Abstract
Polytene chromosomes were prepared from the ovarian nurse cells of semi-gravid females of ten insecticide-resistant strains of Anopheles stephensi. Altogether, 16 heterozygous paracentric inversions, namely b/+ (11D-16C) in alphamethrin; i/+ (14B-18A) and h/+ (27B-28A) in DDT; j/+ (14A-16B) in chlorpyrifos; k/+ (11D-16B) in cyfluthrin; l/+ (11A-16C) in deltamethrin; m/+ (14B-15C) and e/+ (32A-33B) in bifenthrin; n/+ (12D-14B), f/+ (33A-36A) and g/+ (33C-34A) in propoxur; o/+ (11A-12D), h/+ (37A-37C) and i/+ (31C-32C) in temephos; d/+ (33D-35C) in carbofuran and a/+ (41C-43B) in neem strains, were reported. No inversions were observed in X chromosome so far. The frequency of inversions in different insecticides was found to be highest in the 2R arm, followed by the 3R arm. Such inversions were not reported in the corresponding susceptible strains or in the parental stocks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malaria is a major global health problem, with a huge economic and social impact throughout the endemic areas (Wieten et al. 2011). According to the WHO estimates, malaria still accounts for 225 million clinical cases with nearly one million deaths per year mainly among young children (Jortzik et al. 2011). Insecticide-based vector control is a proven method for disease control (van Emden and Service 2004), but in response to the extensive use of insecticides to control mosquitoes, a number of resistant strains have appeared. Insecticide resistance is one of the most spectacular examples of rapid adaptation to strong selection pressure (Paris et al. 2010); such a remarkable ability highly depends on its genetic variability. Information on genetic variation within and among mosquito populations is critical for understanding the evolutionary history and disease epidemiology (Tabachnick and Black 1996).
Chromosomal polymorphism (such as inversions, Robertsonian fusions and fissions, and translocations) has been recognized as a major driving force in local adaptation, speciation processes, and evolution of sex chromosomes (Hoffmann et al. 2004; King 1993; Noor et al. 2001; van Doorn and Kirkpatrick 2007). In insects that possess a highly polymorphic chromosome complement, one might at least expect inversion heterozygotes to exhibit insecticide tolerance through heterosis (White 1974). Chromosomal inversions have been repeatedly regarded as genetic markers for ecotypic adaptation (Coluzzi et al. 1985; Hoffmann and Willi 2008). Correlations between chromosomal inversion frequencies and environmental parameters have been reported in a number of plant and animal species (Coluzzi et al. 1979; Hoffmann et al. 2004; Rodriguez-Trelles et al. 1996).
Afrotropical anopheline mosquitoes are emerging as suitable biological models in understanding the role of chromosomal inversions in ecological adaptation and speciation as exemplified by the rapidly growing literature available on this topic in the malaria mosquito Anopheles gambiae (Coluzzi et al. 2002; della Torre et al. 2002). However, very little work has been done on chromosome polymorphism in Anopheles stephensi, one of the important carriers of urban malaria in India (Gayathri and Shetty 1992). Literature on chromosomal polymorphism and its correlation with insecticide resistance is scarcely available for this species. The study of inversion in the polytene chromosome of A. stephensi is an excellent tool for cytogenetic differentiation of populations. It also helps to determine the role of fixed chromosomal inversions in speciation and to reconstruct the cytogenetic history of the strains (Ghosh and Shetty 2004).
The present paper reports paracentric inversions, their breakpoints and frequencies in the polytene chromosomes of ovarian nurse cells of semi-gravid females of ten insecticide-resistant strains of A. stephensi.
Materials and methods
Mosquito rearing
Insecticide-resistant strains of A. stephensi derived from different classes of insecticides and currently maintained in our laboratory were used for the study. The said strains were maintained at 25 ± 1 °C and 75 ± 5 % of relative humidity with 14-h photoperiods, following the procedure of Shetty (1983). The adults were fed on 10 % sucrose, in 8 in. × 8 in. × 8 in. iron cages, covered with cotton net cloth. Females were provided with restrained mice or pigeon as a source of blood meal. A plastic cup (3-in. diameter) containing clean water lined with filter paper was placed inside the cage for oviposition. The eggs laid were kept for 72 h to ensure complete hatching. The hatched larvae were transferred to an enamel tray and reared. Powdered mixture of fish feed and dog biscuits were given as larval diet. Pupae formed were transferred into wide-mouthed bottles and placed into their respective cages for emergence.
