Abstract
Ascaris nematodes, which cause ascariasis in humans and pigs, are among the most important nematodes from both health and economic perspectives. microRNA (miRNA) is now recognized as key regulator of gene expression at posttranscription level. The public availability of the genome and transcripts of Ascaris suum provides powerful resources for the research of miRNA profiles of the parasite. Therefore, we investigated and compared the miRNA profiles of male and female adult A. suum using Solexa deep sequencing combined with bioinformatic analysis and stem-loop reverse transcription polymerase chain reaction. Deep sequencing of small RNAs yielded 11.71 and 11.72 million raw reads from male and female adults of A. suum, respectively. Analysis showed that the noncoding RNA of the two genders, including tRNA, rRNA, snRNA, and snoRNA, were similar. By mapping to the A. suum genome, we obtained 494 and 505 miRNA candidates from the female and male parasite, respectively, and 87 and 82 of miRNA candidates were consistent with A. suum miRNAs deposited in the miRBase database. Among the miRNA candidates, 154 were shared by the two genders, and 340 and 351 were female and male specific with their target numbers ranged from one to thousands, respectively. Functional prediction revealed a set of elongation factors, heat shock proteins, and growth factors from the targets of gender-specific miRNAs, which were essential for the development of the parasite. Moreover, major sperm protein and nematode sperm cell motility protein were found in targets of the male-specific miRNAs. Ovarian message protein was found in targets of the female-specific miRNAs. Enrichment analysis revealed significant differences among Gene Ontology terms of miRNA targets of the two genders, such as electron carrier and biological adhesion process. The regulating functions of gender-specific miRNAs was therefore not only related to the fundamental functions of cells but also were essential to the germ development of the parasite. The present study provides a framework for further research of Ascaris miRNAs, and consequently leads to the development of potential nucleotide vaccines against Ascaris of human and animal health significance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ascaris spp. nematodes, which cause ascariasis in humans and pigs, are among the most important nematodes from both health and economic perspectives, with an estimated 807 million people infected and 4.2 billion at risk globally (de Silva et al. 2003). Ascaris suum can cause chronic nutritional impairment or acute morbidity including intestinal obstruction of pigs (Crompton 2001; Dold and Holland 2011). Larval migratory of ascariasis has a significant negative impact upon host growth, and billions of dollars are spent annually on the treatment and control of gastrointestinal parasitic nematodes (Lewis et al. 2009). There is a high prevalence of infection of A. suum all over the world, such as 39 % in China (de Silva et al. 2003), 25–35 % in Germany (Joachim et al. 2001), and 10.4 % in Turkey (Uysal et al. 2009).
MicroRNAs (miRNAs) are noncoding small RNAs of 18–24 nucleotides with key regulating functions in gene expression, cell generation, cell differentiation and apoptosis in viruses, animals, and plants (Bartel 2004). Due to the complex life cycle of parasites and the associated regulatory processes, miRNAs are essential for the rapid responses to environmental changes and the regulation of development involved in parasitism. Recently, small RNA classes in gametogenesis and embryo development in A. suum were investigated (Wang et al. 2011), and gender-enriched transcripts of A. suum were reported by Cantacessi et al. (2009), which identified 52 and 157 represented EST being enriched in female and male parasites. The draft genome of A. suum has currently been publically available (Jex et al. 2011). These studies provided powerful tools for comprehensive understanding of the parasite. However, although the body of knowledge regarding miRNA biology in general is rapidly expanding, there is little known about these molecules and their involvement in the biology of Ascaris. In order to investigate the miRNA profile characteristics and differences between genders of A. suum, the miRNA profiles of male and female adult A. suum were characterized and compared using Solexa deep sequencing combined with bioinformatic analysis and stem-loop reverse transcription polymerase chain reaction (RT-PCR).
Materials and methods
Parasites
Male and female adult A. suum were obtained from the small intestines of pigs in a slaughter house in Shenzhen, Guangdong Province, China. The worms were immediately transferred to sterile physiological saline (37 °C) in a sterile beaker for 3 h, followed by washing three times with saline to remove contamination with material derived from the hosts. The worms were then weighted and stored at −70 °C.
