Abstract
Atractolytocestus tenuicollis (Li, 1964) Xi, Wang, Wu, Gao et Nie, 2009 is a monozoic, non-segmented tapeworm of the order Caryophyllidea, parasitizing exclusively common carp (Cyprinus carpio L.). In the current work, the first molecular data, in particular complete ribosomal internal transcribed spacer 2 (ITS2) and partial mitochondrial cytochrome c oxidase subunit I (cox1) on A. tenuicollis from Niushan Lake, Wuhan, China, are provided. In order to evaluate molecular interrelationships within Atractolytocestus, the data on A. tenuicollis were compared with relevant data on two other congeners, Atractolytocestus huronensis and Atractolytocestus sagittatus. Divergent intragenomic copies (ITS2 paralogues) were detected in the ITS2 ribosomal spacer of A. tenuicollis; the same phenomenon has previously been observed also in two other congeners. ITS2 structure of A. tenuicollis was very similar to that of A. huronensis from Slovakia, USA and UK; overall pairwise sequence identity was 91.7–95.2 %. On the other hand, values of sequence identity between A. tenuicollis and A. sagittatus were lower, 69.7–70.9 %. Cox1 sequence, analysed in five A. tenuicollis individuals, were 100 % identical and no intraspecific variation was observed. Comparison of A. tenuicollis cox1 with respective sequences of two other Atractolytocestus species showed that the mitochondrial haplotype found in Chinese A. tenuicollis is structurally specific (haplotype 4; Ha4) and differs from all so far determined Atractolytocestus haplotypes (Ha1 and Ha2 for A. huronensis; Ha3 for A. sagittatus). Pairwise sequence identity between A. tenuicollis cox1 haplotype and remaining three haplotypes followed the same pattern as in ITS2. The nucleotide and amino acide (aa) sequence comparison with A. huronensis Ha1 and Ha2 revealed higher sequence identity, 90.3–90.8 % (96.9 % in aa), while lower values were achieved between A. tenuicollis haplotype and Ha3 of Japanese A. sagittatus—75.2 % (81.9 % in aa). The phylogenetic analyses using cox1, ITS2 and combined cox1 + ITS2 sequences revealed close genetic interrelationship between A. tenuicollis and A. huronensis. Independently of a type of analysis and DNA region used, the topology of obtained trees was always identical; A. tenuicollis formed separate clade with A. huronensis forming a closely related sister group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monozoic, non-segmented tapeworms of the order Caryophyllidea are parasites of cypriniform and siluriform fishes of Europe, North America, Africa, Asia and Australia that are characterised by monozoic body plan and only one set of reproductive organs. Caryophyllidean genus Atractolytocestus Anthony 1958 (family Lytocestidae) comprises three valid species, Atractolytocestus huronensis Anthony 1958; Atractolytocestus sagittatus (Kulakovskaya and Akhmerov 1965); and Atractolytocestus tenuicollis (Li 1964) Xi et al. 2009, all parasitizing exclusively common carp (Cyprinus carpio L.). The first species described within the genus, and so far most intensively studied is A. huronensis. It was originally found in River Huron, Michigan, USA (Anthony 1958), and since then it has been reported in several other North American localities (Hoffman 1999). The species was introduced along with its carp host to Europe, where it has shown its invasive potential and successfully invaded several European countries, such as England (Chubb et al. 1996; Kirk et al. 2003), Hungary (Majoros et al. 2003), Slovakia and the Czech Republic (Oros et al. 2004), Germany (Kappe et al. 2006), Croatia (Gjurcević et al. 2009), and Romania (Bazsalovicsová et al. 2011; Oros et al. 2011). Second species of the genus, A. sagittatus, was originally described from carp from the Amur River basin in Russia (Kulakovskaya and Akhmerov 1965), later on it was also found in Caspian Sea Drainage (Demshin and Dvoryadkin 1981) and Japan (Scholz et al. 2001).
The third and so far the least known species of the genus, A. tenuicollis, was described as Khawia tenuicollis from carp from Lake Wulasuhai, Inner Mongolia, in China (Li 1964), but Xi et al. (2009) transferred it to the genus Atractolytocestus Anthony 1958 according to the morphological characteristics typical to the genus—conical scolex (bulboacuminate type), vitelline follicles continuous alongside uterus and ovary with postovarian vitelline follicles. A. tenuicollis has been accepted as a valid species in recent taxonomic and phylogenetic studies, as a part of complex systematic revision of the order (Oros et al. 2011; Scholz et al. 2011; Xi et al. 2013).
