Abstract
Angiostrongylus cantonensis is a parasitic pathogen whose forth-stage larvae (L4) parasitize in the central nervous system (CNS) of the human cause severe eosinophilic encephalitis or meningoencephalitis. Previous study indicated an impressive anthelmintic efficacy of tribendimidine (TBD) against CNS parasitized L4 of A. cantonensis. Tegument of the larvae is the first physical barrier to protect them from attack by the host immune system. In the present study, tegumental and hypodermic alterations were observed by scanning electron microscopy and transmission electron microscopy after administration of TBD. During treatment of TBD in vivo, L4 presented wizened side sensor, disappearance of mastoids and longitudinal grain, prominent surface coat, heterogeneous tegumental layers, incompact hypodermic cell junctions, blurred myotube, and small scale of vacuole in a basal layer. After incubation with TBD in vitro, L4 exhibited a swollen side sensor and mastoids disappearance in head end. Abundant tegumental blebs and obvious deformation of both cross-grain and longitudinal grain were detected on the surface, and shrinkage of all tegumental layers, chaotic cell junction, turbid muscle cell, disappearance of myotubes, and vacuole-like changes were visible under the electron microscope. The results implied the potential mechanism of the anthelmintic effect of tribendimidine against L4 of A. cantonensis by direct damages to tegumental and hypodermic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiostrongylus cantonensis, a parasitic nematode, was first identified and classified by Chen (1935) in the lung of a rat. After the first-stage larvae (L1) infect their intermediate hosts of fresh water snails, it takes 3 weeks or so for them to develop into third-stage larvae (L3) (Liu et al. 2009). Rat is the permissive definitive host, but humans and mice are nonpermissive hosts. Human could be infected by eating raw or improperly cooked fresh water snails containing L3 (Wallace and Rosen 1966). After ingestion, L3 could penetrate the blood–brain barrier of hosts and migrate to the central nervous system (CNS). The larvae in the permissive host (e.g., rat) develop into fifth-stage larvae (L5) and then migrate to the pulmonary artery of hosts where they develop sexual maturity and lay eggs (Ishii 1987). In nonpermissive hosts (e.g., mouse, guinea pig, rabbit, rhesus monkey, and humans), larvae fail to migrate to the lung and remain in the CNS (Courdurier et al. 1964; Yamashita et al. 1980) causing serious inflammation of the brain (Abrahams-Sandi et al. 2004; Wang et al. 2012).
Tribendimidine (TBD), a broad-spectrum anthelmintic drug developed by the Chinese National Institute of Parasitic Diseases during the 1980s, is a symmetrical diamidine derivative of amidantel (Xiao 2004). TBD showed anthelmintic activities against Ascaris lumbricoides, hookworm, Trichuris trichiura, and Enterobius vermicularis (Xiao et al. 2005, 2007, 2008, 2009; Zhang et al. 2008). We have previously reported an impressive efficacy of TBD against A. cantonensis (Wang et al. 2013), but the mechanism of the elimination of A. cantonensis is still poorly understood. The tegument (syncytial cytoplasmic layer) covers the entire surface of parasites and acts as a major interface between the parasite and its host. Since parasites can survive for a long time within the bloodstream, intestines, or tissues of the host, they obviously can evade host immune recognition and response, which is dependent on the unusual properties of the tegument surface (Pearce and Sher 1987). The aim of this study is to comprehensively observe the tegumental and hypodermic alterations of L4 of A. cantonensis treated with TBD in vivo and in vitro using electron microscopes.
Methods
Experimental animals
Sixteen Balb/c mice aged 6 weeks were supplied by the Center of Animal Experiments of Sun Yat-sen University. The procedures involved the animals approved by the Animal Care and Use Committee of Sun Yat-sen University. Animals were raised in a room with air condition under a 12:12-h light/dark cycle.
Parasite preparation
The third-stage larvae (L3) of A. cantonensis were collected from positive snails (Ampullaria gigas Spix) by the modified method described by Chen and Lai (2007) and Lan and Lai (2009).