RNA preparation and high-throughput sequencing
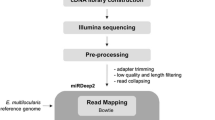
The entire body of one male or female adult A. suum was grounded into fine powder under liquid nitrogen, with 100 mg of which prepared for total RNA preparation with Trizol reagent (Invitrogen). Small RNA isolation was performed as described previously (Xu et al. 2010). Ten micrograms total RNA were used for the isolation of RNA fragments of 18–30 nucleotides by fractionation using a Novex 15 % TBE-Urea gel (Invitrogen). The purified fragments were ligated to 5′ and 3′ adaptors (Illumina) and reversely transcribed using an RT-PCR kit (Invitrogen), before use in high-throughput sequencing employing a Solexa sequencer at Huada Genomics Institute Co. Ltd, China.
Computational analysis of conserved and novel miRNAs
Low quality reads, those representing adaptors, adaptor–adaptors dimers, and reads smaller than 18 nt were all removed from the initial sequence set to obtain a subset of clean reads. These clean reads were searched against GenBank and Rfam databases (version 9.0) (http://www.sanger.ac.uk/software/Rfam) to remove noncoding RNAs, such as rRNA, tRNA, snRNA, and snoRNA with BLAST software (Altschul et al. 1990). To identify the conserved miRNA candidates, the remaining reads were firstly mapped onto the A. suum genome (Jex et al. 2011) by using the Short Oligo nucleotide Analysis Package (SOAP) (Li et al. 2009) with the sequences of pre-miRNA meeting the two criteria: (1) mature miRNAs were present in one arm instead of the loop of hairpin precursors and (2) the free energy hybridization was lower than −18 kcal/mol. And then, the miRNA candidates were searched against the conserved miRNAs deposited in the Sanger miRBase. Unmatched miRNA candidates were regarded as novel miRNAs. The precursors (hairpin) of miRNAs were then inspected manually, and a precursor was deemed “high probability” if there were mature miRNAs on the both arms. Targets of miRNA candidates were predicated with RNAhybrid software (Kruger and Rehmsmeier 2006). To reduce false-positive result, two extra parameters were performed to the analyzed result: 1) the ΔΔG was set as lower than −25 kcal/mol and (2) p value was set as ≤0.01. The Gene Ontology database (GO; http://www.geneontology.org/) was used for functional analysis of the predicted targets.
Transcriptional level analysis of novel miRNAs
Real-time quantitative PCR was performed as described previously (Chen et al. 2005; Ai et al. 2011). An ABI PRISM® 7300 sequence detection system and SYBR Green PCR Master Mix (TOYOBO) were used. The β-actin gene of A. suum (GenBank accession No. BI594141) was used as the endogenous control with primers as follows: forward primer (5′-CTCGAAACAAGAATACGATG-3′) and reverse primer (5′-ACATGTGCCGTTGTATGATG-3′) (Huang et al. 2008). Specific stem-loop primers (5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCCTGAGT-3′) and (5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTGCACAGT-3′) were designed for the reverser transcription of the two selected representative miRNAs named as A. suum miRNAs (Asu-miR)-share-391 and Asu-miR-share-404, respectively. All primers were synthesized by Shenggong Co, Ltd., China. The cycle conditions were as follows: 95 °C for 5 min, followed by 40 cycles of 95 °C for 15 s, 65 °C for 30 s, and 72 °C for 32 s. The relative transcriptional levels of each of the miRNAs investigated was calculated using the equation: \( N={2^{{-\varDelta \mathrm{Ct}}}},\;\varDelta \mathrm{Ct}=\mathrm{C}{{\mathrm{t}}_{\mathrm{miRNA}}}-\mathrm{C}{{\mathrm{t}}_{\mathrm{acin}}} \) (Livak and Schmittgen 2001). All reactions were performed in triplicate.
Results
Discovery of short RNAs in each gender of A. suum
Deep sequencing of total RNAs yielded 11.71 and 11.72 million raw reads from male and female adults of A. suum, respectively. After removal of low quality reads and adaptors, there were 9.81 and 9.76 million clean reads remaining for male and female, which contained 1.53 and 1.21 million unique reads. Length distribution analysis showed the sRNAs profiles were both predominantly focused on reads of 22 and 23 nt length: 19.19 % were 22 nt and 23.62 % were 23 nt in female-derived sRNAs and 16.01 % were 22 nt and 19.25 % were 23 nt in male-derived sRNAs. Of the 19.57 million total reads, 15.24 (77.86 %) million reads were shared by the two genders, and 13.94 (male) and 8.20 % (female) were gender specific.
