Abstract
The current antifilarial treatments are not up to the mark partly due to deep location of filarial parasites in the human lymphatic system. We report here on the improvement in the antifilarial activity of ivermectin (IVM) using chitosan–alginate nanoparticles prepared by modified complex coacervation method. The nanoparticles were spherical having 155 nm size and 4.56 and 75.67 % loading and entrapment efficiency respectively for IVM. The delivery system maintained the sustained release and significantly augmented the microfilaricidal (MIF) activity at a single low dose (200 μg/kg body weight, subcutaneously) in contrast to much higher dose of free ivermectin (400 μg/kg body weight, subcutaneously) against human lymphatic filariid, Brugia malayi in rodent host, Mastomys coucha. To substantiate increase in MIF activity, pharmacokinetics study was designed on Wistar rats which revealed a greater peak plasma concentration (45.3 ± 1.79 ng/ml), area under the concentration curve (298 ± 38.7 ng d/ml) and extended mean residence time (23.4 ± 8.56 days)of IVM in chitosan–alginate nanoparticles. Administration of 25 mg/kg of diethylcarbamazine following nanoparticle therapy significantly improved the MIF and macrofilaricidal action of encapsulated drug and was considered superior in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most of the antifilarial drugs available for the management of lymphatic filariasis are poorly water soluble and exhibit wide biodistribution, low bioavailability at the required site of disease location and side effects (Ali et al. 2013). These limitations often impede the optimal pharmacological activity of antifilarial drugs, resulting in inadequate chemotherapy. Due to deprived antifilarial chemotherapy, the global burden of lymphatic filarial infection poses a major public health problem, placing it second to malaria in causing permanent and long-term disability disease of limbs and genitals that ensue not only in physical crippling but also in psychosocial impairment (World Health Organization 2010). Therefore, we need new or alternative strategy that can substantially improve the antifilarial activity of existing antifilarial drugs. For the effective control of filarial infection, the strategy necessitates targeting of antifilarials to the lymphatic system of the host where the parasites are thriving well by defying the host immune system.
Over the last 20 years, nanomedicines offer new insight for the improvement of existing management strategies with better treatment outcomes and cost effectiveness (Kawashima 2006; Teli et al. 2010; Petrak 2006). With the applications of nanotechnology in medical sciences, new and existing therapeutic agents and even the drugs that had been disqualified during the development process could also be reformulated. Nanomedicines would definitely benefit lymphatic filariasis, by resurrecting some of those drugs or compounds that exhibited promising antifilarial action against the target parasite species, but had to be disqualified, largely due to poor pharmacokinetic issues or acute toxicity (Singh et al. 2010). A number of versatile nanomedical carriers suitable for various routes of administration (intravenous, intramuscular, intraperitoneal, subcutaneous, etc.) and targeted delivery of drugs have been existing (Soppimath et al. 2001; Mohanraj and Chen 2006; Manish and Vimukta 2011). These nano-sized carriers possess wide range of applications to overcome therapeutic limitations. Besides these, highly specific lymphatic targeted drug delivery systems (Maincent 1992; Hauff et al. 2004; Lu et al. 2005; 2006) may prove as the most promising delivery technologies to improve the bioavailability of antifilarial agents close to adult filarial worms in the human lymphatic system and possibly would lead to a novel stratagem for the treatment of lymphatic filariasis.
In the present study, we employed chitosan–alginate (CS–ALG) nanoparticles to improve the antifilarial efficacy of ivermectin (IVM) against human filarial parasite Brugia malayi. CS–ALG nanoparticles were characterized with respect to particle size, morphology, surface charge, drug loading, encapsulation efficiency (%EE) and in vitro release kinetics. DSC and Fourier transform infrared (FT-IR) studies were carried out to determine whether the model drug was incorporated into nanoparticulate formulations as crystalline, amorphous or bound (physically adsorbed) to surface. The characterized nanoparticles were evaluated in vivo in Mastomys coucha model of brugian filariasis.
Materials and methods
Chemicals and reagents
CS, ALG, and dialysis tubes (MW 12,000 kDa) were purchased from Sigma Aldrich (USA). IVM was received as a gift sample from Jubilant Organosys (NOIDA, India). All the solvents used were of analytical grade and purchased from Merck (Mumbai, India).
Animals
M. coucha males (6–8 weeks old) weighing 200–250 g were obtained from the National Laboratory Animal Center, CDRI, Lucknow and used as an experimental animal model. These were fed on rodent diet and water ad libitum and housed in hygienic and standard conditions of light (12 h light/12 h dark) and temperature (28 °C). For pharmacokinetics study of nanoparticles, 6-week-old Wistar rats weighing 200–250 g were obtained from the Central animal house facility of Jamia Hamdard. These were housed in polypropylene cages, with free access to model laboratory food pellets (Bengal gram soaked in water) under standard conditions (temperature, 25 ± 2 °C; relative humidity, 55 ± 5 %). The animal protocols to carry out in vivo studies were approved by the Animal Ethical Committee.