Insecticide-resistant strains
The strains used in this study belong to the “type” biological form of A. stephensi which were originally collected from various areas of urban Bangalore (India) and maintained as a pure line stock in the laboratory (Table 1). Further, these are laboratory-induced insecticide-resistant strains that have been established after continuous selection and inbreeding for several generations for respective insecticides following the standard procedure of WHO (1981; 2005). Discriminating dose (DD) used for each one of the insecticides was WHO-recommended, except for alphamethrin, carbofuran and neem for which DDs were calculated as per WHO guidelines (2006). Resistance tests (WHO 1981; 2005) were carried out on every generation so as to validate and maintain the purity of resistance. Detailed information on each one of the insecticide-resistant strains is presented in Table 1.
Inversion polymorphism studies
Inversion polymorphism studies were carried out in polytene chromosomes from ovarian nurse cells of semi-gravid females of A. stephensi, following the procedures of French et al. (1962) and Gayathri and Shetty (1992). Five- to seven-day-old females of insecticide-resistant strains were blood-fed on restrained mice. After 28 to 30 h, these semi-gravid females were individually separated into a test tube and immobilized by striking the lower end of the test tube gently against the palm. The mosquito was then placed on a slide and a drop of dilute Carnoy's fixative (Carnoy's fixative/distilled water, 1:19) was added. A quick and successful method to remove the ovaries was to hold the anterior of the abdomen with one needle (left hand) and the penultimate segment of the abdomen with the other and then to give a sharp pull posteriorly to cut the last two segments. The abdomen was then gently pressed, spilling the ovaries. The ovaries were separated from the debris and then fixed in Carnoy's fixative (methanol/acetic acid, 3:1) for 2 to 4 min.
For routine staining of polytene chromosomes, synthetic orcein was used for lacto-aceto-orcein (LAO). Orcein was mixed with glacial acetic acid, in the following proportion: 2 g of orcein in 50 ml of 85 % lactic acid and 50 ml of 100 % glacial acetic acid. The stock solution of LAO was stored in a cool and dry place. It was further diluted in lactic acid and acetic acid (1:1) prior to use to avoid overstaining. After fixation, the material was stained with a drop of LAO for 15–20 min. After staining, 60 % acetic acid was added and a clean coverslip was placed on the top of the material. Gentle pressure was applied to obtain an even spread. The coverslip was sealed with nail polish and the slide was examined under the microscope.
The nomenclature of inversions and their frequencies were followed, according to the method of Coluzzi et al. (1973). The zones and sub-zones, carrying various aberrations, were identified and assigned their exact location on the chromosome by using the standard polytene chromosome map (Gayathri and Shetty 1989; Sharakhova et al. 2006).
Results and discussion
The data on chromosomal inversions from ovarian nurse cells of insecticide-resistant strains showing their tentative breakpoint is presented in Table 2. Altogether, 16 heterozygous paracentric inversions were observed in insecticide-resistant strains. These include eight inversions on the 2R arm and six on the 3R arm. Arms 2L and 3L showed the presence of only one inversion in each. No inversions have been observed in X chromosome so far studied.
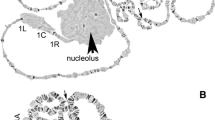
The present study revealed that chromosome arm 2R tends to be rich in polymorphic inversions among insecticide-resistant strains. Eight heterozygous paracentric inversions were found in the 2R arm of different insecticide-resistant strains. These include b/+ (11D-16C) in AM-R, i/+ (14B-18A) in DDT-R, j/+ (14A-16B) in CPF-R, k/+ (11D-16B) in CyF-R, l/+ (11A-16C) in DL-R, m/+ (14B-15C) in BIF-R, n/+ (12D-14B) in PR-R and o/+ (11A-12D) in TR-R with 13.33, 2.2, 0.5, 1.8, 3.4, 19.23, 3.92 and 9.2 % of frequencies, respectively. Six heterozygous inversions were observed in chromosome arm 3R. These include d/+ (33D-35C) in CBF-R, e/+ (32A-33B) in BIF-R, f/+ (33A-36A) and g/+ (33C-34A) in PR-R, h/+ (37A-37C) and i/+ (31C-32C) in TR-R with 0.3, 10.71, 4.09, 6.45, 3.0 and 10.56 % frequencies, respectively. It is pertinent to mention here that though there were overlapping inversions, no two inversions have the same breakpoints and there is involvement of the bands in the polytene chromosome. Inversion h/+ (27B-28A) in DDT-resistant strain and a/+ (41C-43B) in neem-resistant strain each showed one heterozygous inversion with a frequency of 1.5 and 8.67 % in 2L and 3L, respectively. Paracentric inversions in insecticide-resistant strains are presented in Fig. 1.
