also detected against PvMSP10 (prevalence of IgG2, 75 %) (Fig. 2b). Antibody responses in human were predominantly to cytophilic IgG1 and IgG3 responses, indicating that PvMSP10 may induce a high efficacy of host protection. Prevalence of anti-PvMSP-10 IgG subclasses showed that the sensitivity was 90 % (IgG1), 75 % (IgG2), 80 % (IgG3), and 60 % (IgG4) (Table 1).
PvMSP-10 is a merozoite surface protein
To localize native PvMSP-10 protein in vivax parasites, we developed an IFA using anti-PvMSP-10 and PvMSP1-19 sera. In mature schizonts, the PvMSP-10 localizes to the surface of merozoite parasites, which completely merges with PvMSP-1 (Fig. 3). This finding demonstrates that the native PvMSP-10 localizes on the merozoite surface.
Localization of PvMSP-10 in mature schizonts. a Schizont-stage parasites were double-labeled with rabbit antisera against PvMSP-10 (red) and mouse antisera against PvMSP1-19 (merozoite surface marker, green). b Schizont-stage parasites were also double-labeled with mouse antisera against PvMSP-10 (red) and rabbit antisera against PvMSP1-19 (green). Nuclei were visualized using DAPI staining in merged images. Bar represents 5 μm
The isotype distribution of the antibody response in mice
The isotype distribution of anti-PvMSP-10 antibodies from immunized mice was analyzed. As shown in Fig. 2c, noncytophilic antibodies (IgG1 and IgG3) are major components of the antibody response by MSP-10-immunized mice. IgG1 levels were significantly higher than that of IgG2a (p < 0.01), IgG2b (p < 0.01), and IgG3 (p < 0.01). The imbalance was observed in the BALB/c mice group immunized with PvMSP-10 (IgG1/IgG2b ratio: 3.9).
Antigen-specific T-cell cytokine responses
To analyze the proliferation of cytokines in the culture supernatant elicited by PvMSP-10, the frequencies of cells producing IL-2, IL-4, IL-5, IL-10, TNF-α, and IFN-γ at the single-cell level were determined using a Cytometric bead array (CBA) assay. After a 72-h culture, we tested splenocyte proliferation, which was stimulated by various concentrations of recombinant PvMSP-10 protein, ConA (positive control), LPS (positive control), and culture medium alone (negative control) (Fig. 4a). We found that spleen T cells elicited the most powerful cytokine response to stimulation with 10 μg/mL PvMSP-10. IFN-γ, IL-2, IL-10, and TNF could be detected, but not IL-4 or IL-5. Additionally, IL-12p70 was detected at only a background level (data not shown). By contrast, the nonimmunized group showed only TNF (12.2 to 22.9 pg/mL), but no other detectable cytokine response to PvMSP-10 (data not shown). The results obtained from spleen T cells of MSP-10-immunized mice indicate that Th1 response play a more predominant role, with TNF, IFN-γ, and IL-2 secreted in this study (Fig. 4b). In contrast, in Th2 profiling cytokines, only IL-10 levels were measured from 3.2 to 16.0 pg/ml.
Cytokine levels in supernatants from 72-h splenocyte cultures from PvMSP-10-immunized BALB/c mice stimulated with PvMSP-10 ex-vivo. a The levels (OD 450 nm) of spleen T cells from mice immunized with PvMSP-10 or PBS (nonimmunized control) following stimulation with culture medium and purified PvMSP-10 (0, 2.5, 5, and 10 μg/mL). Proliferation of spleen T cells stimulated with Con A or LPS as a positive control are indicated. b Each cytokine level was shown individually for each mouse tested in duplicate with samples taken after the third immunization. The levels of IL-4 and IL-5 were below the level of detection in this study (data not shown). Error bars represent the geometric mean of mice ± SDs. Triple asterisks, p < 0.0001
Mouse and rabbit serum IgG titers against the PvMSP10
After three immunizations with recombinant PvMSP10, the specific IgG titers of immune BALB/c mouse and rabbit sera were determined by ELISA. The titers against PvMSP10 were 480,000 ± 160,000 (mean ± SD, mouse) and 1,600,000 (rabbit), respectively (Fig. 5). Thus, PvMSP10 induced a robust immune response in PvMSP10-immunized mice and rabbit. Together with the strong immunoreactivity of vivax patient sera to PvMSP10, these data strongly suggested that PvMSP10 induces a long-term, potent immune response, supporting its potential use in a rationally-designed vivax malaria vaccine.