Preparation of IVM-encapsulated CS–ALG nanoparticles
CS–ALG nanoparticles were prepared as per our previously described modified complex coacervation method of Motwani et al. (2007). Briefly, an aqueous solution of polysaccharide CS was mixed with an aqueous solution of ALG under controlled magnetic stirring resulting in to the formation of nanoparticles because of ionic interactions. The rate of addition of ALG solution to CS solution was controlled so as to avoid the formation of microparticles or precipitate. CS is insoluble in water. For increasing its solubility, CS was first dissolved in aqueous acetic acid solution (2 % w/v) at various concentrations under stirring condition. Sodium ALG was also dissolved in distilled water at various concentrations. For preparing nanoparticles, about 10 mL of ALG solution was added drop wise into 10 mL of CS solution containing 100 μl of IVM (10 mg/ml) under continuous magnetic stirring at 1800 rpm for 30 min. The solution was ultracentrifuged at 25,000 rpm for 30 min at 4 °C to separate the free polymers from the nanoparticles and drug content in the supernatant was measured by validated high-performance thin-layer chromatography (HPTLC) method (Ali et al. 2011).
Physiochemical characterization of IVM CS–ALG nanoparticles
Morphology, particle size, and zeta potential
Morphological analysis of the polymeric nanoparticles was performed using transmission electron microscopy (TEM, Philips CM-10, USA). Samples of the nanoparticles suspension (5–10 μl) were dropped onto the formvar-coated copper grids. After complete drying, the samples were stained using 2 % w/v phosphotungstic acid and micro graphed at 80–100 kV. Digital Micrograph and Soft Imaging Viewer software were used to perform the image capture and analysis of CS–ALG nanoparticles. The particle size and size distribution of IVM-loaded polymeric nanoparticles were determined by photon correlation spectroscopy using a particle sizer 90 ZS (Malvern Instruments, Southborough, MA). The intensity of scattered light was detected at 90° to an incident beam. The freeze-dried nanoparticle powder was dispersed in an aqueous buffer and the measurements were carried out, after the aqueous micelles solution was passed through a microfilter of an average pore size of 0.2 μm (Millipore). For zeta potential measurement, 50 μL sample was diluted to 5 mL with 0.1 mM KCl for optimal signal intensity and placed in the an electrophoretic cell. All the measurements were performed in triplicate (n = 3) and the standard deviation (SD) was calculated.
Drug loading and entrapment efficiency
The encapsulation efficiency of nanoparticles was determined after separation of drug-loaded nanoparticles from the aqueous medium containing non-associated drug by ultracentrifugation at 25,000 rpm at 4 °C for 30 min. The amount of drug loaded into the nanoparticles was calculated as the difference between the total amount of drug added to the initial formulation medium and the free drug, i.e. non-entrapped drug remaining in the supernatant. The amount of the free IVM drug in the supernatant was measured by a validated HPTLC method as described previously (Ali et al. 2011). The drug loading and entrapment efficiency of nanoparticles was calculated using the following equations:
In vitro release of IVM
The in vitro release study of IVM from polymeric nanoparticles was performed using dialysis sacs (Motwani et al. 2007). The drug-loaded CS–ALG nanoparticles (containing about 10 mg of IVM) were taken in a cellulose membrane dialysis tube (MW 12,000 kDa) and the dissolution study was performed by rotating at 250 rpm using 500 ml each of phosphate buffer saline (PBS) at pH 7.4. Samples (5 ml each) were removed at various time intervals of 0, 0.5, 1, 2, 3, 4, 6, 12, 18, 24 h and suitably diluted to analyze the drug content by UV–spectrophotometric method.
Fourier transform infrared and differential scanning calorimetry (DSC) of CS–ALG nanoparticles
Fourier-transform infrared spectra of CS, ALG, CS–ALG nanoparticles and free IVM were obtained using a FTIR-8300 spectrophotometer (Shimadzu, Japan). A total of 2 % (w/w) of sample, with respect to the potassium bromide (KBr) disc, was mixed with dry KBr. The mixtures were triturated into fine powder using an agate mortar before compressing into KBr disc under a hydraulic pressure at 10,000 psi for 30 s. Each KBr disc was scanned at 4 mm/s at a resolution of 2 cm−1 over a wave number region of 4,000–400 cm−1. The characteristic peaks were recorded for different samples.