Totally, 6.86 and 7.98 % of the total reads were found to be non-coding RNA (tRNA, rRNA, snRNA, snoRNA, repeat, etc.) of female- and male-derived sRNAs, respectively. The total percentages of ncRNA were therefore similar between the two genders and the percentages of each type of RNA were also similar: rRNA and tRNA were 5.19 and 1.54 % in female and 5.77 and 1.80 % in male.
Differences in miRNA profiles of the two genders
By mapping with the A. suum genome, we obtained 494 and 505 miRNA candidates respectively from female and male of the parasite, with precursors of these miRNAs meeting the criteria listed above and having standard stem-loop structures.
By matching the miRNA candidates with known Asu-miR deposited in the miRBase database (Wang et al. 2011), 87 and 82 of miRNA candidates were found as conserved ones for female and male, respectively. Among them, 70 miRNAs were shared by different genders, and 17 and 12 were female and male specific (Additional file 1). The annotated conserved gender-specific miRNAs were shown in Additional file 2. Those unmatched miRNA candidates were marked as novel ones, including 407 of female and 423 of male. Among them, 84 novel ones were shared by the two genders and 323 and 339 novel miRNAs were female and male specific. Totally, among the miRNA candidates, 154 were shared by the two genders and 340 and 351 were female and male specific (Table 1).
For conserved miRNA, 68.97 % (60/87) miRNAs of female had mature miRNAs found on both of the arms of the precursors (miR-5p and miR-3p), and for male adult, the percentage was similar as 68.29 % (56 of 82). For novel miRNAs, 21.62 % (88 of 407) of miRNAs of female had mature miRNAs found on both arms of the precursors, and for male gender, it was 20.01 % (85 of 423).
Target prediction and functional prediction
All of the 57,359 mRNA and EST items of A. suum deposited in the NCBI websites were downloaded and used for target prediction of the miRNA candidates. Besides, the gender-enriched EST of A. suum (Cantacessi et al. 2009) was also specifically used for target prediction. Under the stringent matching criteria, it was found that the target numbers of male and female were ranged from one to thousands. For female-specific miRNAs, the target numbers were ranged from 1 (female-miR-330-5p, female-miR-432-5p, female-miR-081-3p, etc.) to 3,343 targets (female-miR-039-3p) with an average of 176 targets, and for male-specific miRNAs, the target numbers were ranged from 1 (male-miR-051-5p, male-miR-006-3p, male-miR-311-3p, etc.) to 4,725 target (male-miR-301-5p) with 166 targets in average. The same phenomenon was also found in miRNA shared, which ranged from 1 (Asu-miR-1-3p and miR-share-411-5p) to 2,927 (miR-share-320-5p) with average as 149 targets.
Functional prediction with Blast2GO software revealed a set of elongation factors, heat shock proteins, and growth factors from the targets of gender-specific miRNAs, which were essential for the development of the parasite. The targets of male-specific miRNAs included larva development regulatory growth factor daf-7 (gi|324503058), three elongation factors (gi|324532688, gi|324517122, and gi|324508579), eukaryotic translation initiation factor 5 (gi|324516788), heat shock protein 90 (gi|324527949), heat shock protein 70 (gi|298104210), etc. (Additional file 3). Besides, one major sperm protein (MSP) (gi|256665205), three nematode sperm cell motility protein mfp2 (gi|27589088, gi|256665089, and gi|256665108) were also found. While for the targets of female-specific miRNAs, two elongation factors (gi|27926898 and gi|171287708), one growth factor receptor-bound protein (gi|171285982), one eukaryotic initiation factor (gi|256664999), and one heat shock protein 70 were found (Additional file 4). Besides, one ovarian message protein of A. suum (gi|256664985) was also identified. Enrichment analysis showed that most of the functions of targets of gender-specific miRNAs were similar (Fig. 1). The antioxidant function was only found in target of female-specific miRNA, while the electron carrier function was only found in target of male-specific miRNA.
miRNAs quantification
Two representative novel miRNAs named Asu-miR-share-391 and Asu-miR-share-404 that were shared by both genders were verified respectively using qRT-PCR, with the house-keeping gene β-actin of A. suum as inner reference gene (Huang et al. 2008). The two miRNAs had more variants than others and had both mature miRNAs on the both arms of their precursors. The standard stem-loop structure of Asu-miR-share-391 precursors was shown in Fig. 2.
