Abstract
In rubber plantations, tree holes are one of the major types of breeding habitats of Aedes mosquitoes which transmit dengue and chikungunya. A mermithid nematode, Romanomermis iyengari, was evaluated in tree holes for its efficacy in controlling Aedes albopictus. Infection of mosquito larvae by the nematode was determined through microscopic examination on the next day of application, and evaluation of immature density of mosquito was done on the seventh day. After application of the infective stage of the nematode in a host–parasite ratio of 1:3 or 1:4, the infection rates on the different larval instars of mosquito were similar, 85.7–95.8 % in first to third instars and 79.3 % in fourth instar larvae or 100 and 92.9 %, respectively. Parasite burden varied from 1.1 to 2.4, respectively, among first and third instar larvae applied at 1:3. At 1:4, the parasite burden was between 1.6 (fourth instar) and 4 (second instar). The increase in parasite burden due to parasite density was significant in all the larval instars (P < 0.05). High parasite burden is detrimental to parasite recycling as it can cause premature mortality of the host. Hence, the dosage of 1:3 could be considered as suitable for rubber tree hole habitats. In the nematode-applied tree holes, there was a significant level (P < 0.05) of reduction in the immature density of A. albopictus, especially late instars and pupae, confirming the efficacy of R. iyengari in infecting the mosquito and controlling pupal emergence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kerala state, India, with the largest area of rubber plantation in the country, was the worst affected by chikungunya fever during 2006–2010 and is still under the grip of dengue fever. Plantation practices, along with climatic factors, resulted in the proliferation of the most predominant Aedes albopictus species of mosquitoes in rubber plantation areas (Kumar et al. 2011). During the rainy season, water collected in the unused latex collection cups and tree holes become the major habitats for breeding of A. albopictus. Hence, an effective and appropriate environmentally safe method for the control of the vector mosquito is the need of the hour (Maheswaran and Ignacimuthu 2012). Mosquito control strategies, alternative to chemical insecticides, which are harmless to nontarget organisms as well as humans, are being advocated and developed by many workers (Amer and Mehlhorn 2006; Arjunan et al. 2012).
Control methods using sustainable pathogens, parasites, or predators as biocontrol agents are highly promising due to their advantages over chemical control. The use of entomophilic mermithid nematodes, especially Romanomermis iyengari and Romanomermis culicivorax, has been attempted for the control of mosquitoes breeding in different types of habitats (Keiser et al. 2005; Platzer 2007). R. culicivorax is a temperate-zone species and was found effective in rice fields, lakes, and ponds (Petersen et al. 1978; Westerdahl et al. 1982; Rojas et al. 1987; Santamarina and Perez 1997). R. iyengari is a tropical species indigenous to India (Gajanana et al. 1978) and is successful in controlling mosquitoes breeding in rice field, grassland, and other natural habitats (Paily et al. 1991, 1994; Santamarina et al. 1999; Santamarina and Bellini 2000; Pérez et al. 2005). These nematodes parasitize mosquito larvae and kill them before their maturation into adults. Larvae are killed through mechanical rupture of their integument by the emerging parasitic life completed nematodes, and hence, there is no chance of resistance development by the host. They are aquatic, easy to mass produce in the laboratory, safe to nontarget organisms including humans, and are very specific to mosquitoes (Gajanana et al. 1978).
R. iyengari has been shown to be infectious to A. albopictus and recycled in the tree hole habitat for a season (Paily et al. 1991). However, its effectiveness in controlling mosquitoes breeding in rubber tree holes, with the specific type of organic pollution and localized pattern of rainfall, is yet to be studied. Therefore, a field trial of R. iyengari was carried out by introducing its infective stage and monitoring for level of infection and mosquito density.