In the current work, the first molecular data on Chinese A. tenuicollis are provided. In particular, complete ribosomal internal transcribed spacer 2 (ITS2) and partial mitochondrial cytochrome c oxidase subunit I (cox1) were analysed. In order to evaluate molecular interrelationships within Atractolytocestus, the data on A. tenuicollis were compared with relevant data on two other congeners, A. huronensis and A. sagittatus.
Materials and methods
Parasite material
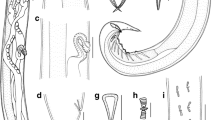
Tapeworms A. tenuicollis were found in the intestine of common carp (C. carpio L.) from Niushan Lake, Wuhan, in China in March 2009 and processed as described in detail by Oros et al. (2011). Live tapeworms were washed and immediately fixed with hot 4 % formalin for morphological studies and five specimens were fixed with ethanol (95–99 %) for DNA analyses. Parasites were identified according to the morphological characters determined by Xi et al. (2009), i.e. bulboacuminate scolex, numerous testes begin posterior to first vitelline follicles, vitelline follicles form an uninterrupted line alongside uterine coils and ovarian arms. Photomicrographs of morphologically analysed tapeworms (Fig. 1) were digitally captured with a Leica DFC 450C camera mounted on a Leica DM 5000B light microscope with differential interference contrast. Voucher specimens are deposited in the Helminthological collection of the Institute of Parasitology, Biology Centre of the Academy of Sciences of the Czech Republic, České Budějovice, Czech Republic (collection no. C-635).
Photomicrographs of A. tenuicollis from Cyprinus carpio in China. a Total view of the whole mounted worm; b anterior part of the body—note the bulboacumminate scolex and the distribution of first vitelline follicles and first testis; c posterior part of the body. Abbreviations: cs, cirrus-sac; fte, first testis; fvf, first vitelline follicles; ov, ovary; pvf, postovarian vitelline follicles; sc, scolex; ut, uterus
Newly obtained molecular data on A. tenuicollis were combined with data on recently published molecular structure of ribosomal ITS2 spacer and mitochondrial cox1 of A. huronensis from several European countries and USA, and A. sagittatus from Japan (Králová-Hromadová et al. 2010; Bazsalovicsová et al. 2011, 2012). The details on Atractolytocestus populations and comparative ITS2 and cox1 sequences are summarised in Tables 1 and 2.
DNA isolation, PCR protocol, sequencing
Genomic DNA was isolated using the QIAamp® DNA Kit (QIAGEN, Hilden, Germany) according to manufacturer’s instructions. Conditions of PCR amplification were described in detail in Králová-Hromadová et al. (2010). Sequencing was performed using automatic genetic analyzer Applied Biosystems 3130xl (Applied Biosystems, Foster City, California, USA) and BigDye Terminator v3.1 Cycle sequencing kit (Applied Biosystems). The sequence alignment was performed using ClustalW (Thompson et al. 1994).
ITS2 amplification, cloning and sequencing
For amplification of complete ITS2 spacer, the 5.8S-2 (5′-GTCGATGAAGAGCGCAGC-3′; Králová-Hromadová et al. 2003) and ITS-2 (5′-AGGAGGCGAATCACTAT-3; Cunningham 1997) primers with annealing positions in the 5.8S and LSU rDNA, respectively, were applied. The PCR products were loaded on the 1.5 % agarose gel and purified using the Wizard PCR purification Kit (Promega, Madison, Wisconsin). Purified PCR products of ITS2 ribosomal spacer amplified from five individuals (AT CH1–AT CH5) were cloned into the pGEM®-T Easy vector (Promega) following the manufacturer’s protocol. Three to five recombinant clones from each individual were purified with the Plasmid miniprep kit (Genomed, Löhne, Germany) and sequenced using universal primers T7 and Sp6 (AT CH1/1–5, AT CH2/1–3, AT CH3/1–3, AT CH4/1–5, AT CH5/1–5). The boundaries of both spacers were determined by sequence alignment using ClustalW (Thompson et al. 1994) according to the sequences of Slovak A. huronensis (Králová-Hromadová et al. 2010).
Partial cox1 amplification
Partial cox1 was amplified in all five A. tenuicollis individuals. The forward primer CFCYT2 (5′-ACTAAGTGTTTTCAAAA-3′), with annealing position in the tRNA-Trp (Tryptophane) and reverse primer CRCYT2 (5′-CCAAAAAACCAAAACAT-3′), annealing about 650 bp inside the cox1 gene, were originally designed by us and described in Bazsalovicsová et al. (2011). The ATG start codon was determined according to the flatworm mitochondrial code (Telford et al. 2000).