We also recorded a higher rate of heterozygous inversions within region 10–18 in the 2R arm, followed by inversions involving region 31 to 36 of chromosome arm 3R. The frequency of inversions was highest in 2R followed by 3R. It was also evident that inversions associated with the synthetic pyrethroid class of insecticide resistance were clustered in arm 2R, whereas inversions associated with carbamates were more in 3R. Schematic diagrams of chromosome arms representing insecticide-associated inversions are presented in Fig. 2.
Chromosome arm II represents a higher gene density, among which 2R has more of the genes involved in cellular response to stress and 2L is enriched with genes involved in the structural integrity of a cuticle (Xia et al. 2010). Arm 2R in A. stephensi is longest in the complement and, hence, has more chances of exposure to cellular stress compared to the other arms in the complement. Arm 2R is more tolerant to disrupting gene orders and generating new evolutionary breakpoints compared to the other arms (Sharakhova et al. 2011). As observed in our study, inversion breakpoints not only cluster in particular regions, but also appear to coincide in association to other insecticides. This may also, probably, play a crucial role in the occurrence of multiple insecticide resistance. As indicated earlier, no inversion was recorded in the X chromosome of A. stephensi. Genetic studies of the insecticide-resistant strains used in the present study reveal that the resistant genes are autosomal and incompletely dominant in nature and follow the monofactorial mode of inheritance (Chandrakala and Shetty 2004, 2006a, b; Hariprasad and Shetty 2013; Rajashree and Shetty 1998; Sanil and Shetty 2009, 2010; Zin et al. 2008, 2009). Most of the inversions associated with resistance were observed among the autosomes.
The first instance of a relationship between environmental stress and giant chromosome inversions in anophelines was reported by Frizzi and Holstein (1956). Coluzzi et al. (1973) observed six heterozygous paracentric inversions in the laboratory-maintained population of A. stephensi that originated from India, Pakistan, Iraq and Iran. However, of these six inversions, three were overlapping inversions, i.e. b/+, c/+ and d/+, observed in chromosome arm 2R; two inversions were observed in arm 3L and one in arm 2L. No inversion was reported in chromosome arm 3R and in sex chromosome. Inversion “b” was found to be more prevalent in a population of A. stephensi (Coluzzi et al. 1973). Similarly, inversions were also reported from the laboratory-maintained populations of A. stephensi from India (Gayathri and Shetty 1992).
The discovery of insecticide resistance in malaria vectors led to a series of investigations on possible relationships of this phenomenon with chromosomal inversions. An attempt to show a relationship between inversions and insecticide selection pressure has yielded equivocal results. Mason and Brown (1963) reported that inversion heterozygosity has no relation to true or specific resistance, but is simply a manifestation of hybrid constitution. D'Alessandro et al. (1957) observed that DDT-tolerant strains of Anopheles atroparvus contained significantly more heterozygotes for a single inversion on chromosome IIIS than the parental strain from which they had been selected with DDT and that the heterozygotes and inversion homozygotes were more DDT tolerant than the normal heterozygote. A. atroparvus resistant to dieldrin showed more heterozygous inversion in IIIS than the parental stock (Mosna et al. 1958). Mosna et al. (1959) confirmed that three DDT-resistant strains of this stock contained more heterozygotes than the original strain. DDT-exposed Anopheles arabiensis showed a higher percentage of inversion than the unexposed stock (Nigatu et al. 1995). Paracentric inversions associated with dieldrin resistance were also reported in a population of A. gambiae (Benedict et al. 1999). In A. stephensi, three paracentric inversions were reported from the fenitrothion-resistant strains, whereas the corresponding susceptible and the original strain did not reveal any inversions (Ghosh and Shetty 2004). Dieldrin-resistant gene in A. gambiae was mapped to linkage group II on chromosome 2, and the said gene was found to be closely located to the microsatellite marker AG2H772, which probably falls within inversion 2La (Hunt 1987; Zheng et al. 1996).