Materials and methods
Study area
The study area is located at Aicompu village of Kottayam district in Kerala, where plantations with 12- to 15-year-old rubber trees were plenty. These rubber trees provided the tree hole habitats with A. albopictus breeding for evaluation of the mosquitocidal efficacy of the nematode. The tree holes were situated at about 2.5 to 3.5 m above the ground level and their sizes were in the range of 175 to 200 cm2. The evaluation was done on three occasions, viz., during the months of April–May (pre-southwest monsoon), August (post-southwest monsoon), and October–November (northeast monsoon) 2011. The period of southwest monsoon was not included because of heavy rain and overflowing of habitats. The number of tree holes with mosquito breeding available for the application was 20, 10, and 15, respectively, with equal numbers of control without application.
Pre-application evaluation for mosquito density, any existing parasitism, and water quality
Data on immature density of the vector in each of the habitat were collected 1 day prior to application of the nematode through standard measurement methods (Service 1976). Briefly, the method involved siphoning out of the entire water into an enamel tray held below the tree hole and counting various stages (first instar to pupa) of the mosquito. After counting, the entire water with the immatures of the mosquito was returned to the same tree hole. However, 5–10 larvae of different instars were examined in the laboratory for any existing natural parasitism by entomophilic nematodes (Paily et al. 1991). This was done for both applied and unapplied control groups of tree holes. Water samples from five randomly selected tree holes were pooled and analyzed for dissolved oxygen, pH, and suspended and dissolved solids (Theroux et al. 1943).
Application with the infective stage pre-parasitic stage of R. iyengari
The required quantity of R. iyengari was obtained from a cyclic colony maintained at the Vector Control Research Centre, Pondicherry, India, following the method of Petersen and Willis (1972). Infective stages of the nematode, known as pre-parasitic nematodes (PPN), hatched from eggs by flooding of 2-month-old culture trays were applied as water suspension. The dosage (PPN density) for the first application was 1:4 host–parasite ratio. Whereas for the second and third applications, it was 1:3 as the first application was found to kill most of the larvae prematurely through heavy parasite burden. The application was done by pouring measured quantity of the PPN water suspension.
Post-application evaluation of parasitism and mosquito density
Depending upon their availability, 5–10 mosquito larvae (different instars) per tree hole were collected on the next day of PPN application and brought to the laboratory. Status of infection by R. iyengari was determined through dissection and microscopic examination of the larvae. For this, each larva was placed individually on a microslide with a drop of 0.9 % saline and gently teased with dissection needles. The slides were examined under a compound microscope (×40) for the presence as well as number of the parasite. Number of mosquito larvae positive for nematode infection and number of parasite present in each positive larva was recorded. From this data, percentage parasitism of the nematode on different larval instars of the mosquito and instar-wise parasite burden per larva (total number of parasites present/total number of larvae positive for parasite) was computed (Paily and Balaraman 2000). Evaluation of immature density of mosquito was done both in PPN applied and unapplied control tree holes on the seventh day after application as it was done for evaluation before application. Mean density was computed for each larval instar.
Chi-square test was applied to test the significance of differences in percentage of infections as well as parasite burden in mosquito larvae applied with both the parasite dosages, 1:3 and 1:4 host–parasite ratio. Student’s t independent test was applied to test the difference in mean number of mosquito larvae between control and the PPN applied habitats. Similarly, Student’s t paired test was used to test the difference in mean number of larvae between pre-application and post-application evaluation. For all the statistical tests, level of significance was taken as P < 0.05.
Results
Mosquito larvae collected from tree holes, prior to the application of R. iyengari pre-parasites, were examined for any existing natural parasitism by entomophilic nematodes. None of the larvae was positive for any parasitic nematodes, proving that natural infection with entomophilic nematodes was not existing among the mosquitoes breeding in the rubber tree hole habitat. Analysis of water samples collected from the PPN applied tree holes showed level of dissolved oxygen at 2.8–6.4 mg/L, suspended solids at 3.5–5.1 mg/L, dissolved solids at 1.5–2.8 mg/L, total solids at 5.00–7.5 mg/L, and pH 6.5–7.8.