Phylogenetic reconstruction
Cox1 sequences were aligned by eye in the Seaview 4.2 (Gouy et al. 2010) and analysed using maximum parsimony (MP), maximum likelihood (ML) and Bayesian inference (BI) approaches. The MP analysis was run in TNT 1.1 (Goloboff et al. 2000). ML was performed in PhyML 3.0 (Guindon et al. 2010) using HKY + I model of molecular evolution with parameters estimated from the data. The same model was used in BI run using MrBayes 3.1.2 (Huelsenbeck and Ronquist 2001). Two independent runs with four chains each were run for 10 million MCMC generations with sampling frequency of 10,000 steps. Twenty-five percent of the samples were discarded as burn in. Tracer 1.5 (Rambaut and Drummond 2005) was used to check convergence between runs. The molecular model was selected in jModeltest 2.1 (Darriba et al. 2012) using AIC criterion. Clade supports for MP and ML trees were obtained with 1000 bootstraps of the data. Sequences of Caryophyllaeides fennica and Breviscolex orientalis downloaded from GenBank (Accession nos. JQ034052.1 and JQ034055.1) were used as outgroups in the analyses.
*BEAST software (Heled and Drummond 2010) was used to reconcile Atractolytocestus species tree using both cox1 and ITS2 sequence data. The alignment of ITS2 was first trimmed by manually deleting numerous gap positions. Trimmed dataset was 607 bp long and contained 100 sequences (21 from China, 21 from Japan, 20 from Slovakia, 19 from UK and 19 from USA). Separate models were used for the two genes. HKY + I was used for cox1. GTR + G with four gamma categories was selected for ITS2 using AIC criterion in jModeltest 2.1. *BEAST was run for 200 million MCMC iterations with parameters recorded every 20,000 steps. Ten percent of the samples were discarded as burn in. Lognormal relaxed clock were selected as the clock prior. Due to lack of fossil record there are no general molecular clock rate estimates available for cestodes, but the absolute values of rates or dating splits were not subject of this study. To avoid fixing relative rates between the two genes with ad hoc values, the rate for cox1 gene was fixed at 1 and the clock rate for ITS2 was allowed to vary using a uniform prior (0.1, 1.0E100) with initial value 1. Yule process was selected as the species tree prior. Two independent runs were made to check for convergence. Tracer 1.5 (Rambaut and Drummond 2005) was used to examine ESS values of parameters and convergence between runs. ESS values for all parameters were above 300 and traces showed good mixing.
Results
Ribosomal ITS2
Divergent intragenomic copies were detected in the ITS2 ribosomal spacer of A. tenuicollis. A total of 21 recombinant clones obtained from five tapeworm individuals yielded 16 different sequence types caused mainly by single nucleotide polymorphisms and varying numbers of short repetitive region (TTGGT)n (Table 3, microsatellite repeat 3). It was present either in three or in five repetitions, which differentiated ITS2 in two ITS2 variants (643 and 653 bp, respectively) (Table 3).
ITS2 structure of A. tenuicollis was similar to that of A. huronensis from Slovakia, USA and UK. Overall pairwise sequence identity was 92.0–95.2 % between A. tenuicollis and British A. huronensis, 91.7–94.8 % between A. tenuicollis and A. huronensis from USA, and 92.0–93.9 % sequence identity was determined between A. tenuicollis and A. huronensis from Slovakia. The most profound difference between A. tenuicollis and all geographic A. huronensis populations was in variation of number of repetitive regions GT (Table 3, microsatellite repeat 1) and AGCC (Table 3, repeat 4) .
ITS2 sequence structure of A. tenuicollis was more different from A. sagittatus than from A. huronensis. Structure and distribution of microsatellite motifs in A. sagittatus had substantial species-specific character and did not correspond to those found in A. tenuicollis and A. huronensis. Pairwise sequence identity between A. tenuicollis and A. sagittatus was 69.7–70.9 %.
Mitochondrial cox1
The two designed primers CFCYT2 and CRCYT2 amplified 672 bp of cox1 gene, covering ATG start codon and the following 5′ part of the gene, encoding for 224 amino acids of the protein. Cox1 sequence was identical for all five A. tenuicollis individuals.
Comparison of A. tenuicollis cox1 with respective sequences of two other Atractolytocestus species showed that the mitochondrial haplotype found in Chinese A. tenuicollis is structurally specific (marked as haplotype 4, Ha4) and differs from all so far determined Atractolytocestus haplotypes, in particular Ha1 (A. huronensis from Slovakia, Hungary, Croatia, Romania), Ha2 (A. huronensis from USA and UK) (Bazsalovicsová et al. 2011), and Ha3 (A. sagittatus from Japan) (Bazsalovicsová et al. 2012).