The stability and maintenance of these inversions as polymorphism provides an explanation for the transmission of disease and control of the vectors. Stable inversion polymorphism also provides a possible mechanism for the continual inheritance of suitable genetic factors that otherwise compromise the fitness of genetically modified malaria vector (Brooke et al. 2002). Insecticide resistance-associated inversions were used in synthesizing genetic sexing strains of mosquito vectors (Curtis et al. 1976; Seawright et al. 1978; Shetty 1987; 1997). Fenitrothion-resistant A. stephensi showed a lower rate of rodent malaria parasite, Plasmodium yoelii nigeriensis, infection when compared to control (Shetty 2002).
Based on the number of ridges on the egg-float, three morpho-ecological variants have been identified within A. stephensi populations. These include type (14–22), mysorensis (9–15) and intermediate (12–17) (Sweet and Rao 1937; Subbarao et al. 1987; Shetty et al. 1999). Of these three variants, the type form is reported to inhabit urban areas and is an efficient vector of malaria. All the insecticide-resistant strains used in the present study belong to the type form.
Apart from the evolutionary interest of chromosomal rearrangements, the investigation of inversion polymorphisms, in relation to the biology and behaviour of malaria vectors, may improve our understanding of mosquito behaviour and of the epidemiology of mosquito-borne diseases. Additional investigations are needed to delineate these polymorphisms more fully in A. stephensi and to evaluate their significance particularly in relation to the transmission of malaria.
References
Benedict MQ, McNitt LM, Cornel AJ, Collins FH (1999) A new marker, black, a useful recombination suppressor in (2)2, and a balanced lethal for chromosome 2 of the mosquito Anopheles gambiae. Am J Trop Med Hyg 61:618–624
Brooke BD, Hunt RH, Chandre F, Carnevalle P, Coetzee M (2002) Stable chromosome inversion polymorphisms and insecticide resistance in the malaria vector mosquito Anopheles gambiae (Diptera: Culicidae). J Med Entomol 39(4):568–573
Chandrakala BN, Shetty NJ (2004) Genetic studies of DDT resistance in the malaria mosquito Anopheles stephensi Liston. J Cytol Genet 5:185–190
Chandrakala BN, Shetty NJ (2006a) Genetic studies of chlorpyrifos resistance in the malaria mosquito Anopheles stephensi Liston. J Cytol Genet 7:155–160
Chandrakala BN, Shetty NJ (2006b) Genetic studies of cyfluthrin resistance in Anopheles stephensi Liston—a malaria mosquito. In: Sobti et al (eds) Proceedings of Prof. G. P. Sharma felicitation: new trends in life sciences. Panjab University, Chandigarh, India, pp 48–52
Coluzzi M, Sabatini A, della Torre A, Di Deco MA, Petrarca V (2002) A polytene chromosome analysis of the Anopheles gambiae species complex. Science 298:1415–1418
Coluzzi M, Sabatini A, Petrarca V, Dideco MA (1979) Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg 73:483–497
Coluzzi M, Di Dicco MA, Cancrini G (1973) Chromosomal inversions in Anopheles stephensi. Parassitologia 15:129–136
Coluzzi M, Petrarca V, Di Deco MA (1985) Chromosomal inversion intergradations and incipient speciation in Anopheles gambiae. Bollettino di Zoologia 52:45–63
Curtis CF, Akiyama J, Davidson G (1976) A genetic sexing system in Anopheles gambiae species A. Mosq News 36:492–498
D'Alessandro G, Frizzi G, Mariani M (1957) Effect of DDT selection pressure on the frequency of chromosomal structures in Anopheles atroparvus. Bull World Health Organ 16:859–864
della Torre A, Costantini C, Besansky NJ, Caccone A, Petrarca V, Powell JR, Coluzzi M (2002) Speciation within Anopheles gambiae—the glass is half full. Science 298:115–117