Differential scanning calorimetric analysis was used to characterize the thermal behavior of the individual polymer, drug-loaded nanoparticles and the selected drug. DSC thermograms were obtained using an automatic thermal analyzer system (Pyris 6 DSC, Perkin Elmer, USA). Temperature calibration was performed using an indium calibration reference standard (transition point, 156.600 °C) as a standard. Samples were placed in a standard aluminum pans and heated from 40 to 4,000 °C at a heating rate of 100 °C/min under constant purging of dry nitrogen at 30 ml/min. An empty pan, sealed in the same way (as the sample) was used as a reference.
Pharmacokinetics of CS–ALG nanoparticles in Wistar rats
Pharmacokinetics study of IVM-encapsulated CS–ALG nanoparticles was carried out on Wistar rats. Rats in the 6–8-week age group, weighing 200–250 g were obtained from the animal house facility of Jamia Hamdard and housed till the end of the experiment. These animals were divided into three groups, containing six rats in each group. All the animals were starved for 12 h prior to the start of the experiment. After 12 h, animals of Group I were administered with sterile phosphate buffer saline solution (control). Animals of Group II received free IVM equivalent to 400 μg/kg body weight subcutaneously and Group III animals were injected with IVM CS–ALG nanoparticles equivalent to 400 μg/kg body weight, subcutaneously. Blood samples were collected from the retro-orbital plexus at 5, 15, 30 min, 1, 2, 4, 8, 12, 24 h post injection and placed in heparinised test tubes. Plasma was immediately separated by centrifugation at 3,000 rpm for 10 min. Analysis of IVM concentration in various samples was determined by High performance liquid chromatography with a fluorescence detector. The chromatographic conditions include a mobile phase of acetic acid 0.2 % in water–methanol–acetonitrile (5:30:65 v/v/v) pumped at a flow rate of 1 ml/min through a C18 column. Fluorescence detection was set at excitation wavelength of 375 nm and emission wavelength of 465 nm (Yong-xin et al. 2010).
Host parasite model, infection transmission, and treatment groups
M. coucha male (6 weeks old) infected with sub-periodic strain of B. malayi was used to study the antifilarial prospective of IVM CS–ALG polymeric nanoparticles. Hundred infective larvae (L3) each of B. malayi parasites recovered from the mosquito vector Ades aegytpii were inoculated subcutaneously to M. coucha. The infected animals were selected on the basis of rising parasitic load in systemic circulation, 4–6 months post larval (L3) inoculation. The animals were divided into treatment groups having six animals in each group and all the experiments were done in duplicate. Animals in Group 1 received a single dose of free IVM subcutaneously at 400 μg/kg body weight and animals in Group 2 received a single low dose of IVM CS–ALG nanoparticles subcutaneously at 200 μg/kg body weight. Group 3 animals received a single dose of IVM CS–ALG nanoparticles subcutaneously at 200 μg /kg body weight followed by an oral dose of diethylcarbamazine (DEC) administrated at 25 mg/kg body weight. Group 4 and Group 5 animals remained as negative controls and received phosphate buffer saline and nanoparticles without the drug (Blank). Animals in Group 6 received an oral dose of free DEC given at 25 mg/kg body weight and served as the DEC control.
Parasitological parameters
The efficacy of free IVM as well as IVM CS–ALG nanoparticles was determined on the basis of microfilarial (mf) load in systemic circulation of each treated animal group. An aliquot of 10 μl of blood sample was taken from the tail vein of treated animals on days 0, 10, 20, 30 and at regular interval of 10 days till day 80. Blood smears were made on a clean glass slide just before initiation of treatment (0 day) and on days 10, 20, and thereafter every 10th day till the end of the experiment. The slides were observed under a compound microscope to see the percent change in microfilaremic density as compared to pretreatment count and were used as parameters to determine the microfilaricidal (MIF) efficacy of various drug formulations as described by Misra et al. (1984). The treated and control M. coucha were subsequently euthanized after 80 days and various tissues (lungs, heart, testes, lymph nodes) were teased gently in phosphate buffer saline (PBS, pH 7.2) to recover adult worms. All the surviving females were teased individually in a drop of saline and the condition of the embryonic stages in the uteri was examined microscopically. Any abnormality or death/distortion detected in the uterine contents, including oocytes, eggs and microfilariae was considered as sterilization effect of the test samples on the females and percentage of sterile females was assessed (Misra et al. 1984).
Statistical analysis
Data were analyzed employing Student’s t test (Mann–Whitney test) and one-way analysis of variance (ANOVA) as appropriate. Individual comparisons following ANOVA were made using a post test (Bonferroni) with the help of statistical software PRISM 3.0. Results of mf density was presented as mean ± SEM.
