Abstract
Concomitant infections of different species of parasites are common in the field. Infection with one parasite species likely triggers host responses that may influence the subsequent infection of another species and alter disease outcomes. So far, the majority of studies have focused on single species parasite infection, and the mechanisms of protection induced by the first parasite infection against the secondary infection remain poorly defined. In this study, we assess the impact of trematode Clonorchis sinensis infection on the course of another tissue nematode Trichinella spiralis challenge. We observed that mice with preexisting C. sinensis infection had lower worm burden of intestinal T. spiralis than those infected with T. spiralis alone; mice with preexisting C. sinensis also had severe enteric histopathological changes and higher counts of intestinal Paneth cells in responses to T. spiralis challenge. The mRNA levels of interleukin (IL)-4, IL-10, IL-13, and tumor necrosis factor (TNF)-α from the small intestine and spleen of the different groups were analyzed using quantitative real-time polymerase chain reaction. Compared with that in mice infected with T. spiralis alone, the mRNA expression of IL-13 was significantly increased in the small intestine tissues and IL-4, IL-13, and TNF-α were significantly increased in the spleen tissues in the dually infected mice. Our findings suggest that a “preexisting” trematode infection of C. sinensis is a factor which contributes to reducing the establishment of T. spiralis adult worms in the small intestine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mixed parasite infections are common in many parts of the world. Nearly one half of the world’s human population is infected with one or more of a variety of parasitic helminthes (Bazzone et al. 2008). More than 72 species of protozoan and helminth parasites can reach humans by contaminated food and water. Food-borne trematodes are important causes of parasitic infections in many Asian countries. Among the helminths, seven trematode species, four cestode species, and seven nematode species can infect humans through the consumption of raw seawater and/or freshwater food (Pozio 2003). The trematode Clonorchis sinensis (liver fluke) causes the important food-borne zoonosis, clonorchiasis. The adult worms lodge in the smaller bile ducts of the liver, causing inflammation and fibrosis of the adjacent tissues. Human hosts become infected through the consumption of raw or poorly cooked fish. Clonorchiasis is endemic in East Asia, especially in China, mainly in Guangdong, Guangxi, and Heilongjiang provinces, and also in Korea (Lai et al. 2008). Trichinella spiralis is a nematode that causes trichinosis, a parasitic disease caused by eating raw or undercooked pork or wild game infected with the larvae of the roundworm. The adult worms live in the small intestine, and the mature female worm releases larvae that travel through the bloodstream to the muscles. It has been recognized that helminths and their antigens have important immunomodulatory activities (Maizels and Yazdanbakhsh 2003). C. sinensis and T. spiralis infections are two of the most prevalent food-borne parasitic diseases in China, but little is known of the effects of concomitant parasite infections on the immune response or severity of clinical disease, and the mechanisms contributing to altered disease outcomes during coinfection also remain poorly defined.
It has been reported that Toxoplasma gondii and T. spiralis coinfection in swine can cause specific serum antibody responses (Bokken et al. 2012). Amelioration of influenza-induced pulmonary pathology has been demonstrated when mice were coinfected with T. spiralis (Furze et al. 2006). Progression of visceral leishmaniasis due to Leishmania infantum in BALB/c mice is markedly slowed by prior infection with T. spiralis (Rousseau et al. 1997). However, so far, there is no report about T. spiralis and C. sinensis coinfection. In this study, we investigated whether preinfection of C. sinensis, a liver fluke, could modulate host anti-T. spiralis response and the development of experimental trichinosis in mice. We compared worm burden of Trichinella parasites, histological changes, and immune responses in mice infected with T. spiralis alone or infected with both parasites and showed that preinfection of C. sinensis could lead to significant changes in mouse intestinal tissues. Our data indicated that the reduced establishment of intestinal T. spiralis during concurrent infections may be caused by both the pathological changes and nonspecific immunological interactions in the intestinal mucosa in Kunming (KM) outbred mice.
Materials and methods
Preparation of C. sinensis metacercariae
C. sinensis metacercariae were isolated from freshwater fish (Pseudorasbora parva) that were kept in our indoor ecologic pool at Sun Yat-sen University. Infected fish were digested with pepsin solution (0.2 % HCl and 0.6 % pepsin, pH 2.0) for 2–3 h at 37 °C to collect metacercariae under stereomicroscope. The C. sinensis metacercariae were kept in 0.1 M phosphate-buffered saline (PBS, pH 7.4) at 4 °C until use (Wang et al. 2009).
Animals and parasite infections
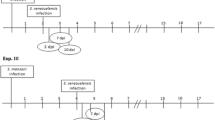
KM (outbred) mice, 6–8 weeks old and specific-pathogen-free, were purchased from the Experimental Animal Center of Sun Yat-sen University (Guangzhou, China). The strain of T. spiralis (pig strain) was maintained in our laboratory via serial passage in mice. T. spiralis infectious larvae were recovered from muscles of mice 60–90 days postinfection (p.i.), and eviscerated mouse carcasses were cut into pieces and then digested in 0.6 % pepsin with 0.2 % hydrochloride digestion fluid 4 h at 37 °C under stirring condition. The larvae were identified from digested solution under microscopy and washed six times with sterile PBS (Park et al. 2011). In total, 104 mice were included in this study. Mice were divided into 4 groups; except for the uninfected control group consisting of 8 mice, the other three groups all consisted of 32 mice per group and 8 mice per group at each time point. (1) Uninfected control; (2) coinfection: mice were infected with 30 encysted metacercaria of C. sinensis 20 days before challenge infection of 300 T. spiralis larvae in 0.1 ml of PBS administered by oral gavage; (3) mice were infected with 300 T. spiralis larvae alone by oral gavage on day 0; (4) mice were infected with 30 encysted metacercaria of C. sinensis alone by oral gavage. C. sinensis infection was confirmed by serological ELISA test. The numbers of adult worms in the small intestine were assessed at 5, 10, 15, and 20 days p.i. of T. spiralis. The Ethical Committee of Animal Experiments of Sun Yat-sen University approved all experiments done in this study.