Parasitological evaluation for R. iyengari on larvae applied at the dosage of 1:4 host–parasite ratio showed 97 % infection on the next day (Table 1). Almost 70 % of the infected larvae showed multiple parasitism and the number of parasites ranged from 1 to 17, with a mean parasite burden of 2.8 per larva. At 1:3 dosage, the first application showed a parasitism of 85.5 %. Multiple parasitism was limited due to the low dosage of application and the maximum number of parasites observed in a single larva was 3. During the second application with the same dosage (1:3), the level of parasitism was 89 % and the mean parasite burden was 2.7 per larva (range, 1–6). Parasites in their early stage of development could be seen within the hemocoel of the mosquito larvae, even without dissection under a compound microscope (Fig. 1a). It could be seen wriggling in the head, thorax, abdomen, or caudal region of the infected larva. When the larvae were dissected, all the infected parasites were found to be released onto the slide (Fig. 1b).
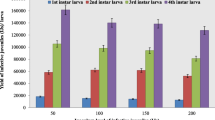
While parasite dosage was 1:3, the percentage of infected mosquito larvae varied between 85.7 and 95.8 % among early instars (first to third) and 79.3 % in late instar (fourth). There seemed to be an increase in the percentage of infection on larvae (instar-wise) when parasite density was 1:4. In the early instars, all larvae were infected, and in the fourth instar, 92.9 % were infected. However, the difference in the level of infection due to different parasite dosages was not significant (chi-square = 3.84; P > 0.05). Parasite burden varied from 1.1 (first instar) to 2.4 (third instar) among the mosquito larvae applied with the parasite density of 1:3 (Fig. 2). Among the larvae applied with the parasite density of 1:4, parasite burden was between 1.6 (fourth instar) and 4 (second instar). There was a significant (t = 2.88; P < 0.05) increase in the parasite burden due to the parasite dosage of 1:4 in all stages of mosquito larvae compared to that of parasite dosage of 1:3 (Fig. 3).
During the pre-application evaluation, instar-wise mean larval density in the unapplied control tree holes varied from 2.0 (±2.2) to 8.1 (±4.3), respectively for the first and fourth instars. The corresponding values in the PPN applied habitats were 1.5 (±2.1) and 7.2 (±3.0). The difference in the instar-wise mean larval density between control and applied habitats was not significant (t values ranged between 0.34 and 1.2; P > 0.05), implying that larval density was almost the same in both habitats. During post-application evaluation, in the control habitats, there was a significant (t values ranged between 2.56 and 8.05; P < 0.05) increase in the density of first to third instar larvae and pupae from the pre-application level (Fig. 4). However, among the PPN applied habitats, there was a significant level (t values ranged between 4.91 and 9.28; P < 0.05) of decrease in the density of larval instars, especially late instars and pupae (Fig. 5). In these tree holes, reduction in the pupal density was from a mean of 2.8 (±2.21) to 0 per tree hole, compared to an increase from 4.0 (±3.1) to 6.3 (±1.6) in the unapplied control tree holes.
Discussion
The natural habitat of R. iyengari is in rice fields and it exerts natural control of mosquitoes breeding in such habitats (Gajanana et al. 1978). In a survey conducted at Pondicherry, India, 0.4–7.5 % of anophelines and 1.1–11.2 % of culicines breeding in rice fields were found infected with this mermithid parasite of mosquitoes (Chandrahas and Rajagopalan 1979). It was very effective in reducing the density of Culex tritaeniorhynchus, Anopheles subpictus, Anopheles pseudopunctipennis, Anopheles albitarsis, and Anopheles rondoni breeding in habitats like grassland, rice fields, stagnant streams, and ditches (Paily et al. 1994; Santamarina et al. 1999; Santamarina and Bellini 2000; Pérez et al. 2005). Application of this nematode to a limited number of tree holes (Poinciana regia) within a park area in Bangalore, India infected A. albopictus larvae (Paily et al. 1991). In the present study, it was evaluated for its habitat suitability and mosquito control potential in rubber tree holes present in plantation areas of Kerala, where they contribute to significant proportion of Aedes mosquito breeding.