French WL, Baker RH, Kitzmiller JB (1962) Preparation of mosquito chromosomes. Mosq News 22:377–383
Frizzi G, Holstein M (1956) Etude cytogenetique d'Anopheles gambiae. Bull World Health Organ 15:425–435
Gayathri DK, Shetty NJ (1989) Polytene chromosomes of Anopheles stephensi Liston—a malaria vector. Vignana Bharathi 12:1–8
Gayathri DK, Shetty NJ (1992) Chromosomal inversions in Anopheles stephensi Liston—a malaria mosquito. J Cytol Genet 27:153–161
Ghosh C, Shetty NJ (2004) Tests for association of fenitrothion resistance with inversion polymorphism in the malaria vector, Anopheles stephensi. The Nucleus 47(3):164–168
Hariprasad TPN, Shetty NJ (2013) Autosomal inheritance of alphamethrin, a synthetic pyrethroid, resistance in Anopheles stephensi – Liston, a malaria mosquito. Bull Entomol Res. doi:10.1017/S0007485313000102
Hoffmann AA, Sgro CM, Weeks AR (2004) Chromosomal inversion polymorphisms and adaptation. Trends Ecol Evol 19:482–488
Hoffmann AA, Willi Y (2008) Detecting genetic responses to environmental change. Nat Rev Genet 9:421–432
Hunt RH (1987) Location of gene on chromosome arms in the Anopheles gambiae group of species and their correlation to linkage data for other anopheline mosquitoes. Med Vet Entomol 1:81–88
Jortzik E, Kehr S, Becker K (2011) Post-translational modifications in apicomplexan parasites. In: Mehlhorn H (ed) Parasitology research monographs. Progress in parasitology, vol 2. Springer, Berlin, p 93
King M (1993) Species evolution: the role of chromosome change. Cambridge University Press, Cambridge
Mason GF, Brown AWA (1963) Chromosome changes and insecticide resistance in Anopheles quadrimaculatus. Bull World Health Organ 28:77–81
Mosna E, Palmieri C, Ascher KRS, Rivosecchi L, Neri I (1959) Studies on insecticide resistant anophelines. 2. Chromosome arrangements in laboratory developed DDT resistant strains of Anopheles atroparvus. Bull World Health Organ 20:63–74
Mosna E, Rivosecchi L, Ascher KRS (1958) Studies on insecticide resistant anophelines. 1. Chromosome arrangements in a dieldrin selected strain of Anopheles atroparvus. Bull World Health Organ 19:297–301
Nigatu W, Curtis CF, Lulu M (1995) Test for association of DDT resistance with inversion polymorphism in Anopheles arabiensis from ethiopia. J Am Mosq Control Assoc 1:238–240
Noor MA, Cunningham AL, Larkin JC (2001) Consequences of recombination rate variation on quantitative trait locus mapping studies. Simulations based on the Drosophila melanogaster genome. Genetics 159:581–588
Paris M, Boyer S, Bonin A, Collado A, David J, Despres L (2010) Genome scan in the mosquito Aedes rusticus: population structure and detection of positive selection after insecticide treatment. Mol Ecol 19:325–337
Rajashree BH, Shetty NJ (1998) Genetic study of deltamethrin resistance in the malaria mosquito Anopheles stephensi Liston. J Parasit Dis 22:140–143
Rodriguez-Trelles F, Alvarez G, Zapata C (1996) Time series analysis of seasonal changes of the O inversion polymorphism of Drosophila subobscura. Genetics 142:179–187
Sanil D, Shetty NJ (2009) Genetic study of temephos resistance (tr), an organophosphate insecticide in the malaria mosquito, Anopheles stephensi Liston. J Cytol Genet 11:15–22
Sanil D, Shetty NJ (2010) Genetic study of propoxur resistance—a carbamate insecticide in the malaria mosquito. Anopheles stephensi Liston. Malaria Research and Treatment. doi:10.4061/2010/502824
Seawright JA, Kaiser PE, Dame DA, Lofgren CS (1978) Genetic method for the preferential elimination of females of Anopheles albimanus. Science 200(4347):1303–1304
Sharakhova MV, Xia A, Leman SC, Sharakhov IV (2011) Arm specific dynamics of chromosome evolution in malaria mosquito. BMC Evolutionary Biology 11:91
