Abstract
The neuromuscular system (NMS) in cercariae of Neoastiotrema trituri, Plagiorchis elegans, Omphalometra flexuosa, Skrjabinoeces similis and Prosthogonimus ovatus was studied with immunocytochemical methods and confocal scanning laser microscopy. The patterns of F-actin in the musculature, 5-HT immunoreactive (IR), FMRFamide-IR neuronal elements and α-tubulin-IR sensory receptors were investigated, and they were found to be rather similar in all the cercariae studied. Four species have seven paired 5-HT-IR neurons in the body, and P. elegans has eight. N. trituri has three 5-HT-IR neurons in each brain ganglion, while the other species have four. A high degree of conformity in the structure of the NMS was observed, probably reflecting the close phylogenetic relationship and the similar strategy of host finding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The organization of the neuromuscular system (NMS) in cercariae reflects the larval adaptations to infect their next host and shows comparatively high variability between species with different behavioural patterns (Tolstenkov et al. 2011). According to their behavioural patterns, the swimming cercariae can be divided into two groups: (a) continuously swimming cercariae and (b) cercariae with an intermittent type of swimming pattern (see Haas 1994). Our recent investigations of the NMS within groups of intermittently swimming furcocercariae revealed features that correlated with the strategies to infect their next host and a trend in the differentiation of the NMS from primitive to advanced forms (Tolstenkov et al. 2012a, b). As far as we know, the NSM of cercariae with a continuous swimming pattern has never before been the subject of a comparative study.
In this study, we focus on Xiphidiocercariae—stylet cercariae—that represent one of the major morphological groups of cercariae that swim continuously (Galaktionov and Dobrovolskij 2003). The structure and the composition of the NMS in five species of Xiphidiocercariae from four different families all belonging to the suborder Plagiorchiata (Tkach et al. 2000) will be compared. We describe the NMS in four species of Plagiorchioidea Lühe, 1901, that represent advanced trematodes of the Plagiorchida branch (Galaktionov and Dobrovolskij 2003)—Neoastiotrema trituri Grabda, 1959, Plagiorchis elegans Rudolphi, 1802, (Plagiorchiidae Lühe, 1901), Omphalometra flexuosa Rudolphi, 1809 (Omphalometridae Looss, 1899), Skrjabinoeces similis Looss, 1899 (Haematoloehidae) and one species of Microphalloidea Ward, 1901—Prosthogonimus ovatus Rudolphi, 1803 (Prosthogonimidae Lühe, 1909). All species studied have similar morphological structures—a stylet in the transformed oral sucker, a simple tail and a well-developed V-shaped excretory bladder. The cercariae use arthropods (insect larvae or crustaceans) as their second intermediate host and terrestrial vertebrates as their definitive host (Grabda 1960; Sudarikov et al. 2002).
The cercariae of N. trituri, P. elegans and O. flexuosa are middle sized. S. similis and P. ovatus are among the smallest freshwater cercaria known so far. In a preliminary study of Plagiorchis sp., no details of the NMS structure of the cercaria were revealed (Tolstenkov et al. 2008). A few studies of the chaetotaxy in Plagiorchiidae have been performed (see Dimitrov et al. 1989) As far as we know, the NMS in cercariae from the families Haematoloehidae, Omphalometridae and Prosthogonimidae has never been described before.
Materials and methods
Samples of snails Planorbis planorbis Linnaeus, 1758, Bithynia tentaculata Linnaeus, 1758 and Lymnaea stagnalis Linnaeus, 1758 were collected in the littoral zone of Lake Naroch and identified at the Scientific and Research Centre “Naroch biological station named after G.G. Vinberg” of the Belarusian State University, Republic of Belarus, in July 2010–2011.
All snails were brought to the laboratory and checked for the release of cercariae by placing them individually in a small amount of filtered water and exposing them to sunlight for a few hours. The morphology of cercariae was investigated on temporary preparations of living individuals. Measurements were taken from cercariae fixed in 70 % ethanol. The larval stages were identified according to Grabda (1960), Faltýnková et al. (2007, 2008) and Filimonova and Chaliapina (1980). Specimens of cercariae of N. trituri, O. flexuosa and S. similis were recovered from P. planorbis, cercariae of P. elegans from L. stagnalis and cercariae of P. ovatus from B. tentaculata. The mollusc nomenclature was applied after Glöer (2002).