Discussion
To screen for an effective vivax malaria vaccine, we focused our studies on blood-stage antigens and targeted those that were highly immunoreactive with vivax patient serum antibodies. These antibodies have a key role in blood-stage immunity (Langhorne et al. 2008). This strategy led us to characterize PvMSP-10, which was identified previously for its antigenicity (sensitivity 45 %) (Chen et al. 2010). However, antibody induced against PvMSP-10 shows no protective efficacy for animals challenged with P. vivax parasites (Giraldo et al. 2009). For this reason, we undertook a detailed study of the immunogenicity of this antigen in mice. Furthermore, antigenicity studies confirm previous findings (Chen et al. 2010) and indicate that this antigen may be the target of immunologically active antibodies.
As demonstrated by SDS-PAGE, we successfully expressed recombinant PvMSP-10 protein using a WGCF system (Tsuboi et al. 2008), which produces properly folded, high-quality protein in quantities sufficient for further study. This high-purity recombinant PvMSP-10 protein was used directly for immunization, and antibodies raised against it were functionally active according to the biochemical, immunocytochemical, and biological analyses performed in this study. Based on the protein used for immunization, the localization of PvMSP-10 was demonstrated clearly on the merozoite surface of parasites using animal immune sera.
Antibodies play an essential role in the protection against blood-stage malaria. However, in previous studies, the induction of antibodies by immunization with full-length recombinant PvMSP-10 antigens did not confer effective protection against an experimental challenge with P. vivax parasites in Aotus monkeys (Giraldo et al. 2009). This reason for such lack of protection is still not clear, but may be associated with imperfect adjustment of the key peptide inside the groove of class II major histocompatibility molecules (Giraldo et al. 2009). In this study, we found that antibodies of all four IgG subclasses (IgG1, IgG2a, IgG2b, and IgG3) in immunized mice appear to recognize PvMSP-10 and that noncytophilic IgG1 plays a predominant role in the mouse immune response, whereas a previous study strongly suggested that anti-malarial antibodies of the cytophilic subclass (IgG2a) are predominantly responsible for protection (White et al. 1991). Furthermore, our data show that the cytokines (Th1 profiling) predominantly induced by PvMSP-10 in vitro are in keeping with the IgG subclass distribution in immunized mice, indicating that a noncytophilic response plays a predominant role in the immune response of PvMSP-10-immunized mice. Although the reason for PvMSP-10-induced antibodies failing to confer protection in animal models is uncertain, the noncytophilic response confirmed in our study may be associated with its lack of effective protection.
In this study, we analyzed the IgG prevalence (42 %) of vivax patient serum antibodies to PvMSP-10. In detail, the IgG1 and IgG3 response were more prevalent than the IgG2 and IgG4 response, whereas the level of IgG2 was the highest. In previous studies, cytophilic subclasses (IgG1 and mainly IgG3) were the most abundant isotypes produced by individuals who are protected from malarial parasites, whereas noncytophilic classes (IgG2) predominated among the anti-malarial antibodies developed by unprotected subjects (Oeuvray et al. 1994; Branch et al. 2000). Conversely, a recent study conducted in Burkina Faso showed an association between the levels of parasite antigen-specific IgG2 and resistance to P. falciparum, suggesting that IgG2 has a cytophilic isotype role and contributes to parasite clearance (Aucan et al. 2000). In agreement with these, PvMSP10 possibly was able to trigger anti-parasitic antibody-dependent cellular inhibition mechanism. Notably, cytophilic classes were predominant in vivax-infected patient against PvMSP10, but noncytophilic classes were found in PvMSP10-immunized mice. These similar finding was also found in other Plasmodium proteins (Daher et al. 2010; Demanga et al. 2010). Nevertheless, IgG1 and IgG3 predominantly responding against PvMSP-10 found in vivax patients suggested that PvMSP-10 should produce antibodies in patients, which may be associated with resistance to vivax malaria.