Results
Physiochemical characterization of nanoparticles
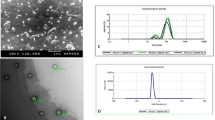
Preparation of CS–ALG nanoparticles was carried out at ambient temperature and the nanoparticles were obtained spontaneously. The preparation process is simple, rapid and reliable. The electron microscopy analysis confirmed the presence of nanoparticles, which appeared to be almost spherical in shape (Fig. 1a). The size of nanoparticles was in range of 122–198 nm with TEM, comparable to the mean particle size of 155 nm observed in the DLS analysis (Fig. 1b). FT-IR and DSC studies indicated that the carboxylic groups of ALG associated with an ammonium group of CS through electrostatic interactions to form the polyelectrolyte complexes and the model drug, IVM was incorporated inside the core of CS–ALG complexes. CS–ALG nanoparticles showed positive zeta potential (+36.23) which may be due to the high positive charge of CS polymer (Motwani et al. 2007). The % EE and percent drug loading of IVM-encapsulated CS–ALG nanoparticles were found to be 75.67 and 4.56 %, respectively. The drug loading and % EE of the nanoparticles are primarily governed by the mechanism of nanoparticle formation and the relative availability of the encapsulate and the polymer. High drug loading and % EE of IVM in CS–ALG nanoparticles may be due to the fact that the drug was first dissolved in the acidic aqueous phase along with the CS polymer following spontaneous formation of CS–ALG nanoparticles upon addition of aqueous ALG solution under stirring. The percent cumulative release curve of IVM (Fig. 2), shows that 78.45 % of the total drug was released from CS–ALG nanoparticles within 24 h. The release profile is characterized by an initial burst at the first 2 h, where a maximum amount of IVM (45.12 %) was released followed by a continuous and controlled release phase within 24 h.
FT-IR and DSC studies
FT-IR spectra of CS, ALG, IVM, and CS–ALG nanoparticles are shown in Fig. 3. The broad band at 3,467 cm−1 in the IR spectra of CS corresponds to the amine and hydroxyl groups whereas peak at 2,916 cm−1 appeared due to –OH stretching. The peaks at 1,640 and 1498 cm−1 correspond to carbonyl (C═O) stretching of secondary amide (amide I band) and the bending vibrations of the N–H (amide II band), respectively (Sankalia et al. 2007). The absorption band of N–H stretching of the amide and ether bonds can be observed at 1,378 cm−1 and N–H stretching (amide III band) at 1208 cm−1. The peaks seen at 1,043 and 1,012 cm−1 were the secondary hydroxyl groups (of –CH–OH in cyclic alcohols) and the primary hydroxyl groups (of –CH2–OH in primary alcohols) respectively as shown in Fig. 3a (Chen et al. 2004). In the IR spectrum of sodium alginate (Fig. 3b), the bands around 1,032 cm−1 (C–O–C stretching) are attributed to saccharide structure. In addition, the bands at 1,567 and 1,423 cm−1 are assigned to asymmetric and symmetric stretching peaks of carboxylate salt groups (Sartori et al. 1997). In the IR spectrum of blank CS–ALG nanoparticles (Fig. 3c), we can observe the asymmetrical stretching of –COO– groups shifted to 1,534 cm−1 and the symmetrical stretching of –COO– groups shifted to 1,420 cm−1 (Li et al. 2007). In addition, the absorption band at 1,498 cm−1 of CS shifts to 1,476 cm−1 after reaction with ALG, the stretching vibration of –OH and –NH2 at 3,475 cm−1 shifts to 3,428 cm−1 and becomes broad. The peak at ~1,640 cm−1 in both the CS and CS–ALG nanoparticles spectra was due to the unreacted –NH2 groups of CS (Motwani et al. 2007). The characteristic strong peaks appeared in the region 700–1,250 cm−1 in the FT-IR spectra of IVM (Fig. 3d) disappeared in the IVM-loaded CS–ALG nanoparticles spectra (Fig. 3e), indicating IVM encapsulation inside the nanoparticles.
DSC thermograms of CS, ALG, CS–ALG nanoparticles, and IVM show exothermic peaks at 302.82, 202.51, 244.01, and 152.51 °C respectively (Fig. 4). The characteristic peak for IVM drug was found to be reduced in intensity and shifted to 87.19 °C in the DSC thermogram of CS–ALG nanoparticles probably due to its encapsulation in nanoparticles (Fig. 4c and d). The characteristic exothermic peaks, each of the CS and ALG at 341.30 and 282.31 °C, respectively, disappeared and could not be seen in CS–ALG nanoparticle thermogram (Fig. 4a and b). The DSC scans of the IVM CS–ALG nanoparticles did not show the characteristic melting exotherms of the drugs, suggesting encapsulation of drugs inside the polymer matrix rather than physical adsorption on nanoparticles surface.