It was found that both of the two miRNAs could be successfully amplified. The relative level of Asu-miR-share-391 (1.46 ± 0.16) was 12.17-fold higher in male than in female (0.12 ± 0.20), while the level of Asu-miR-share-404 was similar in both genders with the relative transcriptional level of 1.25 ± 0.03 in male and 1.31 ± 0.01 in female.
Discussion
It was found that the small RNA yields from each gender were very similar (11.71 and 11.72 million), and the length distributions of sRNA from the two genders were similar to each other, too. Among these sRNAs, 77.86 % of the total sRNAs were shared by the two genders, with only 8.43 % unique reads shared. The noncoding RNAs were also at very similar levels between the two genders. The total number of miRNA candidates were similar to each other of the two genders (494 and 505 for female and male), too. In the present study, we used the entire body of male or female adult for total RNA isolation and miRNA analysis, which was more helpful for obtaining and understanding of global miRNA differences of A. suum than only using reproductive organs, such as testis or ovary of the parasite. This might be the reason why the miRNA profiles of the two genders were so similar. The data indicated very similar metabolism patterns of the two genders.
The targets of the miRNAs distributed in a large range of functions. miRNAs are posttranscriptional regulators and are usually adjoined to the 3′ UTR of mRNA in animals or to the coding regions of mRNA in plants resulting in mRNA degradation or translation inhibition (Uysal et al. 2009; Du and Zamore 2007). More than 1,000 miRNAs have been found in humans, targeting about 60 % of mammalian genes. Therefore, miRNAs show key regulatory functions to gene expression (Chiba and Hijikata 2010; Thorsen et al. 2012). In the present study, we identified a set of elongation factors, heat shock proteins, and growth factors from the targets of gender-specific miRNAs, which indicated that the miRNAs play essential regulating function in the parasite.
Although most terms were similar, enrichment analysis showed significant differences among GO terms of miRNA targets of the two genders, such as male-specific “extracellular region” in “cellular component” analysis, “electron carrier” in “molecular function” analysis, a specific “biological adhesion process” in “biological adhesion process” analysis, and female-specific “antioxidant” as well (Fig. 1). Therefore, although the metabolism pattern of male and female were similar, some cellular components, molecular functions, and biological processes were different and were even gender specific, which might be contributed to the gender differences of the parasite. For example, the daf-7 was found as male specific. The members of DAF family, such as daf-7, daf-9, daf-12, and daf-36, contribute to the gonadal longevity pathway. During larval development, daf-12 is served as a stage selector, which mediated the choice between reproductive developments or at the dauer formation (Antebi 2012), and the same as daf-7. The daf-7 is expressed in the two head sensory neurons named ASIs, and it is required during larval development to inhibit dauer formation (Myers 2012). Under environmental cues, such as starvation and high temperature, the expression of daf-7 is downregulated, mediating the dauer diapauses for a long-lived alternate third larval stage specialized for survival (Antebi 2012). However, the mechanism of the signaling pathways and its sensitivity to environmental signals are still not well understood (Myers 2012). In the present study, we provided a possible approach for the understanding of regulation of daf-7 expression. It was found that the gene of daf-7 were under the regulation of miRNA (miR-413-5p), which is sensitive to the change of environmental changes (Xu et al. 2010). The new miRNA named as miR-413 was found as the male-specific miRNA, which might indicate the difference of metabolism character of the parasite and that the male adult might be more sensitive to the environment change.
We also identified targets as MSP and nematode sperm cell motility protein in male A. suum and ovarian message protein in female A. suum, which were in accordance with the findings of previous analyses of A. suum (Cantacessi et al. 2009) and other parasitic nematodes, such as Brugia malayi (Li et al. 2005) and Trichostrongylus vitrinus (Nisbet and Gasser 2004). Nematode sperm utilize a cytoskeleton composed of MSP, which is considered to form a simpler, yet similar, crawling motility apparatus, and offered a unique perspective for investigating amoeboid cell motility. MSP-filament network generates the forces for movement in the protrusion and retraction of Ascaris sperm (Roberts and Stewart 2012), and its expression content accompany the development of spermatozoa (Laabs et al. 2012). Besides, MSP represents a protein family occurring in nematodes only (Höglund et al. 2008; Strube et al. 2009), which was identified as a diagnostic antigen for onchocerciasis previously (Park et al. 2008), and an evaluated lungworm ELISA detecting method was currently available via recombinant MSP (Schunn et al. 2012; Goździk et al. 2012). The MSP was the target of Asu-miR-46-5p (Additional file 2), indicating that the regulating function of gender-specific miRNAs was not only related to the fundamental functions of cells but also were essential to the germ cell development of the parasite. Some targets obtained via bioinformatics tools and parasite genome as well, will help generate new knowledge on parasite biology and antigens to be used in the disease diagnosis (Fonseca et al. 2012).