Recovery of adult worms from the small intestine
The entire length of the small intestines was removed, opened longitudinally, and incubated in PBS at 37 °C for 4 h to induce migration of worms from the gut epithelium into solution. Worms were counted following incubation. Briefly, the mucosa was separated from the underlying muscularis by scraping with a glass microscope slide and mixed with 4 ml of PBS. All the adult worms were then counted by using a scored Petri dish and under an inverted microscope. The worm counts were expressed as the total number of worms per mouse.
Histology
Consecutive lengths of the small intestine taken 10 cm from the pyloric sphincter were fixed in neutral buffered formalin, embedded in paraffin, and histologically processed using standard methods; 5-μm tissue sections (50- or 100-μm distance between sections) of the organ from each mouse were cut, dewaxed, rehydrated, and stained with hematoxylin and eosin (H&E) (Sigma-Aldrich, Shanghai, China) for mucosa Paneth cells, goblet cells, and eosinophils. The numbers of Paneth and goblet cells per 10 randomly selected villus crypt units (VCUs), and eosinophils per VCU were counted on each section determined under light microscopy from at least two sections per animal, at a magnification of ×40. Villus and crypt lengths were measured using an eyepiece micrometer. Ten villi and crypt areas were measured for each sample, and the mean length was determined for each.
Mucosal mast cell identification
Immunohistochemistry was carried out using a streptavidin–peroxidase conjugation method as described (Huang et al. 2013). Small intestine tissue sections (4-μm) were deparaffinized and rehydrated in distilled water. Heat-induced antigen retrieval was carried out in a 700-W microwave oven for 30 min. Endogenous peroxidase activity was blocked by incubation with 0.3 % hydrogen peroxide in methanol for 10 min at 37 °C. Nonspecific binding was blocked by incubation in PBS containing 10 % normal goat serum and 1 % bovine serum albumin (BSA) (pH 7.4) for 10 min at room temperature. Sections were incubated with anti-tryptase mouse monoclonal antibody (AA1–immunoglobulin [Ig] G1—1:200 dilution; Abcom, Hong Kong, China) overnight at 4 °C. Slides were then rinsed three times with PBS (pH 7.4) and exposed to biotinylated goat anti-mouse IgG (5 mg/ml, 1:200 dilution; Zhongshan, Beijing, China) for 30 min at room temperature. The slides were washed three times in 1 % BSA–PBS and incubated with avidin–biotin–peroxidase complex (Zhongshan, Beijing, China) for 15 min at room temperature before counterstaining with hematoxylin. Mast cells were identified by their dark brown staining and counted at ×40 magnification under light microscopy. Positively stained mast cells were counted in three to five sections per animal. Seven to ten well-oriented VCUs were examined per section (Serna et al. 2006). The results were expressed as the mean value of mast cells per group.
Measurement of mRNA expression by quantitative real-time PCR
Total RNA was extracted from about 100 mg small intestine and spleen samples of each mouse using RNA Extraction Kit (TaKaRa), according to the manufacturer’s protocol. The quality of total RNA was analyzed by running 5 μl of each RNA sample on a 1.0 % agarose gel and visualized with ethidium bromide staining. The quantity of total RNA was measured using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies). First-strand cDNA was constructed from 1.0 μg of total RNA with oligo(dT) as primers (Bian et al. 2005) using PrimeScript II 1st Strand cDNA Synthesis Kit (TaKaRa), following the manufacturer’s protocol. cDNA was stored at −80 °C until use.
To determine the tumor necrosis factor (TNF)-α and interleukin (IL)-4, IL-10, and IL-13 mRNA levels in the tissues of the small intestine and spleen, we performed quantitative real-time polymerase chain reaction (qRT-PCR) using the SYBR Green QPCR Master Mix (TaKaRa), according to the manufacturer’s instructions. The primers are listed in Table 1. Briefly, the total 10-μl reaction mixture contained 5.0 μl of SYBR® Premix Ex Taq™ II (2×), 0.5 μl of each primer (10 pM), 3.0 μl of dH2O, and 1.0 μl of cDNA (0.2 μg/μl). Amplification was initially denaturized for 30 s at 95 °C, followed by 43 cycles of 5 s at 95 °C and 20 s at 60 °C with a LightCycler® 480 instrument (Roche Diagnostics). Specific mRNA expression levels were normalized to the housekeeping gene, β-actin mRNA (Paim et al. 2012), and the results are expressed as the fold change compared to uninfected controls.
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). Differences were analyzed by using Student’s t test or Wilcoxon rank sum test. A P value <0.05 was regarded as statistically significant. The numbers of intestinal worm, mast cells, goblet cells, Paneth cells, and eosinophils and the lengths of villi and crypts were evaluated during two separate infections.
Results
Reduced T. spiralis worm burden in the dually infected mice
Evaluation of parasite load in the small intestine of T. spiralis singly infected and C. sinensis/T. spiralis dually infected mice revealed a time-dependent decrease in worm number in the small intestine in both models over the course of T. spiralis infection or challenge. KM mice were infected with 300 T. spiralis larvae, and worm burdens were assessed at days 5, 10, 15, and 20 p.i. (n = 4). As shown in Fig. 1, compared with T. spiralis singly infected mice, significantly lower numbers of worms were recovered from the small intestine of C. sinensis/T. spiralis dually infected mice at days 5, 10, and 15 after T. spiralis challenges (P < 0.05). However, the worm burdens of the two groups were not significantly different on day 20 after either T. spiralis infection or challenge (p > 0.05). Nevertheless, this result showed that mice coinfected with C. sinensis carried fewer T. spiralis adult worms in the small intestine.