Pharmacokinetics of CS–ALG nanoparticles
In lymphatic filariasis, adult parasites live in the lymphatic system of the host for several years and sexually mature females release millions of mf in the lymph that enter into blood circulation. To communicate disease, these mfs are ingested by a mosquito vector during a blood meal, stay inside the mosquito for one to two weeks, molt twice to produce infective third-stage larvae. Once they reach this stage, they are injected into a new host and migrate to the lymphatic system, enlarge to become adult worms and produce mf which come in the peripheral blood circulation (Paily et al. 2009). Therefore, it is vital to improve the pharmacokinetics of antifilarial drugs that enhance the bioavailability and facilitate long-term drug retention in systemic circulation for effective eradication of mf and interruption of disease transmission. Nanopharmaceuticals are one of the most ornamental systems discovered so far for such application (Marcato and Durán 2008; Ali et al. 2013). In our study, we observed a subsequent enhancement in IVM bioavailability using CS–ALG nanoparticles by administration of single subcutaneous injection of free IVM (0.4 mg/kg) and equivalent IVM nanoformulation in Wistar rats. This can be depicted from a twofold C max (45.3 ± 1.79), AUC0–∞ (298 ± 38.7), and threefold MRT (23.4 ± 8.56) values with IVM CS–ALG nanoparticles in contrast to free IVM (Table 1). Similarly, a liposomal formulation of IVM prepared by Bassissi et al. (2006) exhibited improved pharmacokinetics of this drug as compared to free IVM or ivomec in rabbits. A single subcutaneous injection with 0.3 mg/kg of IVM using Ivomec and liposomal formulation showed a higher C max value (33.33 ng ml−1) of IVM in liposomal formulation in contrast to Ivomec (20.82 ng ml−1) and a significantly faster absorption as indicated by the T max of 0.23 days as compared to 1.13 days for the Ivomec formulation (Bassissi et al. 2006).
Microfilaricidal activity
M. coucha, male (6 weeks old) infected with sub-periodic strain of B. malayi were administered with a single dose of IVM CS–ALG nanoparticles (200 μg/kg body wt) and free IVM (400 μg/kg body wt) subcutaneously and the MIF activity was determined on the basis of mf load in the peripheral circulation of each treated animal group. During initial observation, IVM or CS–ALG nanoparticles did not show any effect on mf up to day 10, however, the microfilarial density started decreasing later on. On day 20, post initiation of treatment, 41.2 % reduction in circulating mf was visible in animals treated with IVM CS–ALG nanoparticles which was statistically significant (P < 0.001) on comparison with the free IVM (13.6 %) animal group and that too required a comparatively lower dose. In addition, this reduction continued till day 50 post treatment and thereafter mf completely disappeared till the end of the experiment (80 days) in IVM CS–ALG nanoparticles. IVM-treated groups, in contrast, demonstrated an increase in the mf counts (Table 2). DEC, a standard filaricide is well known to produce quick reduction in mf density after its administration (Fan 1994) and combination of DEC with IVM promotes the MIF efficacy of IVM (Bajpai et al. 2007). A drastic improvement in the MIF activity of IVM CS–ALG nanoparticles (73.9 %) was therefore observed following oral treatment with a single dose of DEC (25 mg/kg). Moreover, in contrast to DEC or IVM alone, IVM CS–ALG nanoparticles demonstrated complete disappearance of mf by day 60 post treatment that was sustained till the end of the experiment. Blank nanoparticles and untreated control groups did not show any effect on mf density and mf count rose continuously during the period of observation. Thus, the MIF activity of IVM was sufficiently enhanced in CS–ALG nanoparticles against B. malayi in M. coucha. An improvement in the therapeutic efficacy of encapsulated IVM in a liposomal formulation at a lower dose against swine sarcoptiasis and in other applications has also been reported (Clark et al. 2004; Kuang et al. 2006; Borges et al. 2007; Wei and Xia 2008; Guang-Wei et al. 2010).
Macrofilaricidal activity
DEC + IVM nanoparticles or IVM nanoparticles demonstrated maximum female sterilization causing all the females deprived of their developmental stages (Fig. 6), whereas a higher dose of IVM or DEC alone produced 92.78 and 58.9 % female sterilizations respectively. Untreated control or empty nanoparticles did not produce any pronounced effect on female sterilization. Total worm recovery was least in DEC + IVM nanoparticles group followed by DEC and IVM nanoparticle (Fig. 5), however the difference between these groups was not statistically significant.