Th1 responses are important for the clearance of malaria parasite (Angulo and Fresno. 2002; Langhorne et al. 2008), and the secretion of IFN-γ by both CD4+ and CD8+ T cells is associated with a protective immune response, which appears to be critically linked to or to act through IFN-γ production, thereby allowing an early and sustained Th1 response. TNF, as another Th1 cytokine, is protective against the parasite (Taverne et al. 1987). Severe malaria anemia has been associated with low IL-10 responses (Kurtzhals et al. 1998), whereas respiratory distress is associated with abnormally large amounts of IL-10. IL-2 is a major T-cell growth factor and is important for the homeostasis, proliferation, and differentiation of CD4+ and CD8+ T cells (Smith. 1988). IL-2 might regulate the balance between effector Th1 cells and Treg cells during blood-stage malaria (Finney et al. 2010; Scholzen et al. 2010; Berretta et al. 2011). All together, PvMSP-10 induced Th1 profile predominant response in mouse suggesting that PvMSP10 might be able to produce an effective protection for host.
In summary, our data confirmed that PvMSP-10 is a MSP with a different localization to that of PfMSP10 (Black et al. 2003). PvMSP-10 can induce an immune response in vivax patients. Both humoral and cellular immunity profiling indicated that PvMSP-10 had a highly immunogenic antigenicity. High levels of IgG subclasses were found after exposure to the PvMSP-10 protein.
References
Alaro JR, Lynch MM, Burns JM Jr (2010) Protective immune responses elicited by immunization with a chimeric blood-stage malaria vaccine persist but are not boosted by Plasmodium yoelii challenge infection. Vaccine 28:6876–6884
Angulo I, Fresno M (2002) Cytokines in the pathogenesis of and protection against malaria. Clin Diagn Lab Immunol 9:1145–1152
Anstey NM, Russell B, Yeo TW, Price RN (2009) The pathophysiology of vivax malaria. Trends Parasitol 25:220–227
Arumugam TU, Takeo S, Yamasaki T, Thonkukiatkul A, Miura K, Otsuki H, Zhou H, Long CA, Sattabongkot J, Thompson J, Wilson DW, Beeson JG, Healer J, Crabb BS, Cowman AF, Torii M, Tsuboi T (2011) Discovery of GAMA, a Plasmodium falciparum merozoite micronemal protein, as a novel blood-stage vaccine candidate antigen. Infect Immun 79:4523–4532
Aucan C, Traore Y, Tall F, Nacro B, Traore-Leroux T, Fumoux F, Rihet P (2000) High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Infect Immun 68:1252–1258
Bang G, Prieur E, Roussilhon C, Druilhe P (2011) Pre-clinical assessment of novel multivalent MSP3 malaria vaccine constructs. PLoS One 6:e28165
Berretta F, St-Pierre J, Piccirillo CA, Stevenson MM (2011) IL-2 contributes to maintaining a balance between CD4 + Foxp3+ regulatory T cells and effector CD4+ T cells required for immune control of blood-stage malaria infection. J Immunol 186:4862–4871
Black CG, Wang L, Wu T, Coppel RL (2003) Apical location of a novel EGF-like domain-containing protein of Plasmodium falciparum. Mol Biochem Parasitol 127:59–68
Branch OH, Oloo AJ, Nahlen BL, Kaslow D, Lal AA (2000) Anti-merozoite surface protein-1 19-kDa IgG in mother-infant pairs naturally exposed to Plasmodium falciparum: subclass analysis with age, exposure to asexual parasitemia, and protection against malaria. V. The Asembo Bay Cohort Project. J Infect Dis 181:1746–1752