Worm burden in mice singly infected with T. spiralis or dually infected with C. sinensis/T. spiralis. Mice were infected orally with 300 larvae of T. spiralis alone or with 300 larvae of T. spiralis 20 days after infection with 30 encysted metacercaria of C. sinensis. Adult T. spiralis worms were recovered from excised intestine on days 5, 10, 15, and 20 to determine the total worm numbers per mouse. Data are expressed as the mean worm burden ± SEM. Four mice were used per group at each time point and the results shown are representative of two independent experiments. *P < 0.05, **P < 0.01, significantly different compared to T. spiralis-infected mice
Severe enteropathy changes in villus heights and crypt lengths in the dually infected mice
To assess the induction of the intestinal inflammation by helminth infection, the pathological lesions in the small intestine, e.g., the villus height and crypt length (thickness of the mucosa), were observed. The results showed that the villus height and crypt depth were similar between uninfected mice (Fig. 2a) and mice infected with C. sinensis alone (Fig. 2b), which were, however, obviously different between uninfected mice (Fig. 2a) and mice infected with T. spiralis alone (Fig. 2c) or dually infected with C. sinensis/T. spiralis (Fig. 2d). The villus and crypt lengths in duodenal sections were measured in different groups (Fig. 2e). There were no significant differences in both the villus height and crypt depth in the small intestine between mice infected with C. sinensis at day 30 p.i. and the uninfected controls. However, compared with those of uninfected controls, there were significant crypt hyperplasia and villus atrophy in mice infected with T. spiralis alone (10 days p.i.) (P < 0.01) or in mice dually infected with C. sinensis (30 days p.i.)/T. spiralis (10 days p.i.) (P < 0.01); furthermore, there were significantly shorter villus height (P < 0.01) and longer crypt depth (P < 0.01) in dually infected mice than those in T. spiralis singly infected mice.
Histological assessment in duodenal segments of different groups. Mice infected orally with 300 larvae of T. spiralis alone or with 300 larvae of T. spiralis 20 days after infection with 30 encysted metacercaria of C. sinensis. Histological analyses of the duodenum were performed at 10 days p.i. or challenge with T. spiralis. a Naive mice; b C. sinensis singly infected mice; c T. spiralis singly infected mice; and d C. sinensis/T. spiralis dually infected mice. Four mice were used for each group. Magnification, ×20; H&E stain. e Enteropathy changes in duodenal mucosal morphology. The crypt depths (bottom) and villus heights (top) of the sections of duodenal samples were measured in 10 appropriately orientated crypt–villus profiles per sample, using a graticule, at 10 days p.i. or challenge with T. spiralis. Data are presented as the mean villus/crypt length ± SEM. **P < 0.01, significantly different from the value for uninfected mice; § P < 0.01, significantly different from the value for C. sinensis singly infected mice; † P < 0.01, significantly different from the value for T. spiralis singly infected mice. Four mice were used per group at each time point and the results shown are representative of two independent experiments
Mucosal mast cell counts
Mast cells were identified by immunohistochemical staining for tryptase (dark brown appearance), and the number of mucosal mast cells in the duodenum of infected animals was determined. The results showed that few positively stained mast cells were observed in the small intestine tissue of the uninfected control mice (Fig. 3a) and more mast cells were observed in the small intestine tissues of mice both infected with C. sinensis alone at day 30 p.i. (Fig. 3b) and dually infected with C. sinensis (at 30 days p.i.)/T. spiralis (at day 10 post challenge) (Fig. 3d); however, even more mast cells were observed in mice infected with T. spiralis alone at day 10 p.i. (Fig. 3c). As shown in Fig. 3e, there were only few positively stained mast cells observed in the small intestine tissues of naïve mice, while there were significantly higher numbers of mast cells in the small intestine tissues of the mice either infected (P < 0.01) or challenged (P < 0.01) with T. spiralis. Compared with that of C. sinensis alone, the mice with dual infection had significantly higher numbers of mast cells (P < 0.01); however, the mice infected with T. spiralis alone had the highest numbers of mast cells (P < 0.01) among all the groups.
Intestinal mast cell response in the epithelium of the duodenum of different groups. Mice infected orally with 300 larvae of T. spiralis alone or with 300 larvae of T. spiralis 20 days after infection with 30 encysted metacercaria of C. sinensis. Immunohistochemical staining for mast cell tryptase in the duodenum were performed at 10 days p.i. or challenge with T. spiralis. Representative image showing the mast cell in the duodenum in naïve mice (a), increased mast cells in mice singly infected with C. sinensis (at 30 days p.i.) (b) and in mice dually infected with C. sinensis/T. spiralis (d), and markedly increased mast cells in mice singly infected with T. spiralis (c). Four mice were used for each group at each time point. Magnification, ×100. e Mean numbers of mast cells per VCU by immunohistochemical staining for mast cell tryptase per group. **P < 0.01, significantly different from the value for uninfected mice; § P < 0.01, significantly different from the value for C. sinensis singly infected mice; † P < 0.01, significantly different from the value for T. spiralis singly infected mice. Four mice were used per group at each time point and the results shown are representative of two independent experiments
Goblet cells in the small intestines
Goblet cells reside throughout the length of the small and large intestines, and they are responsible for the production and maintenance of the protective mucus blanket by synthesizing and secreting high-molecular-weight glycoproteins known as mucins (Specian and Oliver 1991). Using H&E staining, goblet cells were pale in color. In uninfected mice, only a small number of goblet cells were seen in both the crypts and villi (Fig. 4a) and more goblet cells were observed in the villi in mice infected with C. sinensis alone at day 30 p.i. (Fig. 4b). However, greater increased goblet cells, which were much larger, were observed in the crypt, villi, and villous tips in mice infected with T. spiralis alone at day 10 p.i. (Fig. 4c) and markedly increased goblet cells were observed in the crypt, along the villi, and at villous tips in mice dually infected with C. sinensis (at 30 days p.i.)/T. spiralis (at day 10 post challenge) (Fig. 4d). Goblet cells in duodenal sections were counted in different groups. As shown in Fig. 4e, compared with uninfected mice, all mice infected with C. sinensis (at 30 days p.i.) alone, with T. spiralis (10 days p.i.) alone, and dually infected with C. sinensis (at 30 days p.i.)/T. spiralis (at day 10 post challenge) possessed a significantly greater number of intestinal goblet cells in both the crypt and villus (P < 0.01). Compared with mice infected with C. sinensis alone, greater numbers of goblet cells in the crypt and villus were observed in mice infected with T. spiralis alone (P < 0.01) or in mice dually infected with C. sinensis (at 30 days p.i.)/T. spiralis (at day 10 post challenge) (P < 0.01). However, the goblet cell numbers in both the crypt and villus rose even significantly higher in mice infected with T. spiralis alone at day 10 p.i. (P < 0.01).