Discussion
IVM is a hydrophobic drug that exhibits poor solubility, wide biodistribution (accumulates in fat tissues and liver) and rapid systemic clearance by phagocytic cells (Ali and Hennessy 1996). These properties limit the IVM bioavailability, thus desiring for alternate strategies to promote its therapeutic efficacy. We reported improvements in the antifilarial activity of IVM using polymeric nanoparticles of CS–ALG polyelectrolyte complexes. CS and ALG are biodegradable, biocompatible and nontoxic compounds having ample application in the safe delivery of drugs (George and Abraham 2006; Silva et al. 2011). CS–ALG nanoparticles increase the IVM solubility and enhanced bioavailability to systemic circulating mf as indicated by higher C max or AUC values (Table 1). The out-layer of CS not only provides the nanoparticles a positive surface charge, but also enhances absorption via the para-cellular transport pathway through the tight junctions that can be attained from comparatively smaller T max, suggesting faster absorption (Kotze et al. 1999). These improvements, perhaps account for the increased MIF activity of IVM in CS–ALG nanoparticles as compared to free IVM. The important noticeable observation was that the animals treated with IVM CS–ALG nanoparticles showed disappearance of mf within 60 days that lasted till the end of the observation whereas rise in mf was seen in free IVM- or DEC-treated groups even after 40 days post treatment. The mechanism of mf suppression can be better explained due to extended MRT (23.4 ± 8.56 days) indicative of prolonged retention time of IVM in vivo, resulting in long-term mf suppression in the peripheral blood till the end of the experiment.
Apart from MIF action, the potential value of IVM against human filarial infections ultimately depends on the degree of macrofilaricidal (MAF) activity, which is known to be poor on B. malayi adults worms (Campbell 1993). In the current study, we report on the improved MAF efficacy of IVM demonstrating CS–ALG nanosystem as an important drug carrier for antifilarial chemotherapy. Improvement in MAF activity could be due to safe delivery and sustained release of the drug from nanoparticles in the vicinity of the filariid, B. malayi in the lymphatic system thus leading to a larger number of female worms sterilization at a comparatively lower dose. Drug delivery to the lymphatic dwelling parasites can be better understood by the choice of subcutaneous administration after which nanocarriers are efficiently captured by the macrophages and get accumulated in the regional lymph nodes after being absorbed from the injection site and sustained release of drug from nanocarrier may provide relatively higher and prolonged concentrations in lymphatic tissues (Porter 1997). The present study is the first to our knowledge to use polymeric nanoparticles of CS and ALG containing IVM, leading to passive killing of lymphatic filarial worms.
Interestingly, a synergistic effect on the antifilarial activity of IVM CS–ALG nanoparticles was also observed, when DEC was included in the study. It is well known that both the existing antifilarials, DEC and IVM, are principally microfilaricides and various combinations of these drugs produced a much greater effect on microfilarial density than when treated alone (Moulia-Pelat et al. 1995) and this effect was also substantiated in the investigation. The animals revealed 77.9 % reduction in mf density within a few days of IVM nanoparticles therapy followed by administration of DEC IVM nanoparticles alone, however, did not show any effect on mf density at such an early period post treatment (Table 1). In addition, the synergistic effect continued to be evident at every point of mf detection till the end of experiment. The MIF efficacy of this combination seems to be mostly DEC driven. DEC also promotes the MAF activity of IVM nanoparticles (Figs. 5 and 6). However, female sterilization appeared to be exclusively exerted by IVM nanoparticles demonstrating no synergistic efficacy of DEC and IVM nanoparticles combination (Fig. 6). Almost similar findings have also been observed by Bajpai et al. (2007) who reported that the co-administration of DEC and IVM had no effect on the embryo-static activity of IVM which alone produced 95 % female sterilization. Similarly, synergistic effects on antifilarial activity of liposomal rifampicin, doxycycline, and tetracycline in combination with DEC were also well reported (Dangi et al. 2010; Bajpai et al. 2005). These investigations apparently proved that the nano drug delivery systems not only show the way for improving the therapeutic efficacy of antifilarial drugs but also lead with the possibilities of potent combinations that are more beneficial, efficacious and less harmful than conventional chemotherapy. Nevertheless, rigorous explorations in this line are urgently required to develop an affordable and targeted drug delivery system for this neglected tropical disease.
We are in the process of developing targeted drug delivery system for the treatment of various diseases including lymphatic filariasis. Targeted drug delivery approach is an attractive method for the cure of complex and devastating diseases. The present study reveal that the developed delivery system is safe, biocompatible, easy to prepare and provides good prospects to enhance the antifilarial gamut of IVM for the elimination of lymphatic filariasis. The encapsulation of IVM in CS–ALG polyelectrolyte complexes significantly augmented the adulticidal action at a sub-optimal dose in M. coucha infected with lymphatic filarial parasite, B. malayi and the much enhanced MIF efficacy is supported by improved pharmacokinetic records acquired in a separate study on Wistar rats. Additionally, the combined treatment of DEC with IVM nanoparticles demonstrated superior MIF and marginally improved MAF efficacy thereby recommending explicit use of such formulations in antifilarial treatment for the elimination of this morbid disease.