Pairwise sequence identity between A. tenuicollis cox1 haplotype and remaining three haplotypes followed the same pattern as in ITS2. The nucleotide and amino acid (aa) sequence comparison with A. huronensis Ha1 and Ha2 revealed 90.3–90.8 % (96.9 % in aa) sequence identity. Significantly lower values were achieved between A. tenuicollis Ha4 and Ha3 of Japanese A. sagittatus—75.2 % (81.9 % in aa).
To visualise the sample sizes of our data in phylogenetic analyses, all available sequences for the four cox1 haplotypes were included when building the trees. The MP method produced a single most parsimonious tree, identical in topology with the two other methods of phylogenetic reconstruction (Fig. 2). In accordance with the indices of sequence similarity the most basal split was between Japanese samples of A. sagittatus and the Chinese, US and European samples. The latter group formed two well-supported clusters separating Chinese A. tenuicollis from American and European A. huronensis. Despite very low sequence divergence between Ha1 and Ha2 of A. huronensis the clade separating USA and UK populations from continental Europe received relatively high support in all analyses. Bootstrap support was 86 and 94 % in MP and ML, respectively, and posterior probability was 1 in BI.
Maximum Likelihood phylogeny of Atractolytocestus obtained using mitochondrial sequences. Clade supports (bootstraps and posterior probabilities) are as follows maximum parsimony/maximum likelihood/Bayesian inference. See Table 2 for details on species codes, country codes, haplotype numbering and individual codes
Joint analysis of mtDNA and ribosomal data
Species tree (Fig. 3) produced in the *BEAST analysis showed pattern congruent with mtDNA phylogenies in Fig. 2. Posterior probabilities of A. huronensis and of its inner clade separating UK and USA populations from continental Europe showed high values (1 and 0.95 respectively). The latter split was relatively well-supported despite disagreement between ITS2 and cox1 data caused by occurrence of intragenomic variants in ITS2. The clade separating A. huronensis and A. tenuicollis from A. sagittatus had lower but still relatively high posterior probability (0.88).
Individual gene trees (Electronic supplementary material (ESM), Supplements 1 and 2) showed values of posterior probabilities above 0.9 in all but one clade in cox1 and values equal or very close to 1 in all ITS2 clades. Similarly to earlier studies (Bazsalovicsová et al. 2011, 2012), some ITS2 variants were shared between the samples belonging to UK + US and European mtDNA clades. However, a general pattern of three clearly separated lineages (A. sagittatus, A. tenuicollis and A. huronensis) remained the same in both gene trees.
Discussion
Current paper provides new molecular data on A. tenuicollis and its comparison with relevant data on the only two congeners, A. huronensis and A. sagittatus. Molecular data along with phylogenetic analysis and statistical methods revealed interesting outcomes on current taxonomy and interrelationships within the genus Atractolytocestus.
Morphological and molecular studies on caryophyllidean family Lytocestidae (mainly those of Khawia and Atractolytocestus) revealed that conflict between morphological and genetic data represents a serious problem of the current taxonomy and systematics of the order Caryophyllidea (Scholz et al. 2011; Králová-Hromadová et al. 2012; current work). In a majority of tapeworms (e.g. Bothriocephalus, Taenia), an occurrence of sibling species or cryptic species complexes (morphologically similar or identical species with high molecular variation) should not be underestimated when envisaging their taxonomy (Verneau et al. 1997; Lavikainen et al. 2010). Controversially, monozoic tapeworms of the order Caryophyllidea are characterised by an opposite example; occurrence of morphologically dissimilar taxa (mainly recognised as different species) that display high genetic similarity.
This feature was studied in detail in caryophyllidean genus Khawia. Khawia sinensis, specific parasite of carp (C. carpio—Cyprininae), and Khawia saurogobii, parasite of gudgeons (Saurogobio spp.—Gobioninae) are morphologically clearly different species (Xi et al. 2009; Scholz et al. 2011) that displayed very high molecular sequence identity in ribosomal ITS2, SSU and LSU (ribosomal small and large subunit), and in mitochondrial cox1 and nad3 (nicotinamide dehydrogenase subunit III) (Scholz et al. 2011; Králová-Hromadová et al. 2012). In spite of this, taxonomic status of recently described K. saurogobii was not questioned. It is assumed that K. saurogobii, after switching to a new unrelated fish host, underwent morphological divergence as a result of ongoing sympatric speciation, but this process has not been accompanied by corresponding nucleotide changes (Scholz et al. 2011). Since sympatrically derived species are expected to show profound genetic similarity (Via 2001), striking molecular similarity of K. saurogobii and K. sinensis was explicable (Králová-Hromadová et al. 2012).