Intestinal goblet cell response in the epithelium of the duodenum of infected mice. Mice infected orally with 300 larvae of T. spiralis alone or with 300 larvae of T. spiralis 20 days after infection with 30 encysted metacercaria of C. sinensis. Histological analyses of the duodenum were performed at 10 days p.i. or challenge with T. spiralis. Representative image showing the goblet cell in the duodenum in naïve mice (a), increased goblets in mice singly infected with C. sinensis (at 30 days p.i.) (b), the pronounced mucosal goblet cell hyperplasia in mice singly infected with T. spiralis (at 10 days p.i.) (c), and markedly increased goblet cells also observed in mice dually infected with C. sinensis (30 days p.i.)/T. spiralis (10 days post challenge) (d). Four mice were used for each group at each time point. Magnification, ×40; H&E stain. e Numbers of goblet cells in the epithelium of the duodenum. Goblet cell counts are expressed as the mean goblet cells ± SEM per 10 VCUs. **P < 0.01, significantly different from the value for uninfected mice; § P < 0.01, significantly different from the value for C. sinensis singly infected mice; † P < 0.01, significantly different from the value for T. spiralis singly infected mice. Four mice were used per group at each time point and the results shown are representative of two independent experiments
Increased Paneth cells in the small intestine of dually infected mice
Paneth cells in the small intestine are the main epithelial cell type that secretes antimicrobial peptides and are involved in the mucosal production of immunoglobulin A (Santaolalla and Abreu 2012). Using H&E staining, Paneth cells were identified by the presence of eosinophilic granules. In uninfected mice, Paneth cells were seen only at the base of crypts (Fig. 5a, b), but they were present in the basal and upper portions of the crypt in mice infected with C. sinensis at 30 days p.i. (Fig. 5c, d). Paneth cells were seen in the crypt, along the villi, and at the villous tips in mice infected with T. spiralis alone at 10 days p.i. (Fig. 5e, f) and dually infected with C. sinensis (30 days p.i.)/T. spiralis (10 days post challenge) (Fig. 5g, h). Paneth cells in sections of the duodenum of each group were counted. As shown in Fig. 5i, compared with uninfected mice, mice singly infected with C. sinensis (30 days p.i.), with T. spiralis (10 days p.i.), or dually infected with C. sinensis (30 days p.i.) and T. spiralis (10 days post challenge) possessed significantly greater numbers of intestinal Paneth cells in the crypt–villus (P < 0.01). Compared with mice infected with C. sinensis alone, greater numbers of Paneth cells were observed in the crypt in mice infected with T. spiralis alone at day 10 p.i. (P < 0.01), and the numbers of Paneth cells in both the crypt and villus were even significantly higher in dually infected mice with C. sinensis/T. spiralis (P < 0.01).
Paneth cell responses in the epithelium of the duodenum of different groups. Mice infected orally with 300 larvae of T. spiralis alone or with 300 larvae of T. spiralis 20 days after infection with 30 encysted metacercaria of C. sinensis. Histological analyses of the duodenum were performed at 10 days p.i. or challenge with T. spiralis. Representative image of the duodenum from naïve mice showing the crypts containing few Paneth cells (a, b); duodenum taken 30 days p.i. with C. sinensis, showing crypts containing Paneth cells with some seen along the villi (c, d) (arrows); duodenum taken 10 days p.i. with T. spiralis, showing larger crypts containing many Paneth cells with some seen along the villi (e, f) (arrows); and duodenum taken 10 days post challenge with T. spiralis, showing larger crypts containing a greater number of Paneth cells and many were also seen along the villi and villous tips (g, h). Four mice were used per group at each time point. Magnification, ×100; H&E stain. i Numbers of Paneth cells in the epithelium of the duodenum. Paneth cell counts are expressed as the mean Paneth cells ± SEM per 10 VCUs. **P < 0.01, significantly different from the value for uninfected mice; § P < 0.01, significantly different from the value for C. sinensis singly infected mice; † P < 0.01, significantly different from the value for T. spiralis singly infected mice. Four mice were used per group at each time point and the results shown are representative of two independent experiments
Eosinophils in the small intestines
Eosinophils are granulocytes associated with host defense against parasitic helminths with allergic conditions and, more recently, with immunoregulatory responses (Muniz et al. 2012). Using light microscopy, the eosinophils were identified in the jejunum of mice infected with C. sinensis alone at 30 days p.i., with T. spiralis alone at 10 days p.i., or dually infected with C. sinensis (30 days p.i.)/T. spiralis (10 days post challenge). Eosinophils comprised a significant portion of the inflammatory cells infiltrating the small bowel. Eosinophils were seen more commonly at the base of the jejunal villi and in the crypt regions in T. spiralis singly infected mice, whereas the cellular infiltrate was composed predominantly of granulocytes and a smaller number of eosinophils in the small intestine of C. sinensis/T. spiralis dually infected mice. In contrast, the cellular infiltrate was reduced in the small intestine of mice infected with C. sinensis alone, which was composed predominantly of mononuclear cells, rather than granulocytes. The number of eosinophils was evaluated per VCU. As shown in Fig. 6, compared with those in uninfected mice, all mice infected with C. sinensis (30 days p.i.) alone, with T. spiralis (10 days p.i.) alone, or with C. sinensis (30 days p.i.)/T. spiralis (10 days post challenge) possessed significantly greater numbers of intestinal eosinophils in both the crypt and villus (P < 0.01). Compared with mice infected with C. sinensis alone, greater numbers of eosinophils in both the crypt and villus were observed in the C. sinensis/T. spiralis dually infected mice (P < 0.01); however, the numbers of eosinophils were even higher in mice infected with T. spiralis alone at day 10 p.i. (P < 0.01).
