Abstract
Trypanosoma cruzi, the etiologic agent of Chagas disease, causes an acute myocarditis and chronic cardiomyopathy. The current therapeutic agents for this disease are not always effective and often have severe side effects. Curcumin, a plant polyphenol, has demonstrated a wide range of potential therapeutic effects. In this study, we examined the effect of curcumin on T. cruzi infection in vitro and in vivo. Curcumin pretreatment of fibroblasts inhibited parasite invasion. Treatment reduced the expression of the low density lipoprotein receptor, which is involved in T. cruzi host cell invasion. Curcumin treatment of T. cruzi-infected CD1 mice reduced parasitemia and decreased the parasitism of infected heart tissue. This was associated with a significant reduction in macrophage infiltration and inflammation in both the heart and liver; moreover, curcumin-treated infected mice displayed a 100% survival rate in contrast to the 60% survival rate commonly observed in untreated infected mice. These data are consistent with curcumin modulating infection-induced changes in signaling pathways involved in inflammation, oxidative stress, and apoptosis. These data suggest that curcumin and its derivatives could be a suitable drug for the amelioration of chagasic heart disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trypanosoma cruzi, an intracellular parasite, causes Chagas disease which affects millions of people in Latin America leading to cardiac and gastrointestinal disease. Of those infected, about millions will develop severe cardiac and/or digestive disorders and annually, up to 50,000 people may die and many others will become physically disabled (Romero and Morilla 2010). Recently, Chagas disease has become a public health concern in non-endemic area due to immigration of infected persons from Chagas-endemic regions of Latin America. The clinical manifestations of acute infection include myocarditis, hepatosplenomegaly, and rarely meningoencephalitis. Fifteen to 30% of infected individuals may develop a chronic chagasic cardiomyopathy, arrhythmia, congestive heart failure, and stroke. In addition, individuals with chronic Chagas disease can develop gastrointestinal manifestations such as the development of megasyndrome.
Previous investigations, including those from our laboratory group and others, have demonstrated that T. cruzi infection upregulates markers of inflammatory and oxidative stress including phosphorylated AKT, MAPK, notch signaling, COX2, chemokines (CCl2,CCl3, CCl5, CXCl5, and CXCl10), and proinflammatory cytokines (Combs et al. 2005; Machado et al. 2000; Nagajyothi et al. 2010). T. cruzi infection in a murine model increased the expression in the myocardium of ET-1, ICAM, VCAM, and matrix metallo-peptidase 3 (MMP3) and 9 (MMP9) (Zhang and Tarleton 1996; Gutierrez et al. 2008). Cardioprotective proteins such as adiponectin and PPAR-γ were significantly reduced during infection (Combs et al. 2005; Nagajyothi et al. 2010). Parasitic infection is known to regulate the host NFκB signaling pathway resulting in inflammation and oxidative stress (Heussler et al. 2006). These interacting pathways and the associated inflammatory responses result in cardiac remodeling and cardiomyopathy.
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione (commonly called diferuloylmethane)) is the orange-yellow active component from the herb Curcuma longa, possessing many pharmacological and biological activities. Studies on the safety of curcumin and its derivatives in different animal models (Chiu et al. 2009) have demonstrated that even at high doses curcumin is nontoxic to laboratory animals. Curcumin has antioxidant, anti-inflammatory, anti-infectious immunomodulatory effects, an ability to regulate apoptosis, antiangiogenic properties, and anticarcinogenic properties and has been shown to target several molecules including growth factors, transcription factors, cytokines, and enzymes involved in the etiology of diverse diseases (Chiu et al. 2009; Kang and Chen 2009a; Kuttan et al. 2007; Aggarwal et al. 2006; Arbiser et al. 1998). Published studies on the effect of curcumin in heart disease indicate that curcumin can regulate genes involved in cardiac homeostasis. Curcumin has been demonstrated to prevent and reverse cardiac hypertrophy and failure in animal models (Ghosh et al. 2010).