Animal test
All the mice were infected by L3 (20 per mouse) via a feeding tube inserted into the stomach of the animals. Fourteen days after infection, the mice were randomly divided into two groups (eight per group). Mice in group 1 were administered with TBD (dissolved in normal saline) at a dosage of 100 mg/kg/day per mouse for 7 days by intragastric infusion method (Ren et al. 1987), and the animals in group 2 were treated with normal saline at the same volume. Twenty-one days postinfection, all the mice were sacrificed by cervical dislocation, and the brains were removed from the cranial cavity and torn into small pieces into a petri dish to collect the L4 under a dissecting microscope.
In vitro incubation assay
The L4 collected from the infected animals without treatment of TBD were divided into two parts with the same number. The first part of the larvae were incubated for 24 h with DMEM cell culture medium containing 20 ng/ml TBD, and the second part of the worms was incubated with the same medium without TBD.
All the harvested L4 from “animal test” and “in vitro incubation assay” were washed three times with 0.2 M phosphate buffer solution (PBS, pH 7.2) and fixed in 0.2 M PBS containing 2.5 % glutaraldehyde at 4 °C for 24 h prior to the electron microscopic observation.
Scanning electron microscopy
Specimens were washed three times in pH 7.2 PBS and six times in cold distilled water to remove the glutaraldehyde and were dehydrated through a graded series of ethanol (50–100 %). After the ethanol was exchanged with acetone and isoamyl acetate, respectively, and the worms were dried in the Hitachi HCP-2 critical point drying machine using a transitional medium of liquid carbon dioxide. Thereafter, specimens were mounted on aluminum stubs and coated with platinum in an ion-sputtering apparatus, Hitachi E-102, and were examined and photographed in the Hitachi scanning electron microscope S-2500.
Transmission electron microscopy
The samples were washed as described above, dehydrated by ethanol, and embedded in the Araldite 502. Ultrathin sections were cut using a glass knife, collected on 300-mesh copper grids, and doubly stained with 1 % methanolic uranyl acetate and lead citrate for 30 min each. The sections were viewed and photographed in the Hitachi H-300 transmission electron microscope.
Results
Tegumental alterations observed by scanning electron microscopy
L4 from the infected mice without treatment of TBD
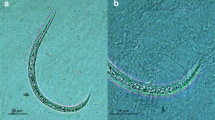
The scanning electron microscopy (SEM) photograph of L4 showed a ring-shaped mouthpart with a mouth sensor on the head end of the larvae. Six pairs of mastoids and a side sensor were distributed around the mouthpart (Fig. 1a), and the surface of L4 is characterized by cross-grain and longitudinal grain (Fig. 1d).
Scanning electron micrographs of tegumental alterations of A. cantonensis L4 after TBD administration. a Head of L4 from a control group with side sensor (SS), mouthpart (MP), mastoids (MT), and mouth sensor (MS) indicated by arrows. Bar = 20 μm. b Head of L4 from TBD-treated group with mouth sensor (MS), mouthpart (MP), mastoids (MT) around the head disappearance, and wizened side sensor (SS). Bar = 30 μm. d The surface of L4 from a control group with cross-grain (CG, white arrows) and longitudinal grain (LG, black arrows). Bar = 30 μm. c Head of L4 from TBD incubation group with swollen side sensor (SS) and mouthpart (MP). Bar = 10 μm. e The surface of L4 from TBD-treated group with longitudinal grain (LG) feature that could rarely be seen, cross-grain (CG, thick arrow), and a small scale of blebs and lesions (the box and thin arrows). Bar = 30 μm. f The surface of L4 from TBD incubation group with serious deformation, both longitudinal grain (LG) and cross-grain (CG) features that were disrupted, and massive scale of blebs (arrow). Bar = 30 μm
L4 from the infected mice treated with TBD
The SEM photograph of L4 from TBD-treated group showed a mouthpart and a mouth sensor on the head of worm, while the mastoids around the head disappeared, and the side sensor became wizened (Fig. 1b). The cross-grain displayed invisible changes after drug treatment, while longitudinal grain disappeared, and small scale of blebs and lesions appeared on the surface (Fig. 1e).