When describing the NSM, we follow the terminology introduced by Reuter and Gustafsson (1995) and Reuter et al. (1998). For methods of staining, see Tolstenkov et al. (2011).
Results
The patterns of staining with TRITC-labelled phalloidin
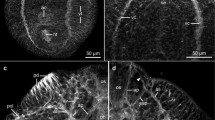
All the cercariae are small (Table 1). In N. trituri cercariae, staining with TRITC-conjugated phalloidin revealed a body wall consisting of a well-organised outer layer of regularly distributed circular muscle fibres and an intermediate layer of longitudinal muscle fibres (Fig. 1a, Table 1). The longitudinal muscle fibres form pairs and run straight. An inner layer of regularly distributed single diagonal muscle fibres also occurs, covering the surface of the body except at the oral sucker. Dorsoventral muscle fibres were also observed. The oral sucker includes outer and inner circular and longitudinal muscle fibres and a well-developed layer of radial muscle fibres (Fig. 1a, b). The stylet pocket is formed by longitudinal muscle fibres similar to those in the body wall and occupies less than a half of the oral sucker (Fig. 1b). Longitudinal and radial fibres form the musculature of the pharynx. In the walls of the oesophagus and intestine, many longitudinal and single circular fibres occur (Fig. 1b). The ventral sucker is formed by radial, longitudinal and equatorial muscle fibres and is attached to the body wall by single longitudinal muscle fibres (Fig. 1a, b). The excretory ducts are lined by a few longitudinal and circular fibres, which become denser in the terminal part. The terminal part of bladder has a characteristic calabash-like form (Fig. 1j). Its wall is formed by circular and longitudinal muscle fibres. A series of three sphincters occurs. The terminal parts of the two excretory ducts open into the first sphincter and it leads to the first, narrow part of the bladder. The second sphincter occurs in between the narrow and the wide part, and the third sphincter lies in the bottom of the wide part of the bladder, leading out (Fig. 1b, j). Many muscle fibres attach to this last sphincter and extend to the surface of the cercariae. The tail contains an outer layer of circular muscle fibres, four narrow bands of smooth muscle fibres and striated muscle fibres organised into the four diffuse bundles (Fig. 1c).
a–l Confocal scanning laser micrographs of the pattern of TRITC-phalloidin-conjugated F-actin in four cercariae. Bar = 20 μm. a Max projection of N. trituri showing the musculature of the body wall, the oral and ventral sucker. b Optical section of N. trituri showing the radial muscle fibres in the oral sucker, the musculature of the stylet pocket (large arrow), pharynx, oesophagus, intestine and ventral sucker. c Max projection of N. trituri, showing the musculature of the tail. d Max projection of P. elegans showing the musculature of the body wall, oral sucker, stylet pocket and ventral sucker. e Max projection of P. elegans showing the musculature of the tail. f Max projection of O. flexuosa showing the musculature of the body wall, oral sucker, stylet pocket and ventral sucker. g Max projection of O. flexuosa showing the musculature of the tail. h Max projection of S. similis showing the musculature of the body wall, oral sucker and stylet pocket. i Max projection of S. similis showing the musculature of the tail. j Max projection of N. trituri showing the musculature of the bladder. The three sphincters are marked with arrows. k Max projection of P. elegans showing the musculature of the bladder. The sphincter is marked with an arrow. l Max projection of O. flexuosa showing the musculature of the bladder. The two sphincters are marked with arrows. b bladder, c circular muscle fibres, d diagonal muscle fibres, dv dorsoventral muscle fibres, es esophagus, ex excretory ducts, in intestine, l longitudinal muscle fibres, os oral sucker, ph pharynx, r radial muscle fibres, s smooth muscle fibres, st striated muscle fibres, vs ventral sucker
The general pattern of the phalloidin-stained F-actin filaments in the musculature of P. elegans is similar to that in N. trituri (Fig. 1d). However, the oral sucker lacks a well-developed layer of radial fibres. The stylet pocket is composed of densely packed longitudinal muscle fibres (Fig. 1d). The terminal part of the bladder is cylindrical and has two sphincters (Fig. 1k). The bundles of striated fibres in the musculature of the tail are more distinct (Fig. 1e). The pattern of the phalloidin-stained F-actin filaments in the musculature of O. flexuosa is similar to that in N. trituri and P. elegans (Fig. 1f). However, the muscle fibres are sparsely distributed, and the sizes of single muscle fibres are smaller, all less than 0.5 μm in diameter (Table 1). The stylet pocket occupies the most part of the oral sucker. It is formed by thick longitudinal fibres (0.8 μm in diameter) that join in the distal part. The distal part of the bladder is spherical (Fig. 1l). In the tail, the striated muscle fibres do not form bundles but are diffusely distributed (Fig. 1g). The pattern and size of the muscle fibres in S. similis are similar to those in O. flexuosa (Fig. 1h, i). The spherical bladder is formed by circular and longitudinal muscle fibres and has two sphincters. Unfortunately, the phalloidin staining in P. ovatus cercariae was unsuccessful.