Recently we reported that T. cruzi utilizes the host low density lipoprotein receptor (LDLr) in its invasion of mammalian cells (Nagajyothi et al. 2011). Curcumin has been demonstrated to have an inhibitory effect on LDLr transcription (Kang and Chen 2009b; Yuan et al. 2008). Therefore, we tested the inhibitory effect of curcumin treatment on T. cruzi invasion and the pathogenesis of Chagas disease in a murine model. Here, we report that not only does curcumin treatment reduce the rate of invasion, but it also modulates the consequences of infection. Curcumin-based compounds represent a new therapeutic approach for the treatment of Chagas disease and should provide an effective and economical therapy to prevent and modulate the pathogenesis of this infection.
Materials and methods
Parasitology and pathology
The Brazil strain of T. cruzi was maintained in C3H/He mice (Jackson Laboratories, ME). Six to 8-week-old male CD-1 mice were obtained from Charles River Laboratories (Wilmington, MA, USA) and infected intraperitoneal with 5 × 104 trypomastigotes. One set (12 mice in each group) of infected mice were treated with curcumin (100 mg/kg body weight/day orally) for 35 days (Reddy et al. 2005). Blood (75 μl) was collected on day 23 postinfection (p.i.) to detect parasitemia. The parasitemia was determined using a Neubauer hemocytometer. Five mice from each group were sacrificed on 20 days p.i., and the heart tissue was analyzed for parasite load, macrophage infiltration, and inflammatory, oxidative, and cardiovascular signaling pathways. In vitro experiments were repeated thrice and the results were represented by standard errors. For each in vivo experiment, a minimum of five mice were used per group and each experiment has been repeated thrice unless otherwise mentioned. Animal experiments were approved by the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine (protocol no. 20100204). For in vitro experiments, parasites were also maintained in L6E9 myoblasts in vitro as previously described (Rowin et al. 1983).
Effect of curcumin on T. cruzi
Epimastigotes of Brazil strain (1.23 × 106 per well) were incubated with different concentrations of curcumin (0–100 uM in Liver Digest Neutralized Tryptone medium (Tyler and Engman 2000)) for different time intervals at 37°C incubator. The number of dead and live parasites was determined by microscopy (total 1,000 parasites).
Mammalian cell culture
Human foreskin fibroblast (HFF) (ATCC CRL 1475) cell line was maintained in our laboratory using standard methods as previously published (Nagajyothi et al. 2011).
Materials
All of the cell culture reagents used in these experiments was obtained from Cellgro (Mediatech Inc.), primary antibodies were obtained from Abcam (MA, USA) and secondary fluorescence antibodies were obtained from Invitrogen (CA, USA) unless other suppliers are specifically mentioned in the text. Curcumin (C1386-5G) was purchased from Sigma (MO, USA).
Immunoblot analysis
Cell lysates were prepared as previously described (Nagajyothi et al. 2008). An aliquot of each sample (40 μg protein) was subjected to 7.5% SDS-PAGE and the proteins were transferred to nitrocellulose filters for immunoblot analysis. AKT, phosphoAKT, GSK3β, and phospho GSK3β primary antibodies were obtained from Cell Signal (1:2,000 dilution, ab52818 Abcam, MA, USA) and horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin (1:5,000 dilution, Amersham Biosciences) were used to detect specific protein bands (see Fig. legends) using a chemiluminescence system (Nagajyothi et al. 2011; Combs et al. 2005).
Immunofluorescence analysis
Fibroblasts were cultured on cover slips to 80% confluence and then infected with trypomastigotes (3.1 × 106/cm2 surface area of culture plates) for 1 h. One set of cells were pretreated with 30-μM curcumin for 24 h. The monolayers were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton ×100 (30 min) and stained for LDLr using specific primary antibodies (rabbit monoclonal to LDLr, Ab52818 ABCAM), with the concentrations as recommended by the manufacturers and Alexa fluor 594 (goat anti-rabbit IgG 1:500 dilution; Invitrogen, CA, USA). Images were obtained and analyzed by fluorescence microscopy using an inverted Olympus IX71 with a HQ2 CCD camera and a Nikon Microphot-FXA with Spot camera software.