Though tree hole-breeding mosquito-specific nematodes like Octomyomermis muspratti has been reported from Africa (Muspratt 1945), there are no reports of tree hole-colonizing mosquito-specific entomophilic nematodes in India. Hence, the nematode parasitism observed in the mosquito larvae, after application, was the result of infection by R. iyengari. Application of R. iyengari to tree holes caused infection in the mosquito sufficient to reduce their density, as the level of infection was above 80 %. The rate of infection by the nematode on mosquito larvae will be the rate of reduction in larval density, as none of the infected early instar larvae survives to emerge into adult (Paily and Balaraman 2000). In this study, it has been proved by the final trial when reduction of mosquito density was also evaluated. Reduction in immature density of the mosquito was significant in PPN applied tree holes compared to that in unapplied control tree holes. The physical and chemical properties of the water were within the favorable ranges and R. iyengari could infect mosquitoes in such types of habitats, where the water is not highly polluted (Paily et al. 1991).
The dosage of 1:4 host–parasite ratio caused the highest level of parasitism. However, the multiple parasitism and high parasite burden per larva is detrimental to parasite recycling, as it may cause premature mortality of the host larvae. Moreover, it may lead to the production of more numbers of males due to the density-dependent sex differentiation commonly observed in mermithid nematodes (Paily and Balaraman 1990). Considering these, the dosage of 1:3 host–parasite ratio is ideal as it showed low levels of parasite burden and substantial levels of parasitism.
This study confirmed the efficacy of R. iyengari in infecting A. albopictus larvae and thereby controlling their emergence into pupae from rubber tree holes present in plantation areas of Kerala, which has its own water quality, because of the organic pollutants and location-specific climatic factors. However, as a biocontrol agent, establishment and recycling of the nematode in the mosquito breeding habitat is expected. This aspect is to be studied through large-scale application of the parasite to tree hole habitats. Once it is proved to be recycling, at least for a season, it can be introduced as a biocontrol agent.
References
Amer A, Mehlhorn H (2006) Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol Res 99:466–472
Arjunan N, Murugan K, Madhiyazhagan P, Kovendan K, Prasannakumar K, Thangamani S, Barnard DR (2012) Mosquitocidal and water purification properties of Cynodon dactylon, Aloe vera, Hemidesmus indicus and Coleus amboinicus leaf extracts against the mosquito vectors. Parasitol Res 110:1435–1443
Chandrahas RK, Rajagopalan PK (1979) Mosquito breeding and the natural parasitism of larvae by a fungus Coelomomyces and a mermithid nematode Romanomermis in paddy fields in Pondicherry. Indian J Med Res 69:63–70
Gajanana A, Kazmi SJ, Bheema Rao US, Suguna SG, Chandrahas RK (1978) Studies on a nematode parasite (Romanomermis sp.: Mermithidae) of mosquito larvae in Pondicherry. Indian J Med Res 68:242–247
Keiser J, Maltese MF, Erlanger TE, Bos R, Tanner M, Singer BH, Utzinger J (2005) Effect of irrigated rice agriculture on Japanese encephalitis, including challenges and opportunities for integrated vector management. Acta Trop 95:40–57
Kumar NP, Suresh A, Vanamail P, Sabesan S, Krishnamoorthy K, Mathew J, Jose VT, Jambulingam P (2011) Chikungunya virus outbreak in Kerala, India, 2007: a seroprevalence study. Mem Inst Oswaldo Cruz 106:912–916
Maheswaran R, Ignacimuthu S (2012) A novel herbal formulation against dengue vector mosquitoes Aedes aegypti and Aedes albopictus. Parasitol Res 110:1801–1813