In genus Atractolytocestus, the first ambiguity was raised by Jones and Mackiewicz (1969), who claimed that A. huronensis is very similar to A. sagittatus; the same objection was later on claimed by Chubb et al. (1996). The reason which had let them to such a conclusion was similar morphology of both congeners, with the only significant morphological difference being in the body size, and variation in number and position of testes. In A. sagittatus, the number of testes considerably exceeds 100–200 (in some specimens reaching to several hundreds) (Scholz et al. 2001). In A. huronensis, the number of testes is significantly lower (up to 20) and, contrary to A. sagittatus, they are always posterior to the first vitelline follicles (Oros et al. 2004, 2011). Comparative molecular analysis of sequences of three DNA regions (ITS1, ITS2, cox1) has revealed conspicuous interspecific differences between the two Atractolytocestus species (Bazsalovicsová et al. 2012). Since differences between A. huronensis and A. sagittatus were significantly higher than well-known intraspecific variation (Bazsalovicsová et al. 2011), the validity of A. sagittatus was not questioned.
A. tenuicollis represent another example in family Lytocestidae where parallel morphological and molecular outcomes do not follow same conclusions. A. tenuicollis (originally described as K. tenuicollis (Li 1964)) was recently transferred to genus Atractolytocestus (Xi et al. 2009) because it possess typical morphological characteristics of the genus (Anthony 1958). However, A. tenuicollis resembles morphological markers of both congeners; similarly to A. sagittatus, it is characterised by numerous testes, although anteriormost testes begin posterior to first vitelline follicles is alike in A. huronensis. Current molecular data confirmed validity of A. tenuicollis which formed separate clade using all, cox1, ITS2 and combined cox1 + ITS2 input data. According to sequence identity values and based on phylogenetic tree topologies, Chinese A. tenuicollis is evidently genetically more closely related to A. huronensis than to A. sagittatus.
With cumulating molecular and genetic data on A. huronensis, the question of its geographic and genetic origin has been raised (Jones and Mackiewicz 1969; Králová-Hromadová et al. 2010; Bazsalovicsová et al. 2011). First genetic data on A. huronensis were achieved by Jones and Mackiewicz (1969) who revealed triploid character of American specimens of the tapeworm, described morphologically abnormal sperm cells and concluded parthenogenesis to be a regular mode of reproduction of the species. Recently, triploidy was confirmed also in Slovak A. huronensis individuals, along with distinct intragenomic ITS1 and ITS2 variants and dispersed chromosomal loci of nucleolar organiser regions (multiple NORs) (Králová-Hromadová et al. 2010; Špakulová et al. 2011). These features are evidently well fixed within the species since intragenomic ITS variants were present besides Slovak also in British and American populations (Bazsalovicsová et al. 2011) and a parthenogenic mode of reproduction was further supported also by ultrastructural studies of spermatic cells of Slovak A. huronensis (Bruňanská et al. 2011). A. huronensis represents so far the only cestode species where triploidy, intragenomic ITS variants, parthenogenesis and multiple NORs were proven to be mutually linked.
Jones and Mackiewicz (1969) hypothesised that if triploidy in A. huronensis arose by genetic and not interspecific hybridization, then immediate ancestor of the triploid line may still exist in carp. According to the authors, A. huronensis is either an old, relatively stable member of the caryophyllidean complex, since the pace of evolution is slowed when meiosis and fertilisation is suppressed, or it represents new species, because polyploidy and parthenogenesis has a limited potential for long-term survival. Further, the authors concluded that if A. huronensis is an ancient species, it seems unlikely that organs of so little usefulness as sterile testes would have persisted at all and that there is a possibility that A. sagittatus with “many testes” might have been the diploid ancestor of A. huronensis. Since the third Atractolytocestus species (A. tenuicollis) was not questioned at that time, the hypothesis of Jones and Mackiewicz (1969) was very rational.