Intestinal eosinophil counts in the epithelium of the duodenum of mice infected with different parasites. Mice were infected orally with 300 larvae of T. spiralis alone or with 300 larvae of T. spiralis 20 days after infection with 30 encysted metacercaria of C. sinensis and were sacrificed at 10 days after infection or challenge with 300 larvae of T. spiralis. Eosinophil counts are expressed as the mean eosinophils ± SEM per VCUs. **P < 0.01, significantly different from the value for uninfected mice; § P < 0.01, significantly different from the value for C. sinensis singly infected mice; † P < 0.01, significantly different from the value for T. spiralis singly infected mice. Four mice were used per group at each time point and the results shown are representative of two independent experiments
Cytokine mRNA expression in small intestine and spleen
To determine whether prior C. sinensis infection alters T helper cell response, some key cytokines representing the local intestinal (small intestine) and systemic (spleen) immune responses were measured using qRT-PCR. Compared with uninfected mice, the mRNA expression levels of the cytokines IL-4, IL-10, IL-13, and TNF-α were all significantly increased in the duodenum and spleen of the mice infected with C. sinensis alone at day 30 p.i., with T. spiralis alone at day 10 p.i., or dually infected with C. sinensis (30 days p.i.) and T. spiralis (10 days post challenge) (P < 0.01). Following C. sinensis infection, T helper 2 (Th2)-type immune responses were characterized by increased mRNA levels of IL-4 and IL-13 in the duodenum (Fig. 7a) and spleen (Fig. 7b) at day 30 p.i. The mRNA levels of IL-4 in both the duodenum and spleen and the levels of IL-13 and TNF-α in the spleen of mice dually infected with C. sinensis (30 days p.i.)/T. spiralis (10 days post challenge) were significantly higher (P < 0.01) than those in the mice infected with C. sinensis alone at day 30 p.i.; however, even higher mRNA levels of IL-4 and TNF-α were observed in the duodenum of mice infected with T. spiralis alone at day 10 p.i. (P < 0.01).
Cytokine mRNA expression in the small intestines (a) and spleens (b) were analyzed by qRT-PCR. Mice were infected orally with 300 larvae of T. spiralis alone or with 300 larvae of T. spiralis 20 days after infection with 30 encysted metacercaria of C. sinensis and were sacrificed 10 days after infection or challenge with 300 larvae of T. spiralis. The values are shown as a fold change to the uninfected control. Values are the means from triplicate measurements and data are presented as the means ± SEM. **P < 0.01, significantly different from the value for uninfected mice; § P < 0.01, significantly different from the value for C. sinensis singly infected mice; † P < 0.01, significantly different from the value for T. spiralis singly infected mice. Four mice were used per group at each time point and the results shown are representative of two independent experiments
Discussion
In Asia, Trichinella spp. infection has been documented in humans in 18 countries, in domestic animals (mainly pigs) in 9 countries, and in wildlife in 14 countries (Owen et al. 2005). A conservative estimation was made that 15 million people were infected with C. sinensis in 2004 in East Asia, especially in China, the Republic of Korea, and Vietnam (Qian et al. 2012). Helminth infections can alter host immunity and susceptibility to other pathogens (Cox 2001). The helminth coinfection in humans is quite common in many Asian countries, and emerging evidence suggests that infection with one parasite can significantly influence the outcome of second parasite infection. Infections with Trypanosoma brucei at the time of vaccination or at the time of infection with T. spiralis significantly reduce the effectiveness of the Th2-mediated responses involved in immunity against gastrointestinal (GI) nematode infections (Onah and Wakelin 1999). In addition, cross-protection can be induced by cross-reactive antigen against Fasciola gigantica and T. spiralis infections (Abel Rahman and Abdel Megeed 2005). However, the interactions between nematode T. spiralis and trematode C. sinensis, including immunological responses and clinical outcomes of the host, are poorly defined. KM mice are the most widely used outbred colony in China, started from Swiss mice brought to Kunming, China, from the Indian Haffkine Institute in 1944 (Shang et al. 2009). Using this murine model of trichinosis in the present study, we show here that preinfection with the parasitic trematode C. sinensis resulted in reduced T. spiralis adult worm burden. Our findings raise the possibility that a “preexisting” trematode infection is a factor that influences disease outcome of T. spiralis infection.