Abbreviations
- IVM:
-
Ivermectin
- DEC:
-
Diethylcarbamizine
- mf:
-
Microfilaria
- CS:
-
Chitosan
- ALG:
-
Alginate
- CS–ALG NPs:
-
Chitosan–alginate nanoparticles
- MIF activity:
-
Microfilaricidal activity
- MAF activity:
-
Macrofilaricidal activity
- TEM:
-
Transmission electron microscopy
- DLS:
-
Dynamin light scattering
- FT-IR:
-
Fourier-transform infrared
- DSC:
-
Differential scanning calorimetry
- AUC:
-
Area under the concentration–time curve
- C max :
-
Maximum concentration
- MRT:
-
Mean residence time
- T max :
-
Time to peak concentration
- %EE:
-
percent entrapment efficiency
References
Ali DN, Hennessy DR (1996) The effect of level of feed intake on the pharmacokinetic disposition and efficacy of ivermectin in sheep. J Vet Pharmacol Ther 19:89–94
Ali M, Alam S, Ahmad S, Dinda AK, Ahmad FJ (2011) Determination of ivermectin stability by high-performance thin-layer chromatography. Int J Drug Dev Res 3:240–247
Ali M, Afzal M, Bhattacharya SM, Ahmad FJ, Dinda AK (2013) Nanopharmaceuticals to target antifilarials: a comprehensive review. Expert Opin Drug Deliv 10(5):665–678
Bajpai P, Vedi S, Owais M, Sharma SK, Saxena PN, Misra-Bhattacharya S (2005) Use of liposomized tetracycline in elimination of Wolbachia endobacterium of human lymphatic filariid Brugia malayi in a rodent model. J Drug Target 13(6):375–381
Bajpai P, Srivastava K, Shakya S, Saxena PN, Misra-Bhattacharya S (2007) Improvement in the efficacy of existing combination of antifilarials by inclusion of tetracycline in rodent model of brugian filariasis. Curr Sci 92(5):655–658
Bassissi F, Lespine A, Alvinerie M (2006) Assessment of a liposomal formulation of ivermectin in rabbit after a single subcutaneous administration. Parasitol Res 98:244–249
Borges FA, Cho HS, Santos E, Oliveira GP, Costan AJ (2007) Pharmacokinetics of a new long acting endectocide formulation containing 2.25% ivermectin and 1.25% abamectin in cattle. J Vet Pharmacol Ther 30(1):62–67
Campbell WC (1993) Ivermectin—an anti-parasitic agent. Med Res Rev 13:61–79
Chen SC, Wu YC, Mi FL, Lin YH (2004) A novel pH-sensitive hydrogel composed of N,O-carboxymethyl chitosan and alginate crosslinked by genipin for protein drug delivery. J Control Rel 96:285–300
Clark SL, Crowley AJ, Schmidt PG, Donoghue AR, Piché CA (2004) Long-term delivery of ivermectin by use of poly (d,l-lactic-co-glycolic) acid microparticles in dogs. Am J Vet Res 65(6):752–757
Dangi A, Dwivedi V, Vedi S, Owais M, Misra-Bhattacharya S (2010) Improvement in the antifilarial efficacy of doxycycline and rifampicin by combination therapy and drug delivery approach. J Drug Target 18(5):343–350
Fan PC (1994) Determination of the earliest appearance and peak count of microfilariae of Wuchereria bancrofti and Brugia malayi after taking a single dose of diethylcarbamazine at noon. J Helminthol 68(4):301–304
George M, Abraham TE (2006) Polyhydrochloride for intestinal delivery of protein drugs: chitosan and alginate. J Control Release 114:1–14
Guang-Wei, Hui D, Xiang-Bing (2010) Preparation of ivermectin liposome and its pharmacokinetics and treatments in goats. Chin J Vet Parasitol 5:123–132
Hauff P, Reinhard M, Briel A, Debus N, Schirner M (2004) Molecular targeting of lymph nodes with L-selectin ligand-specific US contrast agent, A feasibility study in mice and dogs. Radiology 231:667–673
Kawashima Y (2006) Nanoparticulate systems for improved drug delivery. Adv Drug Del Rev 47:11–16
Kotze AF, Thanou MM, Luebetaen HL, de Boer AG (1999) Enhancement of paracellular drug transport with highly quaternized N-trimethyl chitosan chloride in neutral environments: in vitro evaluation in intestinal epithelial cells (Caco-2). J Pharm Sci 88:253–257
Kuang XX, Guang-Wei, Hui D, Xiang-Bing M, Yi L (2006) Study on the acute toxicity of ivermectin liposome. Chin J Vet Parasitol 4:67–72
Li T, Shi XW, Du YM, Tang YF (2007) Quaternized chitosan/alginate nanoparticles for protein delivery. J Biomed Mater Res A 83(2):383–390