Double staining immunofluorescence analysis of infected cells to differentiate bound and invaded parasites
Fibroblasts (curcumin pretreated and untreated as discussed above) were incubated with parasites at a multiplicity of infection (1:3) for 1 h, washed to remove unbound parasites, fed with fresh medium, and incubated at 37°C. At 4, 15, and 24 h postinfection, the cells were fixed with 4% paraformaldehyde, blocked in 3% BSA, incubated with anti-parasite mouse serum (serum of infected CD1 mice 1:20 dilution) and secondary antibody fluorescent Alexa 480 (green) to stain parasites bound to the cell surface. Then the cells were permeabilized with 0.2% Triton X-100, blocked in 3% BSA, incubated with anti-parasite mouse serum and secondary antibody fluorescence Alexa 594 (red) to stain intracellular parasites.
DNA extraction and qPCR analysis of parasite load
HFF cells were pretreated with 15 or 25 uM curcumin for 24 h and then incubated with trypomastigotes (2:1 MOI) for 96 h at 37°C in the presence of curcumin. Curcumin-untreated HFF cells were infected with trypomastigotes as control. DNA was isolated and purified using Qiagen DNeasy Kit (QIAGEN Sciences, MD, USA). Parasite load in these cells was quantitated by real-time PCR as previously described (Combs et al. 2005). We followed the above method to isolate DNA and to quantify parasite load in hearts of 20 days post-infected mice.
RNA extraction and qPCR analysis
Total RNA was extracted from the heart tissue of CD1-infected mice (day 20 p.i.) using Trizol reagent (Invitrogen). RNA was further purified using RNeasy minikit (QIAGEN Sciences, MD, USA) according to the manufacturer’s instructions. Reverse transcription of total RNA and the quantitative PCR were carried out as described earlier using iQ5 BioRad system (Nagajyothi et al. 2008). The LDLr mRNA levels were detected using PCR primers designed by OriGene (Rockville, MD, USA). The mRNA levels of macrophage-specific F4/80 gene and TLR-9 were measured using the published primers and protocol. The measurements were normalized to the mRNA levels of HPRT gene as described earlier (Nagajyothi et al. 2008).
The mRNA levels of the genes involved in the various signaling pathways were measured using the PCR arrays from SABiosciences (PAMM 013, PAMM 012, and PAMM 065) following manufacturer’s instructions. cDNA from curcumin-treated and untreated infected CD1 mice were prepared as described above. A minimum of five hearts were used in each set and pooled the respective cDNAs to perform each PCR array and the experiments were repeated thrice. Uninfected, untreated mice were used as control.
Statistical analysis
Standard errors and Student’s t tests were calculated using the statistic function of Microsoft Excel.
Results and discussion
Curcumin inhibits T. cruzi invasion by regulating LDLr levels of host cells
Our recent studies demonstrated that T. cruzi utilizes host LDLr for invasion (Nagajyothi et al. 2011). Inhibition or disruption of LDLr significantly reduced parasite load in infected cells. Earlier studies demonstrated that curcumin inhibits the nuclear translocation of SREBP1 and SREBP2, negatively regulating LDLr transcription (Kang and Chen 2009a; Yuan et al. 2008). Therefore, to examine the effect of curcumin on T. cruzi invasion, HFF cells were pretreated with curcumin (30 μM) for 24 h and then incubated with trypomastigotes for 1 h. The effect of curcumin on LDLr activation is dose-dependent and curcumin pretreatment at 30-μM concentration maximally reduced LDLr levels (Kang 2009). Immunofluorescence analysis (IFA) was used to determine the inhibitory efficiency of curcumin treatment on parasite binding and internalization. Curcumin pretreatment did not prevent the parasite from binding to the host cells; however, it completely inhibited the internalization of the parasites (Fig. 1a). IFA of LDLr demonstrated a significant reduction in the expression of LDLr in curcumin-pretreated cells (Fig. 1b).