In conclusion, the present study described, for the first time, the global profiling of miRNAs in male and female A. suum and identified a number of gender-specific miRNAs for each gender. A recent review has proposed that A. suum and Ascaris lumbricoides (the human ascarid) represent the same single species (Leles et al. 2012), indicating that the findings of present study should provide a framework for further research of Ascaris gender-specific miRNAs and may lead to the development of potential nucleotide vaccines against Ascaris of human and pig health significance.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Antebi A (2012) Regulation of longevity by the reproductive system. Exp Gerontol. doi:10.1016/j.exger.2012.09.009
Ai L, Xu MJ, Chen MX, Zhang YN, Chen SH, Guo J, Cai YC, Zhou XN, Zhu XQ, Chen JX (2011) Characterization of microRNAs in Taenia saginata of zoonotic significance by solexa deep sequencing and bioinformatics analysis. Parasitol Res 110:2373–2378
Bartel DP (2004) microRNAs genomics, biogenesis, mechanism, and function. Cell 116:281–297
Cantacessi C, Zou FC, Hall RS, Zhong W, Jex AR, Campbell BE, Ranganathan S, Sternberg PW, Zhu XQ, Gasser RB (2009) Bioinformatic analysis of abundant, gender-enriched transcripts of adult Ascaris suum (Nematoda) using a semi-automated workflow platform. Mol Cell Probes 23:205–217
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, Lao KQ, Livak KJ, Guegler KJ (2005) Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res 33:e179
Chiba Y, Hijikata T (2010) MicroRNAs and their therapeutic potential for human diseases: preface. J Pharmacol Sci 114:262–263
Crompton DW (2001) Ascaris and ascariasis. Adv Parasitol 48:285–375
de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L (2003) Soil-transmitted helminth infections: updating the global picture. Trends Parasitol 19:547–551
Dold C, Holland CV (2011) Ascaris and ascariasis. Microbes Infect 13:632–637
Goździk K, Engström A, Höglund J (2012) Optimization of in-house ELISA based on recombinant major sperm protein (rMSP) of Dictyocaulus viviparus for the detection of lungworm infection in cattle. Res Vet Sci 93:813–818
Du T, Zamore PD (2007) Beginning to understand microRNA function. Cell Res 17:661–663
Fonseca CT, Braz Figueiredo Carvalho G, Carvalho Alves C, de Melo TT (2012) Schistosoma tegument proteins in vaccine and diagnosis development: an update. J Parasitol Res 2012:541268
Huang CQ, Gasser RB, Cantacessi C, Nisbet AJ, Zhong W, Sternberg PW, Loukas A, Mulvenna J, Lin RQ, Chen N, Zhu XQ (2008) Genomic-bioinformatic analysis of transcripts enriched in the third-stage larva of the parasitic nematode Ascaris suum. PLoS Negl Trop Dis 2:e246
Höglund J, Engström A, Morrison DA, Mineur A, Mattsson JG (2008) Limited sequence variation in the major sperm protein 1 (MSP) gene within populations and species of the genus Dictyocaulus (Nematoda). Parasitol Res 103:11–20
Jex AR, Liu S, Li B, Young ND, Hall RS, Li Y, Yang L, Zeng N, Xu X, Xiong Z, Chen F, Wu X, Zhang G, Fang X, Kang Y, Anderson GA, Harris TW, Campbell BE, Vlaminck J, Wang T, Cantacessi C, Schwarz EM, Ranganathan S, Geldhof P, Nejsum P, Sternberg PW, Yang H, Wang J, Wang J, Gasser RB (2011) Ascaris suum draft genome. Nature 479:529–533
Joachim A, Dulmer N, Daugschies A, Roepstorff A (2001) Occurrence of helminths in pig fattening units with different management systems in Northern Germany. Vet Parasitol 96:135–146
Kruger J, Rehmsmeier M (2006) RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res 34:W451–W454
Laabs EM, Schnieder T, Strube C (2012) In vitro studies on the sexual maturation of the bovine lungworm Dictyocaulus viviparus during the development of preadult larvae to adult worms. Parasitol Res 110:1249–1259
Leles D, Gardner SL, Reinhard K, Iniguez A, Araujo A (2012) Are Ascaris lumbricoides and Ascaris suum a single species? Parasit Vectors 5:42