L4 incubated by TBD in vitro
The SEM photograph of L4 incubated in TBD exhibited a swollen mouthpart and an expended side sensor with an inconspicuous mouth sensor. All mastoids disappeared (Fig. 1c), and the surface of the larvae was seriously deformed. Both cross-grain and longitudinal grain were disrupted, and irregular blebs could be found on the surface (Fig. 1f).
Hypodermic alterations observed by transmission electron microscopy
L4 from the infected mice without treatment of TBD
The transmission electron microscopy (TEM) photograph of L4 showed that the cuticle of larva could be divided into four homogeneous layers: surface coat layer, medial layer, fiber layer, and basal layer (Fig. 2a). The surface coat and the medial layer were relatively thin when compared to fiber layer and basal layer. Hypodermic tissue, which consists of muscle cells with abundant myotubes, was attached to the basal layer. The cell junction of muscle cells and the dividing cells could be found in the hypodermic tissue (Fig. 2b–d).
Transmission electron micrographs of A. cantonensis L4 from infected mice without treatment of TBD. a Surface layer (SL), medial layer (ML), fiber layer (FL), basal layer (BL), muscle cells (M), muscle cell junctions (CJ). Bar = 1 μm. b Higher magnification of muscle cells and myotubes (MT, box). Bar = 200 nm. c Higher magnification of hypoderm, fiber layer (FL), basal layer (BL), muscle cells (M), and cell junction (CJ, box). Bar = 200 nm. d Hypodermic cells just finished division, nucleolus (N), and nuclear membrane (NM). Bar = 1 μm
L4 from the infected mice treated with TBD
The TEM photograph of L4 larvae from TBD-treated group showed that all the layers of the cuticle were heterogeneous, and prominences could be found on the surface coat (Fig. 3a). Blurred cell junction and myotubes of muscle cells and a few vacuole-like changes in the basal layer were apparent (Fig. 3b, c). Cells of hypoderm in L4 from the infected mice treated with or without TBD showed no significant difference (Fig. 3d).
Transmission electron micrographs of A. cantonensis L4 from in vivo TBD-treated group. a Surface layer (SL), medial layer (ML), fiber layer (FL), basal layer (BL), muscle cells (M), and muscle cell junctions (CJ). Bar = 2 μm. b Higher magnification of hypoderm, fiber layer (FL), basal layer (BL), and cell junction (CJ). Bar = 500 nm. c Higher magnification of basal layer, small scale of vacuoles (white arrow), surface layer (SL), medial layer (ML), fiber layer (FL), and basal layer (BL). Bar = 1 μm. d Hypodermic cells, nucleolus (N), and nuclear membrane (NM). Bar = 1 μm
L4 incubated by TBD in vitro
The TEM photograph of L4 larvae from TBD-incubated group showed that all the layers (especially fiber layer and basal layer) of the cuticle became thinner (Fig. 4a). Most of the muscle cells were disintegrated, and the cell junction of cells was incompact. The myotubes disappeared, and massive number of vacuole bodies appeared in the cells (Fig. 4b–d).
Transmission electron micrographs of A. cantonensis L4 from in vitro TBD incubation group. a Surface layer (SL), medial layer (ML), fiber layer (FL), basal layer (BL), muscle cells (M), large scale of vacuoles (black arrows), nucleolus (N), and nuclear membrane (NM). Bar = 1 μm. b Higher magnification of hypoderm, fiber layer (FL), basal layer (BL), muscle cells (M), cell junction (CJ), and vacuoles (V). Bar = 200 nm. c Disrupted muscle cells and apoptosis body (black arrows). Bar = 1 μm. d Hypodermic cells, large scale of vacuoles (V), nucleolus (N), and nuclear membrane (NM). Bar = 1 μm
Discussion
TBD decomposed immediately into p-(1-dimethylamino ethylimino) aniline and terephthalaldehyde in vivo after taken by humans (Yuan 2010). The protein binding ratio of TBD is more than 80 % (Xiao 2004). Recent research found that TBD metabolites acted like an l-subtype nicotinic acetylcholine receptor agonist and paralyzed somatic muscle of Caenorhabditis elegans. The mechanism of its action against nematodes was the same as levamisole and pyrantel (Hu et al. 2009). Although no studies reported how TBD transported through the blood–brain barrier. It has been reported that A. cantonensis larvae induced apoptosis and dysfunction in blood–brain barrier (Hu et al. 2012). So during the infection of A. cantonensis, TBD metabolites might penetrate in the CNS though the dysfunctional blood–brain barrier.