The patterns of 5-HT immunoreactivity
In N. trituri cercariae, the 5-HT immunoreactive (5-HT-IR) nerve fibres form a symmetrical pattern and occur in the bilobed brain, the pair of main nerve cords (MCs) along the ventral surface, in the pairs of lateral and dorsal minor cords and in the tail (Fig. 2a). The total number of neurons in the body is 14. The neurons occur in pairs, and they are numbered in the pictures. Three 5-HT-IR neurons (number 1–3) occur in each brain ganglion and four (number 4–7) along the MCs. Neurons number 1 are situated in the anterior lateral corner of each brain ganglion, apart from the other neurons, and send processes towards the oral sucker. Neurons number 3 are located on both sides of the pharynx (Fig. 2a). Neurons number 4 lie close to the base of the brain ganglia, in the beginning of the MCs. Neurons number 6 and 7 occur in the vicinity of the ventral sucker and send nerves toward it. The longitudinal cords are connected by large brain and caudal commissures and six thin transverse commissures. From the ventral side of the brain ganglia, two 5-HT-IR nerve fibres extend towards the oral sucker (Fig. 2a). Lateral anterior cords also occur. A rich plexus exists in the oral sucker forming two nerve rings. Thin 5-HT-IR nerves innervate the ventral sucker. A rich nervous plexus occurs in the caudal end of the body, surrounding the excretory bladder. In the tail, two pairs of 5-HT-IR nerve cords run along the lateral sides. Two large multipolar 5-HT-IR neurons occur in the middle of the tail (Table 2). They are connected to both pairs of nerve cords. About ten commissures connect the nerve cords in the tail.
a–e Confocal scanning laser micrographs of four cercariae stained with anti-5-HT. Bar = 20 μm. a Max projection showing the 5-HT-IR nervous system in N. trituri. The paired 5-HT-IR neurons are numbered. b Max projection showing the 5-HT-IR nervous system in the body of P. elegans. c Max projection showing the 5-HT-IR nervous system in the body of O. flexuosa. d Max projection showing the 5-HT-IR nervous system in the body of P. ovatus. e Max projection of O. flexuosa tail showing two pairs of 5-HT-IR nerve cords along the lateral sides and two multipolar neurons. Note plexus of 5-HT-IR nerve fibres (arrow). bc brain commissure, dc dorsal nerve cord, g ganglion, lc lateral nerve cord, mc main nerve cord, mn multipolar neuron, os oral sucker, tn tail nerve, vs ventral sucker
In P. elegans cercariae, the general pattern of the 5-HT immunostaining (IS) is similar to that of N. trituri (Fig. 2b). However, the total number of neurons in the body is 16. This species has four neurons in each brain ganglion and two pairs of neurons in the beginning of the MCs (number 5 and 6) as in N. trituri. The neurons in the body are slightly larger than in N. trituri (Table 2). The longitudinal cords are connected by large brain and caudal commissures and six thin transverse commissures. In O. flexuosa cercariae, the general pattern of 5-HT IS is similar to that in P. elegans. The total number of the neurons in the body is 14 (Fig. 2c, Table 2). Four neurons occur in each brain ganglion. Three paired neurons are evenly distributed along the MCs in the middle part of the body; one of them (number 7) sends processes to the plexus in the ventral sucker. Two neurons occur in the tail (Fig. 2e). In P. ovatus cercariae, the general pattern of the 5-HT IS is similar to that of O. flexuosa. The total number of neurons is 14. Three neurons, linked with the MCs, lie in vicinity of the ventral sucker. The neurons are very small (Fig. 2d, Table 2). In the cercariae of S. similis, the staining with anti-5-HT was unsuccessful. The controls were negative.