It can be hypothesised that A. huronensis (triploid parthenogen with few testes) emerged through genetic hybridization from a common ancestor (diploid sexual with many testes) that resided in Asia (China) for a long time. Latest molecular data on all three Atractolytocestus species (Králová-Hromadová et al. 2010; Bazsalovicsová et al. 2011, 2012; current work) strongly indicate that the sought-after species, sharing close ancestry with A. huronensis, is A. tenuicollis. The following so far achieved data on both Atractolytocestus species support this theory: (1) morphology; strikingly similar morphology of both species, with the most profound difference being in number of testes, numerous in A. tenuicollis, several in A. huronensis, however, posterior position of testes relative to the anteriormost vitelline follicles is common for both species (Oros et al. 2004; Xi et al. 2009); (2) molecular data; sequential differences between A. huronensis and A. tenuicollis and their phylogenetic relationships indicate evolutionarily close bonds (current data); (3) karyology; triploidy/ parthenogenesis have been well documented in A. huronensis (Jones and Mackiewicz 1969; Králová-Hromadová et al. 2010), contrary, two sets of chromosomes in A. tenuicollis observed in mitotically dividing cells point to its diploid character (M. Orosová and M. Oros, unpublished data); (4) geographic distribution of both species and invasive character of A. huronensis (details see below).
It is evident that common carp, as the specific host of Atractolytocestus spp., plays an important role in its introduction to novel territories. Common carp is the world’s oldest domesticated and most frequent aquaculture species whose domestication commenced over 4,000 years ago in China. It is supposed that the relatively continuous area of the wild carp ranged from the Black Sea and the River Danube drainage to the Far East, China and Japan during Neogene Period, and that it had been disrupted into western (Europe) and eastern (Asia) parts during the Ice Ages (Baruš and Oliva 1995). Throughout the history, carp has been cultured, wide-scale translocated and restocked; consequently the fish has spread to all continents except for Antarctica (Thai et al. 2004; Mabuchi et al. 2008).
Non-overlapping spatial distribution pattern and other biological features of A. tenuicollis and A. huronensis are consistent with characteristics of sexual and parthenogen conspecifics (see Pongratz et al. 2003 and references therein). The ancestral sexual population (represented here by state preserved in A. tenuicollis) used to be located in the distribution centre of the species, while parthenogens (here A. huronensis) are present at the margin of the distribution. It has been proposed that parthenogenetic descendant might fail to establish itself in the presence of sexual ancestor but it may have better colonising capacities which allow it to subjugate areas where sexuals have difficulties establishing a population.
A noteworthy feature of the triploid A. huronensis is its apparent ability to reproduce successfully and occupy new regions. As discussed by Jones and Mackievicz (1969), polyploid and parthenogenetic animals were often referred to exploit disturbed regions, like posglacial fringe areas. Even though the polyploidy and parthenogenesis are considered to have restrict potential for long-term survival, A. huronensis apparently represents a very successful exception. The species managed to colonise USA, Great Britain and continental Europe what was undoubtedly enhanced by intensive fish trade.
The pattern of virtually missing intrapopulation genetic variability in A. huronensis, where all individuals in each of the populations comprise a single mitochondrial cox1 haplotype, fits well their recent invasive origin and parthenogenetic way of reproduction. Although A. tenuicollis and A. sagittatus samples studied here comprised a single haplotype too, more specimens need to be sequenced to infer whether the extremely low level of mitochondrial diversity is a general feature of the genus and is inherent due to its biology.
Carp, as the specific host of Atractolytocestus spp. and China, as a very probable historic cradle of some caryophyllideans, play apparently an important role in spatial distribution and speciation processes within this genus. The conspicuous genetic similarity of A. tenuicollis with A. huronensis is very interesting and indicates that future studies of the genus might be very helpful. First of all, larger sample sets especially from the Asian area of distribution need scrutinising for morphological re-description and possible taxonomic re-evaluation. Besides, multilocus molecular markers, such as microsatellites or SNPs, may help to consolidate the taxonomic status of A. tenuicollis and its interrelationship with A. huronensis.