Our previous study (Wang et al. 2013) revealed that after the mice infected by A. cantonensis were treated at different dosages of TBD, the number of larvae in the mice brain decreased to 5.33 ± 2.49 (48.3 % reduction, 25 mg/kg/day), 4.67 ± 4.49 (54.8 % reduction, 50 mg/kg/day), 2.33 ± 1.25 (774.% reduction 100 mg/kg/day), and 0 (100 % reduction, 200 mg/kg/day), which demonstrated that TBD was a valid anthelmintic for the elimination of A. cantonensis in the CNS. Ren et al. (1987) reported TBD anthelmintic effects on Necator americanus, leading to transversal texture disappearance, hypodermic tissue shrinkage, and myotube disappearance. Laboratory investigations also showed that TBD exhibited the same activity in vivo against Strongyloides ratti (Keiser et al. 2008a) and several species of trematodes, such as Echinostoma caproni (Keiser et al. 2006), Opisthorchis viverrini (Keiser et al. 2008b), and Clonorchis sinensis (Xiao et al. 2008). These results are consistent to our results, in which abnormal alterations were observed both in the tegument and hypoderm of L4 after TBD administration to the infected mice or a direct incubation by TBD in vitro, and confirm TBD as the promising drug candidate for angiostrongyliasis.
References
Abrahams-Sandi E, Hoffmann WH, Graeff-Teixeira C, Schulz-Key H, Geiger SM (2004) Long-term observations on mouse strains experimentally infected with Angiostrongylus costaricensis. Parasitol Res 93(3):230–234
Chen HT (1935) Un nouveau nematoda pulmonaire: Pulmonema cantonensis, n.g.n. sp. des rats de Canton. Ann Parasitol Hum Comp 13:312–317
Chen KM, Lai SC (2007) Biochemical and pathological evaluation of albendazole/thalidomide co-therapy against eosinophilic meningitis or meningoencephalitis induced by Angiostrongylus cantonensis. J Antimicrob Chemother 59(2):264–276
Courdurier J, Guillon J, Malarde L, Laigret J, Desmoulins G, Schollhammer (1964) Demonstration of the cycle of Angiostrongylus cantonensis in the laboratory: observations on this cycle and anatomo-pathology caused by this parasite in various laboratory animals. Bull Soc Pathol Exot Fil 57(6):1255–1262
Hu X, Li JH, Lan L, Wu FF, Zhang EP, Song ZM, Huang HC, Luo FJ, Pan CW, Tan F (2012) In vitro study of the effects of Angiostrongylus cantonensis larvae extracts on apoptosis and dysfunction in the blood–brain barrier (BBB). PLoS One 7(2):e32161
Hu Y, Xiao SH, Aroian RV (2009) The new anthelmintic tribendimidine is an l-type (levamisole and pyrantel) nicotinic acetylcholine receptor agonist. PLoS Negl Trop Dis 3(8):e499
Ishii AI (1987) Pathogenic factors in the later pulmonary phase of Angiostrongylus cantonensis-infected rats. Parasitol Res 73(5):458–465
Keiser J, Shu-Hua X, Utzinger J (2006) Effect of tribendimidine on adult Echinostoma caproni harbored in mice, including scanning electron microscopic observations. J Parasitol 92(4):858–862
Keiser J, Thiemann K, Endriss Y, Utzinger J (2008a) Strongyloides ratti: in vitro and in vivo activity of tribendimidine. PLoS Negl Trop Dis 2(1):e136
Keiser J, Utzinger J, Xiao SH, Odermatt P, Tesana S (2008b) Opisthorchis viverrini: efficacy and tegumental alterations following administration of tribendimidine in vivo and in vitro. Parasitol Res 102(4):771–776