The patterns of FMRFamide immunoreactivity
In N. trituri cercariae, the nervous system stains strongly with anti-FMRFamide and follows the same general pattern as the 5-HT IS (Fig. 3a). The longitudinal cords are connected by large brain and caudal commissures and six thin transverse commissures. In the oral sucker, three nerve rings occur. The ventral sucker is well innervated. On both sides of the excretory bladder, two FMRFamide-IR neurons (size, 5.3 × 3.5 μm) occur (Fig. 3a).
a–d Confocal scanning laser micrographs of four cercariae stained with anti-FMRFamide. Bar = 20 μm. a Max projection showing the FMRFamide-IR nervous system in the body of N. trituri. Note the FMRFamide-IR neurons (arrow) close to the bladder. b Max projection showing the FMRFamide-IR nervous system in the body of P. elegans. Note the FMRFamide-IR neurons (arrow) close to the bladder. c Max projection showing the FMRFamide-IR nervous system in the body of O. flexuosa. Note the FMRFamide-IR neurons (arrow) close to the bladder. d Max projection of the FMRFamide-IR nervous system in the body of P. ovatus. bc brain commissure, c commissures, cc caudal commissure, dc dorsal nerve cord, g ganglion, lc lateral nerve cord, mc main nerve cord, os oral sucker, vs ventral sucker
In P. elegans cercariae, the general patterns of FMRFamide-IR nerve fibres and neurons are similar to that in N. trituri. In addition to the brain and caudal commissures, five thin commissures are found in the body. On both sides of the excretory bladder, two FMRFamide-IR neurons (size, 4.4 × 2.4 μm) occur (Fig. 3b). In O. flexuosa cercariae, the FMRFamide IS follows the same pattern as the 5-HT IS. Four thin transverse commissures connecting the MCs are observed (Fig. 3c). The ventral sucker is well innervated by FMRFamide-IR fibres. Two large FMRFamide-IR neurons occur in the vicinity of the bladder is (size, 6.5 × 3.8 μm). In P. ovatus cercariae, the FMRFamide IS follows the pattern of the 5-HT IS. No FMRFamide-IR neurons were observed in the distal part of the body (Fig. 3d). In the cercariae of S. similis, the staining with anti-FMRFamide was unsuccessful. The controls were negative.
The patterns of α-tubulin immunoreactivity
The pattern of α-tubulin-IR in the body of N. trituri cercariae is shown in Fig. 4a. The relatively few α-tubulin-IR processes are distributed symmetrically (Table 3). They are concentrated around the oral sucker and are slighly less frequent around the ventral sucker. Processes were also observed along the lateral sides of the body. A pair of sensory receptors is situated in the middle of the tail (Fig. 4a). The distribution of the α-tubulin-IR processes in the body of P. elegans, O. flexuosa, S. similis and P. ovatus cercariae was similar to that in N. trituri (Fig. 4a–e; Table 3). At the base of the oral sucker of P. ovatus cercariae, a group of four to five rather long processes (5–5.5 μm) were observed (Fig. 4e). In the tail of P. elegans and S. similis cercariae, the paired processes are located in the middle of the tail (Fig. 4b, d; Table 3). In O. flexuosa cercariae, the paired processes are situated in the end of the first third of the tail (Fig. 4c; Table 3). In P. ovatus cercariae, the paired processes are located close to the tip of the tail.
a–e. Confocal scanning laser micrographs of five cercariae stained with anti-α-tubulin. Bar = 20 μm. a Max projection showing the α-tubulin IS in N. trituri. b Max projection showing the α-tubulin IS in P. elegans. c Max projection showing the α-tubulin IS in O. flexuosa. d Max projection showing the α-tubulin IS in S. similis. e Max projection showing the α-tubulin IS in P. ovatus. arrows α-tubulin-IR processes, lcp long ciliated processes, os oral sucker, vs ventral sucker, large arrows flame cells
The flame cells of the cercariae react strongly when stained with anti-α-tubulin (Fig. 4a–e). The controls were negative.