In the present study, coinfected mice had significantly reduced establishment of intestinal T. spiralis worm burdens by days 5, 10, and 15 as compared with mice infected with T. spiralis alone. However, we cannot explain the result that the worm burdens were at similar levels between the two groups at day 20 p.i. Infection of mice with T. spiralis leads to a Th2-dependent enteropathy characterized by villus atrophy, crypt hyperplasia, and goblet cell hyperplasia (Ishikawa et al. 1997; Patel et al. 2009). Worm expulsion is CD4+ T cell-dependent and requires Th2 cytokines, in particular, IL-4 and IL-13 (Shea-Donohue and Urban 2004). IL-4 has been shown to be important in the development of protective responses to GI nematode infections (Lawrence et al. 1998), and expulsion of T. spiralis from the small intestine is delayed in IL-4-deficient mice (Else et al. 1994). IL-13, an important mediator during Th2 responses, stimulating B cell proliferation, antibody class switch (to IgE), and eosinophilia, is also required for worm expulsion (Gessner et al. 2005; McDermott et al. 2005). In addition, IL-4Rα, a receptor subunit common to IL-4 and IL-13 signal transduction, has been found to be necessary for worm expulsion (Urban et al. 2001). Mice infected with C. sinensis have increased the production of Th2-associated anti-inflammatory cytokines and upregulation of chemokines (Kim et al. 2012). Our data also showed that, compared with T. spiralis singly infected mice, the mRNA expression of IL-13 was significantly increased in small intestine, and the expressions of IL-13 and TNF-α were significantly increased in spleen in dually infected mice, which suggests that C. sinensis may have some immunomodulatory properties. However, conflicting results demonstrated that neither IL-4/IL-4Rα signaling on CD4+ T cells nor IL-4/IL-13 responsiveness on macrophages/neutrophils are required to induce Th2-type immune responses to GI nematodes (Michels et al. 2009). We propose that the reduced establishment of T. spiralis may be due to the nonspecific inflammatory component of the host’s response to infection with T. spiralis. However, there must be other mechanisms involved in the protection against T. spiralis infection in the concurrent infection condition. Thus, further cytokine characterization will be needed to understand the complex interactions between trematode and nematodes in the host.
T. spiralis dwells within the small intestinal epithelium and actively invades epithelial cells (Wright 1979), leading to major pathological changes such as villus atrophy and crypt hyperplasia, goblet cell and Paneth cell hyperplasia, and infiltration by mucosal mast cells (Kamal et al. 2001).The complexity of epithelia-derived molecules responsible for worm loss include lectin intellectin-2 (Pemberton et al. 2004) and protein RELMβ/FIZZ2 from goblet cell (Abel Rahman and Abdel Megeed 2005). Goblet cells also produce intestinal trefoil factor (Podolsky et al. 1993) that enhances defense in the viscoelastic mucus layer (Kindon et al. 1995). Here, we showed that the enteropathy, represented as significant crypt hyperplasia and villus atrophy, were more severe in C. sinensis/T. spiralis dually infected mice than that in T. spiralis singly infected KM mice. It has been reported that days 6 to 12 of T. spiralis infection shows prominent crypt hyperplasia and villous atrophy in BALB/c, NIH, and C57BL6/129 mice infected with T. spiralis (Kamal et al. 2001; Furze et al. 2006; Walsh et al. 2009), and there is also pronounced increase in the number of Paneth and intermediate cells during this time (Walsh et al. 2009). Our data showed that there were more mast cells, goblet cells, and eosinophils in the epithelial monolayers of the small intestine in T. spiralis singly infected mice than that in C. sinensis/T. spiralis dually infected mice. In contrast, there were more Paneth cells in C. sinensis/T. spiralis dually infected mice than that in T. spiralis singly infected mice at day 10 post challenge. Mast cell is one of the prominent effector cells involved in T. spiralis parasite expulsion in rats as well as in mice (Suzuki et al. 2008). It has been well established that mucosal mast cells are involved in the host protective response to T. spiralis, and the expulsion of the parasite from the gut is associated with intestinal mastocytosis (Donaldson et al. 1996). Secretory products of goblet cells and Paneth cells have a role in innate host defense in the intestine, which inhibits the interaction between luminal microorganisms and surface epithelial cells (Lamont 1992). In addition, T. spiralis infection promotes an initial increase in small intestinal epithelial proliferation and an increase in numbers of Paneth cells at the crypt base causes the proliferative zone to move up the crypt–villus axis (Walsh et al. 2009). The Paneth cells have been implicated in mucosal defense, which synthesize several molecules with potent biological activities, including TNF-α (Tan et al. 1993), epidermal growth factor (Poulsen et al. 1986), guanylin (de Sauvage et al. 1992), and matrilysin (Wilson et al. 1995), and increases in the numbers of Paneth cells correlate with Th2 immune responses (Kamal et al. 2002). Our data indicate that the increased enteropathy and increased number of Paneth cells may contribute in part to the reduced intestinal T. spiralis worm burden in dually infected mice.
Eosinophils have been shown to be associated with resistance to helminth parasites infecting humans and animals (Behm and Ovington 2000; Klion and Nutman 2004). Infection with T. spiralis provides a powerful stimulus for eosinophilia (Herndon and Kayes 1992; Lammas et al. 1992). Eosinophils can kill nematodes in vitro, either alone or in conjunction with other immune components such as antibody or complement (Kazura and Aikawa 1980; Lee 1991). In this study, compared with that of C. sinensis singly infected mice, we observed that the numbers of eosinophils were significantly increased in both the crypt and villus in the C. sinensis/T. spiralis dually infected mice; however, the numbers of eosinophils in the crypt and villus were even significantly increased in T. spiralis singly infected mice at day 10 p.i. Recent study suggests that eosinophils support parasite growth and survival by promoting the accumulation of Th2 cells and preventing the induction of iNOS in macrophages and neutrophils at an extraintestinal site of T. spiralis infection, in which the eosinophil functions as a pivotal regulator of immunity (Gebreselassie et al. 2012). However, whether eosinophils actually contribute to host defense against these parasites is still unclear.