Muspratt J (1945) Observations on the larvae of tree-hole breeding Culicini (Diptera: Culicidae) and two of their parasites. J Entomol Soc South Africa 8:13–20
Paily KP, Balaraman K (1990) Effect of temperature and host–parasite ratio on sex differentiation of Romanomermis iyengari (Welch), a mermithid parasite of mosquitoes. Indian J Exp Biol 28:470–474
Paily KP, Balaraman K (2000) Susceptibility of ten species of mosquito larvae to the parasitic nematode Romanomermis iyengari and its development. Med Vet Entomol 14:426–429
Paily KP, Arunachalam N, Somachary N, Balaraman K (1991) Infectivity of a mermithid nematode Romanomermis iyengari (Welch) in different conductivity levels under laboratory and field conditions. Indian J Exp Biol 29:579–581
Paily KP, Arunachalam N, Reddy CMR, Balaraman K (1994) Effect of field application of Romanomermis iyengari (Nematoda: Mermithidae) on the larvae of Culex tritaeniorhynchus and Anopheles subpictus breeding in grassland. Trop Biomed 11:23–29
Pérez PR, Rodríguez HC, Lara RZ, Montes BR, Ruiz JV (2005) Control of the mosquito Anopheles pseudopunctipennis (Diptera: Culicidae) with Romanomermis iyengari (Nematoda: Mermithidae) in Oaxaca, Mexico. Biol Control 32:137–142
Petersen JJ, Willis OR (1972) Procedure for the mass rearing of mermithid parasite of mosquitoes. Mosq News 32:226–230
Petersen JJ, Chapman HC, Willis OR, Fukuda T (1978) Release of Romanomermis culicivorax for the control of Anopheles albimanus in El Salvador. II. Application of the nematode. Am J Trop Med Hyg 27:1268–1273
Platzer EG (2007) Mermithid nematodes. Am Mosq Control Assoc Bull 7:58–64
Rojas W, Northup J, Gallo O, Montoya AE, Montoya F, Restrepo M, Nimnich G, Arango M, Echavarria M (1987) Reduction of malaria prevalence after introduction of Romanomermis culicivorax (Mermithidae: Nematoda) in larval Anopheles habitats in Colombia. Bull Wld Hlth Org 65:331–337
Santamarina MA, Bellini AC (2000) Mass produced Romanomermis iyengari (Nematoda: Mermithidae) applied to anopheline breeding sites in Boa Vista (Roraima), Brazil. Pan Am J Pub Health 7:155–161
Santamarina MA, Perez PR (1997) Reduction of mosquito larval densities in natural sites after introduction of Romanomermis culicivorax (Nematoda: Mermithidae) in Cuba. J Med Entomol 34:1–4
Santamarina MA, Perez PR, Tomas-Martinez SH, Enrique CL, Flores AG (1999) The Romanomermis iyengari parasite for Anopheles pseudopunctipennis suppression in natural habitats in Oaxaca State, Mexico. Pan Am J Pub Health 5:23–28
Service MW (1976) Mosquito ecology—field sampling methods. Elsevier Applied Science Publishers, London
Theroux FR, Eldrige EF, Mallmann WL (1943) Laboratory manual for chemical and bacterial analysis of water and sewage. McGraw-Hill Book Company, London
Westerdahl BB, Washino RK, Platzer EG (1982) Successful establishment and subsequent recycling of Romanomermis culicivorax (Mermithidae: Nematoda) in a California rice field following post-parasite application. J Med Entomol 19:34–41
Acknowledgments
The authors are grateful to the staff of VCRC Field Station, Kottayam, Kerala, India for their help in the field work. They are also thankful to Mr. A. Ramamoorthy and Mr. S. Rajkumar, VCRC, Pondicherry, India for their assistance in the mass production and evaluation of the nematode.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paily, K.P., Chandhiran, K., Vanamail, P. et al. Efficacy of a mermithid nematode Romanomermis iyengari (Welch) (Nematoda: Mermithidae) in controlling tree hole-breeding mosquito Aedes albopictus (Skuse) (Diptera: Culicidae) in a rubber plantation area of Kerala, India. Parasitol Res 112, 1299–1304 (2013). https://doi.org/10.1007/s00436-012-3265-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-012-3265-3