Lan KP, Lai SC (2009) Differences of proteolytic enzymes and pathological changes in permissive and nonpermissive animal hosts for Angiostrongylus cantonensis infection. Vet Parasitol 165(3–4):265–272
Liu HX, Zhang Y, Lv S, Hu L, Zhou XN (2009) Establishment and observation of the life cycle of Angiostrongylus cantonensis in a laboratory setting. Chin J Pathog Biol 4(11):836–839 (in Chinese)
Pearce EJ, Sher A (1987) Mechanisms of immune evasion in schistosomiasis. Contrib Microbiol Immunol 8:219–232
Ren HN, Cheng BZ, Zhuang ZN (1987) Experimental therapeutic efficacy of a new anti hookworm drug tribendimidine. Chin J Parasitol Parasit Dis 5(4):262–264 (in Chinese)
Wallace GD, Rosen L (1966) Studies on eosinophilic meningitis.2.Experimental infection of shrimp and crabs with Angiostrongylus cantonensis. Am J Epidemiol 84(1):120–131
Wang J, Wei J, Zeng X, Liang JY, Wu F, Li ZY, Zheng HQ, He HJ, Wu ZD (2013) Efficacy of tribendimidine against Angiostrongylus cantonensis infection in the mice. Parasitol Res 112(3):1039–1046
Wang QP, Wu ZD, Wei J, Owen RL, Lun ZR (2012) Human angiostrongyliasis cantonensis: an update. Eur J Clin Microbiol Infect Dis 31(4):389–395
Xiao SH (2004) Tribendimidine—a new anthelmintic. Chin J Parasitol Parasit Dis 22(5):312–315 (in Chinese)
Xiao SH, Wu HM, Tanner M, Utzinger J, Wang C (2005) Tribendimidine: a promising, safe and broad-spectrum anthelmintic agent from China. Acta Trop 94(1):1–14
Xiao SH, Wu ZX, Zhang JH, Wang SQ, Wang SH, Qiu DC, Wang C (2007) Clinical observation on 899 children infected with intestinal nematodes and treated with tribendimidine enteric coated tablets. Chin J Parasitol Parasit Dis 25:372–375 (in Chinese)
Xiao SH, Xue J, Tanner M, Zhang YN, Keiser J, Utzinger J, Qiang HQ (2008) Artemether, artesunate, praziquantel and tribendimidine administered singly at different dosages against Clonorchis sinensis: a comparative in vivo study. Acta Trop 106(1):54–59
Xiao SH, Xue J, Xu LL, Zheng Q, Qiang HQ, Zhang YN (2009) The in vitro and in vivo effect of tribendimidine and its metabolites against Clonorchis sinensis. Parasitol Res 105(6):1497–1507
Yamashita T, Aiba H, Oya H, Fukuda Y (1980) Angiostrongylus cantonensis: development following pulmonary arterial transfers into permissive and nonpermissive hosts. Exp Parasitol 49(3):339–352
Yuan G (2010) Metabolism and disposition of tribendimidine and its metabolites in healthy Chinese volunteers. Drugs RD 10(2):83–90
Zhang JH, Xiao SH, Wu ZX, Qiu DC, Wang SH, Wang SQ, Wang C (2008) Tribendimidine enteric coated tablet in treatment of 1,292 cases with intestinal nematode infection—a phase IV clinical trial. Chin J Parasitol Parasit Dis 26:6–9 (in Chinese)
Acknowledgments
This work was supported by the National Basic Research Program of China (grant no. 2010CB530004) and the National Nature Science Foundation of China (grant nos. 81271855 and 81261160324). We would like to appreciate Professor Guosheng He from the Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences, for his kind gift of tribendimidine.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zeng, X., Wang, J., Wei, J. et al. Angiostrongylus cantonensis: tegumental and hypodermic alterations of the fourth-stage larvae following administration of tribendimidine in vivo and in vitro. Parasitol Res 112, 3035–3040 (2013). https://doi.org/10.1007/s00436-013-3479-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3479-z