Lu B, Xiong SB, Yang H (2005) Mitoxantrone- loaded BSA nanospheres and chitosan nanospheres for local injection against breast cancer and its lymph node metastases II: tissue distribution and pharmacodynamics. Int J Pharm 5:561–57
Lu B, Xiong SB, Yang H (2006) Solid lipid nanoparticles of mitoxantrone for local injection against breast cancer and its lymph node metastases. Eur J Pharm Biopharm 56:86–95
Maincent P (1992) Lymphatic targeting of polymeric nanoparticles after intraperitoneal administration in rats. Pharm Res 9(12):1534–1539
Manish G, Vimukta S (2011) Targeted drug delivery system: a review. Res J Chem Sci 2:135–141
Marcato PD, Durán N (2008) New aspects of nanopharmaceutical delivery systems. J Nanosci Nanotechnol 8:1–14
Misra S, Chaterjee RK, Sen AB (1984) The response of Litomosides carinii to antifilarial agents in cotton rats Sigmodon hispidus and multimammate rat. Indian J Med Res 79:749–752
Mohanraj VJ, Chen Y (2006) Nanoparticles—a review. Trop J Pharm Res 5(1):561–567
Motwani SK, Chopra S, Talegaonkar S, Kohli K, Ahmad FJ, Khar RK (2007) Chitosan–sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: formulation, optimization and in vitro characterization. Eur J Pharm Biopharm 68:513–525
Moulia-Pelat JP, Glaziou P, Weil GJ, Nguyen LN, Gaxotte P, Nicolas L (1995) Combination ivermectin plus diethylcarbamazine, a new effective tool for control of lymphatic filariasis. Trop Med Parasitol 46(1):9–12
Paily KP, Hoti SL, Das PK (2009) A review of the complexity of biology of lymphatic filarial parasites. J Parasit Dis 33(1–2):3–12
Petrak K (2006) Nanotechnology and site-targeted drug delivery. J Biomater Sci Polym Ed 17(11):1209–1219
Porter CJH (1997) Drug delivery to the lymphatic system. Crit Rev Ther Drug Carrier Syst 14:333–393
Sankalia MG, Mashru RC, Sankalia JM, Sutariya VB (2007) Reversed chitosan–alginate polyelectrolyte complex for stability improvement of alpha-amylase: optimization and physicochemical characterization. Eur J Pharm Biopharm 65:215–232
Sartori C, Finch DS, Ralph B (1997) Determination of the cation content of alginate thin films by FTIR spectroscopy. Polymer 38:43–51
Silva MS, Cocenza DS, Grillo R, Silva de Melo NR, Tonello PS, Oliveira LC, Cassimiro DL, Rosa AH, Fraceto LF (2011) Paraquat-loaded alginate/chitosan nanoparticles: preparation, characterization and soil sorption studies. J Hazard Mater 190:366–374
Singh PK, Ajay A, Kushwaha S (2010) Towards novel antifilarial drugs: challenges and recent developments. Futur Med Chem 2(2):251–283
Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE (2001) Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release 70:1–20
Teli MK, Mutalik S, Rajanikant GK (2010) Nanotechnology and nanomedicine: going small means aiming big. Curr Pharm Des 16(16):1882–1892
Wei L, Xia X (2008) Study on the effect of ivermectin liposome in treating swine sarcoptidosis. Chin J Vet Parasitol 3:44–50
World Health Organization (2010) WHO global programme to eliminate lymphatic filariasis progress report for 2000–2009 and strategic plan 2010–2020. World Health Organization, Geneva
Yong-xin, Wangdui B, Zhu SE, Shu-hai HE, Jing W, Lu-de D (2010) Determination of content and entrapment efficiency for ivermectin liposomes by HPLC. China Anim Husb Vet Med 4:190–196
Acknowledgments
The authors are thankful to Indian Council of Medical Research (ICMR), New Delhi, India for providing financial support in the form of Senior research fellowships to M.A. and M.V.
Declaration of Interest
The authors report no declarations of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, M., Afzal, M., Verma, M. et al. Improved antifilarial activity of ivermectin in chitosan–alginate nanoparticles against human lymphatic filarial parasite, Brugia malayi . Parasitol Res 112, 2933–2943 (2013). https://doi.org/10.1007/s00436-013-3466-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3466-4