Lewis R, Behnke JM, Stafford P, Holland CV (2009) Dose-dependent impact of larval Ascaris suum on host body weight in the mouse model. J Helminthol 83:1–5
Li BW, Rush AC, Crosby SD, Warren WC, Williams SA, Mitreva M, Weil GJ (2005) Profiling of gender-regulated gene transcripts in the filarial nematode Brugia malayi by cDNA oligonucleotide array analysis. Mol Biochem Parasitol 143:49–57
Li R, Yu C, Li Y, Lam TW, Yiu SM, Kristiansen K, Wang J (2009) SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics 25:1966–1967
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25:402–408
Myers EM (2012) Gαo and Gαq regulate the expression of daf-7, a TGFβ-like gene, in Caenorhabditis elegans. PLoS One 7:e40368
Nisbet AJ, Gasser RB (2004) Profiling of gender-specific gene expression for Trichostrongylus vitrinus (Nematoda: Strongylida) by microarray analysis of expressed sequence tag libraries constructed by suppressive–subtractive hybridisation. Int J Parasitol 34:633–643
Park J, Dickerson TJ, Janda KD (2008) Major sperm protein as a diagnostic antigen for onchocerciasis. Bioorg Med Chem 16:7206–7209
Roberts TM, Stewart M (2012) Role of major sperm protein (MSP) in the protrusion and retraction of Ascaris sperm. Int Rev Cell Mol Biol 297:265–293
Schunn AM, Forbes A, Schnieder T, Strube C (2012) Validation of a Dictyocaulus viviparus MSP-ELISA and cut-off adjustment in a one-year longitudinal field study in dairy cattle herds. Vet Parasitol 189:291–298
Strube C, Buschbaum S, Schnieder T (2009) Molecular characterization and real-time PCR transcriptional analysis of Dictyocaulus viviparus major sperm proteins. Parasitol Res 104:543–551
Thorsen SB, Obad S, Jensen NF, Stenvang J, Kauppinen S (2012) The therapeutic potential of microRNAs in cancer. Cancer J 18:275–284
Uysal HK, Boral O, Metiner K, Ilgaz A (2009) Investigation of intestinal parasites in pig feces that are also human pathogens. Turkiye Parazitol Derg 33:218–221
Wang J, Czech B, Crunk A, Wallace A, Mitreva M, Hannon GJ, Davis RE (2011) Deep small RNA sequencing from the nematode Ascaris reveals conservation, functional diversification, and novel developmental profiles. Genome Res 21:1462–1477
Xu MJ, Liu Q, Nisbet AJ, Cai XQ, Yan C, Lin RQ, Yuan ZG, Song HQ, He XH, Zhu XQ (2010) Identification and characterization of microRNAs in Clonorchis sinensis of human health significance. BMC Genomics 11:521
Acknowledgments
This work was supported by The Science Fund for Creative Research Groups of Gansu Province (grant No. 1210RJIA006), the National S & T Major Program (grant No. 2012ZX10004220), the Open Funds of the State Key Laboratory of Veterinary Etiological Biology, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences (grant No. SKLVEB2011KFKT004 and SKLVEB2011KFKT010), the China Postdoctoral Science Foundation (grant No. 201104363 and 20090460064), and the Guangdong Provincial Program for Excellent Ph.D. theses (grant No. sybzzxm201037). The experiments comply with the current laws of the country in which the experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional file 1
Conserved gender-specific miRNAs of A. suum. (DOCX 18 kb)
Additional file 2
Annotated functions of conserved gender-specific miRNAs of A. suum. (DOCX 17 kb)
Additional file 3
Predicated female targets of female-specific miRNAs by using mRNA and EST deposited in the GenBank. (DOCX 52 kb)
Additional file 4
Predicated male targets of male-specific miRNAs by using mRNA and EST deposited in the GenBank. (DOCX 50 kb)
Rights and permissions
About this article
Cite this article
Xu, MJ., Fu, JH., Nisbet, A.J. et al. Comparative profiling of microRNAs in male and female adults of Ascaris suum . Parasitol Res 112, 1189–1195 (2013). https://doi.org/10.1007/s00436-012-3250-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-012-3250-x