Discussion
The patterns of phalloidin-stained F-actin filaments in the muscle fibres of the cercariae studied here and the size of their muscle fibres are similar to those observed in other cercariae (see Halton and Maule 2004; Tolstenkov et al. 2012a, b). In N. trituri, the stylet pocket occupies less than a half of the volume of the oral sucker and is formed by muscle fibres of the same size as the fibres in the body wall. However, in all the other species studied here, the stylet pocket occupies the major part of the transformed sucker and is formed by muscle fibres that are wider than those of body wall. This indicates that a process of specialisation of the stylet musculature has taken place.
As expected, the 5-HT-IR fibres and neurons in all the investigated species form orthogonal patterns that are similar to those that have been reported from cercariae of other species (Tolstenkov et al. 2012a, b). Despite the twofold difference in size, the number of the 5-HT-IR neurons was the same in four of the five species studied. However, the size of the neuronal cell bodies varied, being very small in P. ovatus (Tables 1 and 2). Slight differences in the distribution of the 5-HT-IR neurons were observed. In P. elegans, O. flexuosa and P. ovatus, the number of paired neurons in the brain ganglia was four, compared to three in N. trituri. There is a discussion about the systematical position of N. trituri within Plagiorchiidae (Bray et al. 2008). The question about the conservatism of the distribution and number of 5-HT-IR neurons needs further research. No major structural differences in the 5-HT-IR nervous systems were observed between the cercaria studied here. The presence of a rich network of 5-HT-IR nerve fibres around the bladder and the exit sphincter of N. trituri and O. flexuosa indicates that 5-HT might have a role in the control of the bladder musculature.
In all the investigated species, the IS for FMRFamide was more intense than that for 5-HT and followed the same patterns. FMRFamide and 5-HT occupied separate sets of neurons and fibres, as is the case in most flatworms (see Halton and Gustafsson 1996). No major structural differences in the FMRFamide-IR nervous systems were noticed between the cercariae investigated here. The presence of paired FMRFamide-IR neurons in the vicinity of distal part of the well-developed excretory system of all species of Plagiorchiidae studied here has never been described before in other groups of cercariae (see Halton and Maule 2004; Tolstenkov et al. 2012a, b) and indicates a role for FMRFamide in the regulation of the excretory system or its musculature.
In our study, IS towards α-tubulin was observed in surface processes of different lengths of sensilla. In general, our results are in agreement with the previous studies (Dimitrov et al. 1989; Tolstenkov et al. 2012a, b). In the tails of all species studied here, one pair of short sensilla was found. Sensillas in the similar position have been described in cercarial tails of many species that have to catch a swiftly moving second intermediate host and may be considered as mechanoreceptors (see Tolstenkov et al. 2011, 2012a, b). However, the plagiorchioid cercariae do not respond to water turbulence (own observation). Further research is needed.
Because of their high content of tubulin, the flame cells of the cercariae react strongly when stained with anti-α-tubulin. Unfortunately, this has led to a few mistakes in our three latest articles (Tolstenkov et al. 2011, 2012a, b). In Tolstenkov et al. (2011), the yellow structures marked with arrowheads in Fig. 4f are not sensory receptors but flame cells. In Tolstenkov et al. (2012a), the arrow in Fig. 4b points at a flame cell, and the arrowhead in Fig. 4d also points at a flame cell. In Tolstenkov et al. (2012b), the notation for large processes in Fig. 4f, h points to flame cells. We apologize for these mistakes.
Thus, the structure of the NMS in cercaria of the studied Plagiorchioidea shows a high degree of conformity that reflects the close phylogenetic relations of the species studied and, regarding the musculature and sensillas, their strategic similarity of host finding.