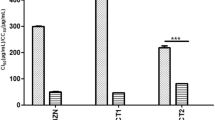
Curcumin treatment inhibits Trypanosoma cruzi invasion a Invasion of T. cruzi in curcumin-pretreated cells evaluated using double-stained IFA demonstrated a significant reduction of internalized parasites (bright red, arrow) in curumin-treated (30 μM) HFF cells compared to untreated cells. Curcumin inhibits T. cruzi internalization but does not affect the binding of parasites (which appear as yellow/green straining) to host cells. b IFA of LDLr demonstrated a significant reduction in LDLr expression in 30 μM curcumin-pretreated HFF cells compared to untreated cells. (×40 magnification, bar represents 50 μm). c Both trypomastigotes (2:1 MOI) and curcumin were incubated with HFF cells for 96 h displaying lower levels of infection compared to untreated cells as demonstrated by qPCR analysis (p < 0.05) (Each experiment was performed three times. Similar results were obtained with each replicate)
Curcumin treatment reduces T. cruzi infection in vitro
We quantified the parasite load in 25 and 15 μM curcumin-treated HFF cells after 96 h of infection by qPCR analysis (Combs et al. 2005). Cells treated with 25 μM curcumin displayed a 75% reduction in parasite load while cells treated with 15 μM displayed a 50% reduction in parasite load compared to untreated cells (Fig. 1c).
Effect of curcumin treatment on parasite load and other parameters in acutely infected mice
Curcumin treatment (100 mg/kg body weight/day orally) of T. cruzi-infected CD1 mice decreased parasitemia to 30% (day 23 postinfection) of that observed in untreated infected mice. qPCR analysis demonstrated a significant reduction of LDLr mRNA (40% of control, p < 0.05) and parasite load (37% of control, p < 0.05) in the heart of curcumin-treated infected mice compared with the untreated infected mice (control mice). Curcumin concentrations ≥15 μM in vitro were trypanocidal over time (Fig. 2a); however, the level of curcumin achieved in serum and tissue were below 15 μM in our in vivo experiments. The bioavailability of orally supplemented curcumin in tissue/serum has been reported to be poor (Vareed et al. 2008). The reduction in parasite load in these treated mice is most likely not a direct effect of curcumin as a trypanocidal agent, as achievable serum and tissue levels are much lower than trypanocidal levels of this drug. Infected CD1 mice treated with curcumin had a 100% survival whereas control untreated infected mice had a 60% survival rate (Fig. 2b, p < 0.05). Increased body weight due to edema (secondary to heart failure) was observed in infected CD1 mice; however, curcumin treatment decreased the body weight in infected treated mice (Fig. 2c). Thus curcumin treatment of infected mice reduced the parasite load and the mortality.
Curcumin treatment kills parasites in vitro and increased survival in infected CD1 mice. a Curcumin has trypanosidal effect on epimastigotes: treatment above 15-μM concentrations of curcumin effectively killed parasites with time. b Curcumin treatment increased the survival of infected mice. c Infection-induced increase in body weight is decreased by curcumin treatment (p < 0.005). Control uninfected, untreated; untreated-infected untreated with curcumin; treated-infected treated with curcumin (a minimum of five mice were used per group and each experiment was performed three times. Similar results were obtained with each replicate)
Multifunctional properties of curcumin attenuate the regulatory effects of T. cruzi infection on various pathways
T. cruzi infection is known to cause inflammation in many organs including the heart where it causes myocarditis. T. cruzi infection increases macrophage infiltration and upregulates NFκB, TNF-α, and a series of chemokines and cytokines in infected cells/tissue (Combs et al. 2005; Machado et al. 2000; Nagajyothi et al. 2010). Curcumin treatment of infected mice significantly reduced macrophage infiltration to 38% (p < 0.05) of untreated infected mice as determined by qPCR analysis using primers specific to macrophage F4/80 mRNA (Fig. 3a). Significant reductions in heart and liver inflammation were also observed in curcumin treatment during acute T. cruzi infection compared to untreated mice (Fig. 3b, c). qPCR analysis demonstrated a significant reduction in the mRNA levels of inflammatory markers such as TNF-α, IL-19, IL-22, Bcl2 like1, COX-2, and TLR-9 compared to curcumin-untreated mice infected with T. cruzi (Table 1).