References
Anthony JD (1958) Atractolytocestus huronensis n. gen., n. sp. (Cestoda: Lytocestidae) with notes on its morphology. Trans Am Microsc Soc 77:383–390
Baruš and Oliva (1995) Mihulovci a ryby (Lampreys and Fish). Fauna Czech Slovak Republic, Academia, Praha 28:234–261 (in Czech)
Bazsalovicsová E, Králová-Hromadová I, Štefka J, Scholz T, Hanzelová V, Vávrová S, Szemes T, Kirk R (2011) Population study of Atractolytocestus huronensis (Cestoda: Caryophyllidea), an invasive parasite of common carp introduced to Europe: mitochondrial cox1 haplotypes and intragenomic ribosomal ITS2 variants. Parasitol Res 109:125–131
Bazsalovicsová E, Králová-Hromadová I, Štefka J, Scholz T (2012) Molecular characterization of Atractolytocestus sagittatus (Cestoda: Caryophyllidea), monozoic parasite of common carp, and its differentiation from the invasive species Atractolytocestus huronensis. Parasitol Res 110:1621–1629
Bruňanská M, Nebesářová J, Oros M (2011) Ultrastructural aspects of spermatogenesis, testes, and vas deferens in the parthenogenetic tapeworm Atractolytocestus huronensis Anthony, 1958 (Cestoda: Caryophyllidea), a carp parasite from Slovakia. Parasitol Res 108:61–68
Chubb JC, Kirk R, Wellby, I (1996) Caryophyllaeid tapeworm Atractolytocestus huronensis Anthony, 1958 (=Markevitschia sagittata Kulakovkaya et Akhmerov, 1965) in carp Cyprinus carpio L. in British Isles—another translocation? In: Abstract of the Spring Meeting of the British Society of Parasitology, University of Wales, 1996, p. 66
Cunningham CO (1997) Species variation within the internal transcribed spacer (ITS) region of Gyrodactylus (Monogenea: Gyrodactylidae) ribosomal RNA genes. J Parasitol 83:215–219
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9(8):772
Demshin NI, Dvoryadkin VA (1981) The development of Markevitschia sagitatta (Cestoidea: Caryophyllidae), a parasite of the Amur wild carp, in the external medium and in the intermediate host. Parazitologiya 15:113–117
Gjurcević E, Bambir S, Beck A (2009) Atractolytocestus huronensis Anthony, 1958 from farmed common carp in Croatia. In: 14th EAFP International Conference, Abstract Book. Diseases of fish and shellfish, Prague, Czech Republic
Goloboff P, Farris S, Nixon K (2000) TNT (Tree analysis using New Technology) (BETA) Published by the authors. Tucumán, Argentina
Gouy M, Guindon S, Gascuel O (2010) SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27:221–224
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321
Heled J, Drummond AJ (2010) Bayesian inference of species trees from multilocus data. Mol Biol Evol 27:570–580
Hoffman GL (1999) Parasites of North American freshwater fishes, Secondth edn. Comstock Publishing, London, 539 pp
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Jones AW, Mackiewicz JS (1969) Naturally occurring triploidy and parthenogenesis in Atractolytocestus huronensis Anthony (Cestoidea: Caryophyllidea) from Cyprinus carpio L. in North America. J Parasitol 55:1105–1118
Kappe A, Seifert T, El-Nobi G, Bräuer G (2006) Occurrence of Atractolytocestus huronensis (Cestoda: Caryophyllaeidae) in German pond-farmed common carp Cyprinus carpio. Dis Aquat Organ 70:255–259
Kirk RS, Veltkamp CJ, Chubb JC (2003) Identification of Atractolytocestus huronensis (Caryophyllidea: Lytocestidae) from carp (Cyprinus carpio) using histological and ashing techniques. Abstract of the Spring Meeting of the British Society of Parasitology, Manchester, pp 45–46
Králová-Hromadová I, Scholz T, Shinn AP, Cunningham CO, Wootten R, Hanzelová V, Sommerville C (2003) A molecular study of Eubothrium rugosum (Batsch, 1786) (Cestoda: Pseudophyllidea) using ITS rDNA sequences, with notes on the distribution and intraspecific sequence variation of Eubothrium crassum (Bloch, 1779). Parasitol Res 89:473–479
Králová-Hromadová I, Stefka J, Spakulová M, Orosová M, Bombarová M, Hanzelová V, Bazsalovicsová E, Scholz T (2010) Intraindividual ITS1 and ITS2 ribosomal sequence variation linked with multiple rDNA loci: a case of triploid Atractolytocestus huronensis, the monozoic cestode of common carp. Int J Parasitol 40:175–181
Králová-Hromadová I, Bazsalovicsová E, Oros M, Scholz T (2012) Sequence structure and intragenomic variability of ribosomal ITS2 in monozoic tapeworms of the genus Khawia (Cestoda: Caryophyllidea), parasites of cyprinid fish. Parasitol Res 111:1621–1627
Kulakovskaya OP, Akhmerov AC (1965) Markevitschia sagittata n. gen. n. sp. (Cestoda, Lytocestidae) from common carp in the Amur River. In: Parasites and parasitoses of man and animals. Naukova Dumka, Kiev, pp 264–271 (in Russian)