In conclusion, the results reported here clearly showed that intestinal T. spiralis worm burden could be influenced by prior infection with C. sinensis, and our data suggests that the reduced establishment of T. spiralis may be due to both the enteric pathological changes and the nonspecific components of host protective intestinal immune responses elicited by prior infection with C. sinensis. It indicates that the phenomenon of a “preexisting” trematode infection prior to a T. spiralis infection may have a direct impact on the epidemiological situation of the disease. Therefore, more studies are necessary to elucidate the molecular mechanism in protection against T. spiralis infection under C. sinensis-concurrent infections.
References
Abel Rahman EH, Abdel Megeed KN (2005) Cross-protection induced by cross-reactive antigen against Fasciola gigantica and Trichinella spiralis infections. J Egypt Soc Parasitol 35:281–294
Bazzone LE et al (2008) Coinfection with the intestinal nematode Heligmosomoides polygyrus markedly reduces hepatic egg-induced immunopathology and proinflammatory cytokines in mouse models of severe schistosomiasis. Infect Immun 76:5164–5172
Behm CA, Ovington KS (2000) The role of eosinophils in parasitic helminth infections: insights from genetically modified mice. Parasitol Today 16:202–209
Bian K, Zhong M, Harari Y, Lai M, Weisbrodt N, Murad F (2005) Helminth regulation of host IL-4Ralpha/Stat6 signaling: mechanism underlying NOS-2 inhibition by Trichinella spiralis. Proc Natl Acad Sci USA 102:3936–3941
Bokken GC et al (2012) Specific serum antibody responses following a Toxoplasma gondii and Trichinella spiralis co-infection in swine. Vet Parasitol 184:126–132
Cox FE (2001) Concomitant infections, parasites and immune responses. Parasitology 122(Suppl):S23–S38
de Sauvage FJ, Keshav S, Kuang WJ, Gillett N, Henzel W, Goeddel DV (1992) Precursor structure, expression, and tissue distribution of human guanylin. Proc Natl Acad Sci USA 89:9089–9093
Donaldson LE, Schmitt E, Huntley JF, Newlands GF, Grencis RK (1996) A critical role for stem cell factor and c-kit in host protective immunity to an intestinal helminth. Int Immunol 8:559–567
Else KJ, Finkelman FD, Maliszewski CR, Grencis RK (1994) Cytokine-mediated regulation of chronic intestinal helminth infection. J Exp Med 179:347–351
Furze RC, Hussell T, Selkirk ME (2006) Amelioration of influenza-induced pathology in mice by coinfection with Trichinella spiralis. Infect Immun 74:1924–1932
Gebreselassie NG et al (2012) Eosinophils preserve parasitic nematode larvae by regulating local immunity. J Immunol 188:417–425
Gessner A, Mohrs K, Mohrs M (2005) Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol 174:1063–1072
Herndon FJ, Kayes SG (1992) Depletion of eosinophils by anti-IL-5 monoclonal antibody treatment of mice infected with Trichinella spiralis does not alter parasite burden or immunologic resistance to reinfection. J Immunol 149:3642–3647
Huang S, Lu F, Chen Y, Huang B, Liu M (2013) Mast cell degranulation in human periodontitis. J Periodontol 84:248–255
Ishikawa N, Wakelin D, Mahida YR (1997) Role of T helper 2 cells in intestinal goblet cell hyperplasia in mice infected with Trichinella spiralis. Gastroenterology 113:542–549
Jash A et al (2011) Topical application of porcine placenta extract inhibits the progression of experimental contact hypersensitivity. J Ethnopharmacol 133:654–662
Jones LA et al (2010) IL-33 receptor (T1/ST2) signalling is necessary to prevent the development of encephalitis in mice infected with Toxoplasma gondii. Eur J Immunol 40:426–436
Kamal M, Wakelin D, Ouellette AJ, Smith A, Podolsky DK, Mahida YR (2001) Mucosal T cells regulate Paneth and intermediate cell numbers in the small intestine of T. spiralis-infected mice. Clin Exp Immunol 126:117–125
Kamal M, Dehlawi MS, Brunet LR, Wakelin D (2002) Paneth and intermediate cell hyperplasia induced in mice by helminth infections. Parasitology 125:275–281
Kazura JW, Aikawa M (1980) Host defense mechanisms against Trichinella spiralis infection in the mouse: eosinophil-mediated destruction of newborn larvae in vitro. J Immunol 124:355–361
Kim EM, Bae YM, Choi MH, Hong ST (2012) Cyst formation, increased anti-inflammatory cytokines and expression of chemokines support for Clonorchis sinensis infection in FVB mice. Parasitol Int 61:124–129
Kindon H, Pothoulakis C, Thim L, Lynch-Devaney K, Podolsky DK (1995) Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology 109:516–523
Klion AD, Nutman TB (2004) The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol 113:30–37
Lai DH et al (2008) Molecular genetic profiles among individual Clonorchis sinensis adults collected from cats in two geographic regions of China revealed by RAPD and MGE-PCR methods. Acta Trop 107:213–216
Lammas DA, Wakelin D, Mitchell LA, Tuohy M, Else KJ, Grencis RK (1992) Genetic influences upon eosinophilia and resistance in mice infected with Trichinella spiralis. Parasitology 105(Pt 1):117–124
Lamont JT (1992) Mucus: the front line of intestinal mucosal defense. Ann N Y Acad Sci 664:190–201
Lawrence CE, Paterson JC, Higgins LM, MacDonald TT, Kennedy MW, Garside P (1998) IL-4-regulated enteropathy in an intestinal nematode infection. Eur J Immunol 28:2672–2684
Lee TD (1991) Helminthotoxic responses of intestinal eosinophils to Trichinella spiralis newborn larvae. Infect Immun 59:4405–4411
Maizels RM, Yazdanbakhsh M (2003) Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol 3:733–744