Multifunctional properties of curcumin attenuate immunopathology of T. cruzi infection measured on day 20 p.i. a Curcumin treatment reduced the load of macrophages in infected heart demonstrated by the qPCR analysis of F4/80 mRNA compared to untreated mice. b, c Bar graphs represent the measured difference in the weights of hearts (b) and liver (c) upon infection and curcumin treatment. d Curcumin treatment modulated the effect of infection on the phosphorylation of AKT and GSK3β. e Effect of curcumin treatment on the mRNA levels of adiponectin and PPARγ in the WAT of infected mice. mRNA levels of control mice were considered as onefold (p < 0.005). f Curcumin treatment attenuated the mRNA levels of ICAM-1, VCAM-1, ITG-b1, TGF-b, MMP-1, and MMP-9, which were regulated by T. cruzi infection. mRNA levels of control mice were considered as onefold (p < 0.005). Control uninfected, untreated; untreated-infected untreated with curcumin; treated-infected treated with curcumin (a minimum of five mice were used per group and each experiment was performed three times. Similar results were obtained with each replicate)
The metabolism of arachidonic acid in cell membranes plays an important role in the inflammatory response by generating potent chemical messengers, eicosanoids. Membrane phospholipids are hydrolyzed by phospholipase A2 (PLA2), releasing arachidonic acid, which may be metabolized by cyclooxygenases (COX) to form prostaglandins and thromboxane A2 (TXA2) or by lipoxygenases (LOX) to form leukotrienes. Curcumin has been found to inhibit PLA2, COX-2, and 5-LOX activities in cultured cells (Hong et al. 2004). Previously we demonstrated that TXA2 contributes to the pathogenesis of T. cruzi infection (Ashton et al. 2007). qPCR analysis demonstrated a significant decrease in COX-2 mRNA levels in curcumin-treated mice upon infection (Table 1). Overall, these data are consistent with the idea that infection-induced inflammation is blunted by the anti-inflammatory property of curcumin.
Reactive oxygen species (ROS) play a key role in enhancing inflammation through the activation of stress kinases and redox sensitive transcription factors such as NF-κB. Oxidative stress-induced injuries are a common finding in chagasic myocardium. Sustained ROS generation of inflammatory and mitochondrial origin, coupled with an inadequate antioxidant response, results in the inefficient scavenging of ROS in the heart and lead to long-term oxidative stress and damage of the cardiac cellular components (Gupta et al. 2009). qPCR analysis demonstrated a significant upregulation at day 15 postinfection of the mRNA levels of NOS-2 (200-fold increase) and superoxide dismutase (Sod1: 7.5-fold increase) in hearts obtained from infected mice compared to uninfected mice. T. cruzi infection has been previously reported to upregulate NOS2, NOS3, and NOS1 levels (Durand et al. 2009). Mice treated with curcumin demonstrated a significant decrease in the mRNA levels of enzymes involved in oxidative signaling such as catalase, peroxidase, SOD, NOS2, and NOS1 in heart tissue compared to untreated infected mice heart as determined by qPCR analysis at day 20 p.i. (Table 2). There was a corresponding increase in the mRNA levels of cardioprotective proteins with curcumin treatment in infected mice such as ApoE (2,040-fold increase) and myoglobin (20,284-fold increase) compared to untreated mice. Myoglobin has been reported to protect heart NOS2 (Hendgen-Cotta et al. 2008). These data indicate that curcumin can regulate stress-induced signaling pathways during infection.