References
Bray RA, Gibson DI, Jones A (2008) Keys to the Trematoda: volume 3. CAB Internat and Nat His Museum, London, p 824
Dimitrov V, Busta J, Kanev I (1989) Chaetotaxy of cercariae of Opisthioglyphe ranae (Frölich, 1791) (Trematoda: Plagiorchidae). Folia Parasitol (Praha) 36:265–274
Faltýnková A, Našincová V, Kablasková L (2007) Larval trematodes (Digenea) of the great pond snail, Lymnaea stagnalis (L.), (Gastropoda: Pulmonata) in Central Europe: a survey of species and key to their identification. Parasite 14:39–51
Faltýnková A, Našincová V, Kablásková L (2008) Larval trematodes (Digenea) of planorbid snails (Gastropoda: Pulmonata) in Central Europe: a survey of species and key to their identification. Syst Parasitol 69:155–178
Filimonova L, Chaliapina VI (1980) Cercariae of trematodes in prosobranchid molluscs Bithynia inflata from the lakes of the Northern Kulunda. In: Worms of aquatic and terrestrial biocoenosis: Nauka, Moscow, Proc Lab Helminthology 30:113–123
Galaktionov KV, Dobrovolskij AA (2003) Biology and evolution of trematodes. An essay on the biology, morphology, life cycles, transmission, and evolution of digenetic trematodes. Kluwer, Boston, p 620
Glöer P (2002) Die Süßwassergastropoden Nord- und Mitteleuropas. Bestimmungschlüssel, Lebesweise, Verbreitung. Die Tierwelt Deutschlands 73:1–237
Grabda B (1960) Life cycle of Haematoloechus similis (Looss, 1899) (Trematoda-Plagiorchiidae). Acta Parasitol 8:357–367
Haas W (1994) Physiological analyses of host-finding behavior in trematode cercariae: adaptations for transmission success. Parasitology 109:15–29
Halton DW, Gustafsson MKS (1996) Functional morphology of the platyhelminth nervous system. Parasitology 113:S47–S72
Halton DW, Maule AG (2004) Flatworm nerve-muscle: structural and functional analysis. Can J Zool 82:316–333
Reuter M, Gustafsson MKS (1995) The flatworm nervous system: pattern and phylogeny. In: Breidbach O, Kutsch W (eds) The nervous system of invertebrates: an evolutionary and comparative approach. Birkhäuser Verlag, Basel, pp 25–59
Reuter M, Mäntylä K, Gustafsson MKS (1998) Organization of the orthogon—main and minor nerve cords. Hydrobiologia 383:175–182
Sudarikov VE, Shigin AA, Kurochkin Y, Lomakin V, Stenka RP, Yurlova N (2002) Metacercariae of trematodes—parasites of freshwater hydrobionts in Central Russia. Nauka, Moscow, p 298
Tkach V, Pawlowski J, Mariaux J (2000) Phylogenetic analysis of the suborder plagiorchiata (Platyhelminthes, Digenea) based on partial lsrDNA sequences. Int J Parasitol 30:83–93
Tolstenkov OO, Terenina NB, Gustafsson MKS, Serbina EA, Kreshchenko ND, Maklakova LM, Jashinа AV (2008) Immunocytochemical study of cercariae trematodes from different taxonomic groups—a preliminary study. Acta Biol Hung 59:221–225
Tolstenkov OO, Prokofiev VV, Terenina NB, Gustafsson MKS (2011) The neuro-muscular system in cercaria with different patterns of locomotion. Parasitol Res 108:1219–1227
Tolstenkov OO, Akimova LN, Chrisanfova GG, Terenina NB, Gustafsson MKS (2012a) The neuro-muscular system in fresh-water furcocercaria from Belarus. I Schistosomatidae. Parasitol Res 110:185–193
Tolstenkov OO, Akimova LN, Chrisanfova GG, Terenina NB, Gustafsson MKS (2012b) The neuromuscular system in freshwater furcocercaria from Belarus. II Diplostomidae, Strigeidae and Cyathocotylidae. Parasitol Res 110:583–592
Acknowledgments
The authors thank the staff and students of the Biological Station of Belarus State University for the friendly atmosphere and help. The study was supported by RFBR grants 12-04-01051-а, 12-04-01086-а, grant of the President of Russian Federation МК-1093.2011.4, The Research Institute of the Foundation of the Åbo Akademi University and the Academy of Finland. The authors want to thank Mr. Esa Nummelin for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tolstenkov, O.O., Akimova, L.N., Terenina, N.B. et al. The neuro-muscular system in continuously swimming cercariae from Belarus. I Xiphidiocercariae. Parasitol Res 111, 1977–1983 (2012). https://doi.org/10.1007/s00436-012-3044-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-012-3044-1