Lavikainen A, Haukisalmi V, Lehtinen MJ, Laaksonen S, Holmström S, Isomursu M, Oksanen A, Meri S (2010) Mitochondrial DNA data reveal cryptic species within Taenia krabbei. Parasitol Int 59:290–293
Li MM (1964) A new genus and three new species of cestodes (Caryophyllaeidae) from Cyprinus carpio in China. Acta Zootax Sin 1:355–366
Mabuchi K, Senou H, Nishida M (2008) Mitochondrial DNA analysis reveals cryptic large-scale invasion of non-native genotypes of common carp (Cyprinus carpio) in Japan. Mol Ecol 3:796–809
Majoros G, Csaba G, Molnár K (2003) Occurrence of Atractolytocestus huronensis Anthony, 1958 (Cestoda: Caryophyllaeidae), in Hungarian pond-farmed common carp. Bull Eur Assoc Fish Pathol 23:167–175
Oros M, Hanzelová V, Scholz T (2004) The cestode Atractolytocestus huronensis (Caryophyllidea) continues to spread in Europe: new data on the helminth parasite of the common carp. Dis Aquat Organ 62:115–119
Oros M, Králová-Hromadová I, Hanzelová V, Bruňanská M, Orosová M (2011) Atractolytocestus huronensis (Cestoda): a new invasive parasite of common carp in Europe. In: Carp: habitat, management and diseases. Nova Science Publisher, USA, pp 63–94
Pongratz N, Storhas M, Carranza S, Michiels NK (2003) Phylogeography of competing sexual and parthenogenetic forms of a freshwater flatworm: patterns and explanations. BMC Evol Biol 3:23
Rambaut A, Drummond A (2005) Tracer. http://tree.bio.ed.ac.uk/software/tracer/
Scholz T, Shimazu T, Olson PD, Nagasawa K (2001) Caryophyllidean tapeworms (Platyhelminthes: Eucestoda) from freshwater fishes in Japan. Folia Parasitol 48:275–288
Scholz T, Brabec J, Králová-Hromadová I, Oros M, Bazsalovicsová E, Ermolenko A, Hanzelová V (2011) Revision of Khawia spp. (Cestoda: Caryophyllidea), parasites of cyprinid fish, including a key to their identification and molecular phylogeny. Folia Parasitol 58:197–223
Špakulová M, Orosová M, Mackiewicz JS (2011) Cytogenetics and chromosomes of tapeworms (Platyhelminthes, Cestoda). Adv Parasitol 74:177–230
Telford MJ, Herniou EA, Russell RB, Littlewood DT (2000) Changes in mitochondrial genetic codes as phylogenetic characters: two examples from the flatworms. Proc Natl Acad Sci USA 9:11359–11364
Thai BT, Burridge CP, Pham TA, Austin CM (2004) Using mitochondrial nucleotide sequences to investigate diversity and genealogical relationships within common carp (Cyprinus carpio L.). Anim Genet 36:23–28
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment throughout sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Verneau O, Renaud F, Catzeflis F (1997) Evolutionary relationships of sibling tapeworm species (Cestoda) parasitizing teleost fishes. Mol Biol Evol 14:630–636
Via S (2001) Sympatric speciation in animals: the ugly duckling grows up. Trends Ecol Evol 16:381–390
Xi BW, Wang GT, Wu SG, Gao D, Nie P (2009) New record of genus Atractolytocestus in China with redescription of A. sagittatus (Cestoda, Caryophyllidea) from Cyprinus carpio. Acta Zootax Sin 34:407–410
Xi BW, Oros M, Wang TG, Scholz T, Xie J (2013) Khawia abbottinae n. sp. (Cestoda: Caryophyllidea) from the Chinese false gudgeon Abbottina rivularis (Cyprinidae: Gobioninae) in China: morphological and molecular data. Folia Parasitol 60:141–148
Acknowledgments
M. O. greatly appreciates Dr. Xi Bing Wen (Freshwater Fisheries Research Centre, Wuxi, China) for invaluable help during material collection. This study was financially supported by the Grant Agency of the Slovak Republic (projects VEGA 2/0014/10 and 2/0129/12), by the Slovak Research and Development Agency under contract APVV-0653-11, by the Czech Science Foundation (project P505/12/G112), and by the Institute of Parasitology (RVO: 60077344). The study was realised as part of the project “Centre of Excellence for Parasitology” (Code ITMS: 26220120022), based on support of the Operational Programme “Research & Development” funded from the European Regional Development Fund (rate 0.1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Note: Nucleotide sequence data reported in this paper are available in the GenBank™, EMBL and DDBJ databases under the accession numbers KC834609-KC834634.
Rights and permissions
About this article
Cite this article
Králová-Hromadová, I., Štefka, J., Bazsalovicsová, E. et al. The tapeworm Atractolytocestus tenuicollis (Cestoda: Caryophyllidea)—a sister species or ancestor of an invasive A. huronensis?. Parasitol Res 112, 3379–3388 (2013). https://doi.org/10.1007/s00436-013-3516-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3516-y