There is evidence that curcumin is both pro-apoptotic and antiapoptotic (Li et al. 2007; Chan and Wu 2004). T. cruzi is known to alter the host cellular signaling (Chuenkova and PereiraPerrin 2009). In vivo studies have shown that the parasite targets AKT in host cells as an intracellular antiapoptotic strategy. T. cruzi induces AKT phosphorylation regulating cell survival signaling in infected mouse heart; curcumin treatment reduced the phosphorylation of AKT and GSK3β significantly (Fig. 3d). qPCR analysis showed a significant increase (61-fold) in the mRNA levels of Bnip3l (BCL2/adenovirus E1B 19kD-interacting protein 3-like) in curcumin-treated infected mice heart compared to infected but untreated mice (day 20 p.i.). Bnip3l induces apoptosis by interacting with viral and cellular antiapoptotic proteins.
Infection with T. cruzi results in acute myocarditis and chronic cardiomyopathy and vasculopathy. Caveolin-1 (Cav-1) and Cav-3 null mice develop cardiomyopathy. Previously, we reported that there are reduced levels of Cav-1 and Cav-3 in the hearts of T. cruzi-infected mice (Medina et al. 2007; Nagajyothi et al. 2006; Adesse et al. 2010). Curcumin regulates Cav-1 expression and can inhibit atherosclerosis (Yuan et al. 2008). The levels of adiponectin, a protein hormone derived from adipocytes, and PPARγ are significantly reduced in mice with acute T. cruzi infection (Combs et al. 2005; Nagajyothi et al. 2008, 2010). Adiponectin and PPARγ modulate inflammation and are cardioprotective (Wang and Nakayama 2010). Curcumin treatment increased adiponectin and PPARγ levels in infected mice epidydimal white adipose tissues (WAT) compared to untreated infected mice (Fig. 3e). There was an increase in mRNA levels of leukocyte adhesion molecules such as ICAM, VCAM, and ITG- β-1, the receptor for VCAM, as well as TGF- β-1, MMP1, and MMP9 in the heart of infected CD1 mice (Fig. 3e). ICAM and VCAM are known inducers of atherosclerosis. Interestingly, coronary atherosclerosis and atherosclerotic lesions are detected in chagasic patients. Curcumin-treated infected mice showed a significant decrease in the mRNA levels of all of these genes (Fig. 3e).
Increased expression of cytokines, chemokines, production of reactive oxygen species, inducible nitric oxide synthase, and adhesion molecules are all significant contributors to the development of chagasic cardiomyopathy (Combs et al. 2005; Machado et al. 2000). Therefore, while inflammatory cells in the heart control parasite growth, they are also involved in the pathogenesis of chagasic heart disease. Inflammation induced cytokine and chemokine upregulation, the expression of inflammation modulators like metalloproteinases, and changes in the expression of stress response proteins all result in alterations of the cardiac cytoskeleton and vasculature ultimately leading to morbidity. Both TNF-α blockers and anti-cytokine/chemokine treatment have been reported to ameliorate and prevent myocarditis in T. cruzi infection models. Therefore, modulation of macrophage activation may be central in providing therapeutic benefits for Chagas disease control (Melo 2009).
Recent studies on the beneficial effects of curcumin in heart diseases indicate a role for curcumin in the regulation of cardiac homeostasis. For example, it has been demonstrated to prevent and reverse cardiac hypertrophy and failure in animal models (Ghosh et al. 2010). In vitro and in vivo research on curcumin has demonstrated it has a diverse profile of activities, including anti-inflammatory, cytokine release, antioxidant effects, immunomodulatory effects, an ability to regulate apoptosis, and the presence of antiangiogenic properties (Kuttan et al. 2007; Aggarwal et al. 2006; Arbiser et al. 1998). Our in vivo studies with curcumin-treated T. cruzi-infected mice demonstrated a significant reduction in parasitemia and tissue parasite load, increased survival rate, and improved pathological conditions in comparison to untreated infected mice. In summary, we believe the reduction in parasite load in organs is the result of a reduction in LDLr levels. Curcumin is trypanocidal; however, the dose of curcumin (effective bioavailability) used in in vivo experiments are very low and below the level required for a direct trypanocidal effect. Thus the amelioration of the disease may be the result of decreased parasite load as well as an indirect effect by modulating various signaling pathways. The results of these studies are significant and indicate that curcumin may be useful in the treatment of Chagas disease. The multifunctional properties of curcumin both directly on the parasite and on the modulation of host responses to T. cruzi infection altering the pathophysiology of infection merits further investigation.