Sharakhova MV, Xia A, McAlister SI, Sharakhov IV (2006) A standard cytogenetic photomap for the mosquito Anopheles stephensi (Diptera: Culicidae): application for physical mapping. J Med Entomol 43:861–866
Shetty NJ (1983) Chromosomal translocation and semisterility in the malaria vector Anopheles fluviatilis. James Ind J Malariol 20:45–48
Shetty NJ (1987) Genetic sexing system for the preferential elimination of females in Culex quinquefasciatus. J Am Mosq Control Assoc 3(1):84–86
Shetty NJ (1997) Genetic control of mosquito vectors of diseases. J Parasit Dis 21:113–121
Shetty NJ (2002) The genetic control of Anopheles stephensi—a malaria mosquito. In: Raghunath D, Nayak R (eds) Trends in malaria and vaccine research: the current scenario. Tata McGraw-Hill, New Delhi, pp 44–79
Shetty NJ, Madhyastha AD, Ghosh C, Rajasree BH (1999) Egg float ridge number in Anopheles stephensi: ecological variation. J Parasitic Dis 23:45–48
Subbarao SK, Vasantha K, Adak T, Sharma VP, Curtis CF (1987) Egg-float ridge number in Anopheles stephensi: ecological variation and genetic analysis. Med Vet Entomol 1:256–271
Sweet WC, Rao BA (1937) Races of Anopheles stephensi Liston, 1901. Indian Medical Gazette 72:665–674
Tabachnick WJ, Black WC (1996) Population genetics in vector biology. In: Beaty BJ, Marquardt WC (eds) The biology of disease vectors. University Press of Colorado, Niwot, pp 417–437
van Doorn GS, Kirkpatrick M (2007) Turnover of sex chromosomes induced by sexual conflict. Nature 449:909–912
van Emden HF, Service MW (2004) Pest and vector control. Cambridge University Press, Cambridge
White GB (1974) Biological effects of intraspecific chromosomal polymorphism in malaria vector populations. Bull World Health Organ 50:299–306
World Health Organization (WHO) (1981) Instruction for determining the susceptibility or resistance of mosquito larvae to insecticide. WHO/VBC/81.807. WHO, Geneva
World Health Organization (WHO) (2005) Guidelines for laboratory and field testing of mosquito larvicides. WHO/CDS/WHOPES/GCDPP/2005.13. WHO, Geneva
World Health Organization (WHO) (2006) Mosquito adulticides for indoor residual spraying and treatment of mosquito nets. Guidelines for testing. WHO/CDS/NTD/WHOPES/GCDPP/2006.3. WHO, Geneva
Wieten RW, van Vugt M, van Leth F, Grobusch MP (2011) Highlights of a symposium, malaria: where are we today, where are we going? Open Infectious Diseases Journal 5:99–106
Xia A, Sharakhova M, Leman S, Tu Z, Bailey J, Smith C, Sharakhov IV (2010) Genome landscape and evolutionary plasticity of chromosomes in malaria mosquitoes. PLoS ONE 5:e10592
Zheng L, Benedict MQ, Cornel AJ, Collins FH, Kafatos FC (1996) An integrated genetic map of the human malaria vector mosquito, Anopheles gambiae. Genetics 143:941–952
Zin T, Minn MZ, Shetty NJ (2008) Estimation of proteins and enzymes in different developmental stages of neem susceptible and resistant strains of Anopheles stephensi Liston, 1901. Universities Research Journal 1:185–193
Zin T, Minn MZ, Shetty NJ (2009) Biochemical basis of bifenthrin resistance in Anopheles stephensi Liston 1901, a malaria mosquito. Journal of Myanmar Academy of Arts & Science 2:121–130
Acknowledgments
This paper has been supported by financial assistance from the Department of Science and Technology (DST) and University Grants Commission (UGC), New Delhi, to Professor N. J. Shetty.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shetty, N.J., Hariprasad, T.P.N., Sanil, D. et al. Chromosomal inversions among insecticide-resistant strains of Anopheles stephensi Liston, a malaria mosquito. Parasitol Res 112, 3851–3857 (2013). https://doi.org/10.1007/s00436-013-3575-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3575-0