Chen JH, Jung JW, Wang Y, Ha KS, Lu F, Lim CS, Takeo S, Tsuboi T, Han ET (2010) Immunoproteomics profiling of blood stage Plasmodium vivax infection by high-throughput screening assays. J Proteome Res 9:6479–6489
Cheng Y, Lu F, Tsuboi T, Han ET (2013a) Characterization of a novel merozoite surface protein of Plasmodium vivax, Pv41. Acta Trop 126:222–228
Cheng Y, Wang Y, Ito D, Kong DH, Ha KS, Chen JH, Lu F, Li J, Wang B, Takashima E, Sattabongkot J, Tsuboi T, Han ET (2013b) The Plasmodium vivax merozoite surface protein 1 paralog is a novel erythrocyte-binding ligand of P. vivax. Infect Immun 81:1585–1595
Collins WE, Kaslow DC, Sullivan JS, Morris CL, Galland GG, Yang C, Saekhou AM, Xiao L, Lal AA (1999) Testing the efficacy of a recombinant merozoite surface protein (MSP-1(19)) of Plasmodium vivax in Saimiri boliviensis monkeys. Am J Trop Med Hyg 60:350–356
Daher LJ, Demanga CG, Prieur E, Perignon JL, Bouharoun-Tayoun H, Druilhe P (2010) Toward the rational design of a malaria vaccine construct using the MSP3 family as an example: contribution of immunogenicity studies in models. Infect Immun 78:477–485
Demanga CG, Daher LJ, Prieur E, Blanc C, Perignon JL, Bouharoun-Tayoun H, Druilhe P (2010) Toward the rational design of a malaria vaccine construct using the MSP3 family as an example: contribution of antigenicity studies in humans. Infect Immun 78:486–494
Finney OC, Riley EM, Walther M (2010) Regulatory T cells in malaria—friend or foe? Trends Immunol 31:63–70
Genton B, D'Acremont V, Rare L, Baea K, Reeder JC, Alpers MP, Muller I (2008) Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med 5:e127
Giraldo MA, Arevalo-Pinzon G, Rojas-Caraballo J, Mongui A, Rodriguez R, Patarroyo MA (2009) Vaccination with recombinant Plasmodium vivax MSP-10 formulated in different adjuvants induces strong immunogenicity but no protection. Vaccine 28:7–13
Herrera S, Corradin G, Arevalo-Herrera M (2007) An update on the search for a Plasmodium vivax vaccine. Trends Parasitol 23:122–128
Kurtzhals JA, Adabayeri V, Goka BQ, Akanmori BD, Oliver-Commey JO, Nkrumah FK, Behr C, Hviid L (1998) Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet 351:1768–1772
Langhorne J, Ndungu FM, Sponaas AM, Marsh K (2008) Immunity to malaria: more questions than answers. Nat Immunol 9:725–732
Lima-Junior JC, Tran TM, Meyer EV, Singh B, De-Simone SG, Santos F, Daniel-Ribeiro CT, Moreno A, Barnwell JW, Galinski MR, Oliveira-Ferreira J (2008) Naturally acquired humoral and cellular immune responses to Plasmodium vivax merozoite surface protein 9 in Northwestern Amazon individuals. Vaccine 26:6645–6654
Nogueira PA, Alves FP, Fernandez-Becerra C, Pein O, Santos NR, Pereira da Silva LH, Camargo EP, del Portillo HA (2006) A reduced risk of infection with Plasmodium vivax and clinical protection against malaria are associated with antibodies against the N terminus but not the C terminus of merozoite surface protein 1. Infect Immun 74:2726–2733
Oeuvray C, Bouharoun-Tayoun H, Gras-Masse H, Bottius E, Kaidoh T, Aikawa M, Filgueira MC, Tartar A, Druilhe P (1994) Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84:1594–1602
Pacheco MA, Elango AP, Rahman AA, Fisher D, Collins WE, Barnwell JW, Escalante AA (2012) Evidence of purifying selection on merozoite surface protein 8 (MSP8) and 10 (MSP10) in Plasmodium spp. Infect Genet Evol 12:978–986
Perez-Leal O, Sierra AY, Barrero CA, Moncada C, Martinez P, Cortes J, Lopez Y, Salazar LM, Hoebeke J, Patarroyo MA (2005) Identifying and characterising the Plasmodium falciparum merozoite surface protein 10 Plasmodium vivax homologue. Biochem Biophys Res Commun 331:1178–1184