McDermott JR, Humphreys NE, Forman SP, Donaldson DD, Grencis RK (2005) Intraepithelial NK cell-derived IL-13 induces intestinal pathology associated with nematode infection. J Immunol 175:3207–3213
Michels CE et al (2009) Neither interleukin-4 receptor alpha expression on CD4+ T cells, or macrophages and neutrophils is required for protective immunity to Trichinella spiralis. Immunology 128(1 Suppl):e385–e394
Muniz VS, Weller PF, Neves JS (2012) Eosinophil crystalloid granules: structure, function, and beyond. J Leukoc Biol 92:281–288
Onah DN, Wakelin D (1999) Trypanosome-induced suppression of responses to Trichinella spiralis in vaccinated mice. Int J Parasitol 29:1017–1026
Owen ILGMM, Pezzotti P, Pozio E (2005) Trichinella infection in a hunting population of Papua New Guinea suggests an ancient relationship between Trichinella and human beings. Trans R Soc Trop Med Hyg 99:618–624
Paim RM et al (2012) Validation of reference genes for expression analysis in the salivary gland and the intestine of Rhodnius prolixus (Hemiptera, Reduviidae) under different experimental conditions by quantitative real-time PCR. BMC Res Notes 5:128
Park MK et al (2011) Protease-activated receptor 2 is involved in Th2 responses against Trichinella spiralis infection. Korean J Parasitol 49:235–243
Patel N, Kreider T, Urban JF Jr, Gause WC (2009) Characterisation of effector mechanisms at the host:parasite interface during the immune response to tissue-dwelling intestinal nematode parasites. Int J Parasitol 39:13–21
Pemberton AD et al (2004) Innate BALB/c enteric epithelial responses to Trichinella spiralis: inducible expression of a novel goblet cell lectin, intelectin-2, and its natural deletion in C57BL/10 mice. J Immunol 173:1894–1901
Podolsky DK et al (1993) Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem 268:6694–6702
Poulsen SSNE, Olsen PS, Hess J, Kirkegaard P (1986) Immunohistochemical localization of epidermal growth factor in rat and man. Histochemistry 85:389–394
Pozio E (2003) Foodborne and waterborne parasites. Acta Microbiol Pol 52(Suppl):83–96
Qian MBCY, Liang S, Yang GJ, Zhou XN (2012) The global epidemiology of clonorchiasis and its relation with cholangiocarcinoma. Infect Dis Poverty 1:4
Rousseau D, Le Fichoux Y, Stien X, Suffia I, Ferrua B, Kubar J (1997) Progression of visceral leishmaniasis due to Leishmania infantum in BALB/c mice is markedly slowed by prior infection with Trichinella spiralis. Infect Immun 65:4978–4983
Santaolalla R, Abreu MT (2012) Innate immunity in the small intestine. Curr Opin Gastroenterol 28:124–129
Serna H, Porras M, Vergara P (2006) Mast cell stabilizer ketotifen [4-(1-methyl-4-piperidylidene)-4h-benzo[4,5]cyclohepta[1,2-b]thiophen-10(9H)-one fumarate] prevents mucosal mast cell hyperplasia and intestinal dysmotility in experimental Trichinella spiralis inflammation in the rat. J Pharmacol Exp Ther 319:1104–1111
Shang H, Wei H, Yue B, Xu P, Huang H (2009) Microsatellite analysis in two populations of Kunming mice. Lab Anim 43:34–40
Shea-Donohue T, Urban JF Jr (2004) Gastrointestinal parasite and host interactions. Curr Opin Gastroenterol 20:3–9
Specian RD, Oliver MG (1991) Functional biology of intestinal goblet cells. Am J Physiol 260:C183–C193
Suzuki T, Sasaki T, Takagi H, Sato K, Ueda K (2008) The effectors responsible for gastrointestinal nematode parasites, Trichinella spiralis, expulsion in rats. Parasitol Res 103:1289–1295
Tan X, Hsueh W, Gonzalez-Crussi F (1993) Cellular localization of tumor necrosis factor (TNF)-alpha transcripts in normal bowel and in necrotizing enterocolitis. TNF gene expression by Paneth cells, intestinal eosinophils, and macrophages. Am J Pathol 142:1858–1865
Urban JF Jr, Noben-Trauth N, Schopf L, Madden KB, Finkelman FD (2001) Cutting edge: IL-4 receptor expression by non-bone marrow-derived cells is required to expel gastrointestinal nematode parasites. J Immunol 167:6078–6081
Walsh R, Seth R, Behnke J, Potten CS, Mahida YR (2009) Epithelial stem cell-related alterations in Trichinella spiralis-infected small intestine. Cell Prolif 42:394–403
Wang X et al (2009) Experimental model in rats for study on transmission dynamics and evaluation of Clonorchis sinensis infection immunologically, morphologically, and pathologically. Parasitol Res 106:15–21
Wilson CL, Hepper KJ, Rudolph LA, Matrisian LM (1995) The metalloproteinase matrilysin is preferentially expressed by epithelial cells in a tissue-restricted pattern in the mouse. Mol Biol Cell 6:851–861
Wright KA (1979) Trichinella spiralis: an intracellular parasite in the intestinal phase. J Parasitol 65:441–445
Zhao J, Endoh I, Hsu K, Tedla N, Endoh Y, Geczy CL (2011) S100A8 modulates mast cell function and suppresses eosinophil migration in acute asthma. Antioxid Redox Signal 14:1589–1600
Acknowledgments
We thank Dr. Xin-zhuan Su (LMVR/NIAID/NIH, USA) for his valuable advice and critical reading of the manuscript. This work was supported, in part, by grants from the National Basic Research Program of China (973 Program) (No. 2010CB530000) and the Natural Science Foundation of China to F. Lu (nos. 81071387 and 81271854).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, Y., Huang, B., Huang, S. et al. Coinfection with Clonorchis sinensis modulates murine host response against Trichinella spiralis infection. Parasitol Res 112, 3167–3179 (2013). https://doi.org/10.1007/s00436-013-3493-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3493-1