Scholzen A, Minigo G, Plebanski M (2010) Heroes or villains? T regulatory cells in malaria infection. Trends Parasitol 26:16–25
Sirima SB, Cousens S, Druilhe P (2011) Protection against malaria by MSP3 candidate vaccine. N Engl J Med 365:1062–1064
Smith KA (1988) Interleukin-2: inception, impact, and implications. Science 240:1169–1176
Soares IS, Levitus G, Souza JM, Del Portillo HA, Rodrigues MM (1997) Acquired immune responses to the N- and C-terminal regions of Plasmodium vivax merozoite surface protein 1 in individuals exposed to malaria. Infect Immun 65:1606–1614
Taverne J, Tavernier J, Fiers W, Playfair JH (1987) Recombinant tumour necrosis factor inhibits malaria parasites in vivo but not in vitro. Clin Exp Immunol 67:1–4
Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, Karyana M, Lampah DA, Price RN (2008) Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med 5:e128
Tsuboi T, Takeo S, Iriko H, Jin L, Tsuchimochi M, Matsuda S, Han ET, Otsuki H, Kaneko O, Sattabongkot J, Udomsangpetch R, Sawasaki T, Torii M, Endo Y (2008) Wheat germ cell-free system-based production of malaria proteins for discovery of novel vaccine candidates. Infect Immun 76:1702–1708
Tsuboi T, Takeo S, Sawasaki T, Torii M, Endo Y (2010) An efficient approach to the production of vaccines against the malaria parasite. Methods Mol Biol 607:73–83
Valderrama-Aguirre A, Quintero G, Gomez A, Castellanos A, Perez Y, Mendez F, Arevalo-Herrera M, Herrera S (2005) Antigenicity, immunogenicity, and protective efficacy of Plasmodium vivax MSP1 PV200l: a potential malaria vaccine subunit. Am J Trop Med Hyg 73:16–24
Vargas-Serrato E, Barnwell JW, Ingravallo P, Perler FB, Galinski MR (2002) Merozoite surface protein-9 of Plasmodium vivax and related simian malaria parasites is orthologous to p101/ABRA of P. falciparum. Mol Biochem Parasitol 120:41–52
White WI, Evans CB, Taylor DW (1991) Antimalarial antibodies of the immunoglobulin G2a isotype modulate parasitemias in mice infected with Plasmodium yoelii. Infect Immun 59:3547–3554
Zeyrek FY, Babaoglu A, Demirel S, Erdogan DD, Ak M, Korkmaz M, Coban C (2008) Analysis of naturally acquired antibody responses to the 19-kd C-terminal region of merozoite surface protein-1 of Plasmodium vivax from individuals in Sanliurfa, Turkey. Am J Trop Med Hyg 78:729–732
Acknowledgments
We are grateful to Deok-Hoon Kong, Hye-Yoon Jeon, Jung-Yeon Lee, Jin-Hee Han, Seong-Kyun Lee, and Professor Kwon-Soo Ha for their technical assistance with the protein array. This work was supported by a Mid-Career Researcher Program through a NRF grant funded by the MEST (2011-0016401). This work was also supported in part by MEXT KAKENHI (23117008), JSPS KAKENHI (23406007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Eun-Taek Han and Takafumi Tsuboi are co-corresponding authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. S1
Simple scatter regression was used for generation of mouse IgG subclass standard curves (PPTX 54 kb)
Rights and permissions
About this article
Cite this article
Cheng, Y., Wang, B., Sattabongkot, J. et al. Immunogenicity and antigenicity of Plasmodium vivax merozoite surface protein 10. Parasitol Res 113, 2559–2568 (2014). https://doi.org/10.1007/s00436-014-3907-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3907-8