Abbreviations
- MAPK:
-
Mitogen activated protein kinase
- COX 2:
-
Cyclooxygenase 2
- CCL:
-
Chemokine C-C ligand motif
- CXCL:
-
Chemokine C-X-C ligand
- ET1:
-
Endothelin 1
- ICAM:
-
Intercellular adhesion molecule
- VCAM:
-
Vascular cell adhesion molecule
- SREBP:
-
Sterol regulatory element binding protein
- PPAR-γ:
-
Peroxisome proliferators activated receptor γ
- TNF:
-
Tumor necrosis factor
- BCL2:
-
B cell lymphoma 2
- TLR:
-
Toll-like receptor
- IL:
-
Interleukin
References
Adesse D, Lisanti MP, Spray DC, Machado FS, de Nazareth MM, Tanowitz HB, Garzoni LR (2010) Trypanosoma cruzi infection results in the reduced expression of caveolin-3 in the heart. Cell Cycle 9(8):1639–1646
Aggarwal S, Kim SW, Cheon K, Tabassam FH, Yoon JH, Koo JS (2006) Nonclassical action of retinoic acid on the activation of the cAMP response element-binding protein in normal human bronchial epithelial cells. Mol Biol Cell 17(2):566–575. doi:10.1091/mbc.E05-06-0519
Arbiser JL, Klauber N, Rohan R, van Leeuwen R, Huang MT, Fisher C, Flynn E, Byers HR (1998) Curcumin is an in vivo inhibitor of angiogenesis. Mol Med 4(6):376–383
Ashton AW, Mukherjee S, Nagajyothi FN, Huang H, Braunstein VL, Desruisseaux MS, Factor SM, Lopez L, Berman JW, Wittner M, Scherer PE, Capra V, Coffman TM, Serhan CN, Gotlinger K, Wu KK, Weiss LM, Tanowitz HB (2007) Thromboxane A2 is a key regulator of pathogenesis during Trypanosoma cruzi infection. J Exp Med 204(4):929–940. doi:10.1084/jem.20062432
Chan WH, Wu HJ (2004) Anti-apoptotic effects of curcumin on photosensitized human epidermal carcinoma A431 cells. J Cell Biochem 92(1):200–212. doi:10.1002/jcb.20059
Chiu J, Khan ZA, Farhangkhoee H, Chakrabarti S (2009) Curcumin prevents diabetes-associated abnormalities in the kidneys by inhibiting p300 and nuclear factor-kappaB. Nutrition 25(9):964–972. doi:10.1016/j.nut.2008.12.007
Chuenkova MV, PereiraPerrin M (2009) Trypanosoma cruzi targets Akt in host cells as an intracellular antiapoptotic strategy. Sci Signal 2(97):ra74. doi:10.1126/scisignal.2000374
Combs TP, Nagajyothi MS, de Almeida CJ, Jelicks LA, Schubert W, Lin Y, Jayabalan DS, Zhao D, Braunstein VL, Landskroner-Eiger S, Cordero A, Factor SM, Weiss LM, Lisanti MP, Tanowitz HB, Scherer PE (2005) The adipocyte as an important target cell for Trypanosoma cruzi infection. J Biol Chem 280(25):24085–24094. doi:10.1074/jbc.M412802200
Durand JL, Mukherjee S, Commodari F, De Souza AP, Zhao D, Machado FS, Tanowitz HB, Jelicks LA (2009) Role of NO synthase in the development of Trypanosoma cruzi-induced cardiomyopathy in mice. Am J Trop Med Hyg 80(5):782–787
Ghosh SS, Salloum FN, Abbate A, Krieg R, Sica DA, Gehr TW, Kukreja RC (2010) Curcumin prevents cardiac remodeling secondary to chronic renal failure through deactivation of hypertrophic signaling in rats. Am J Physiol Heart Circ Physiol 299(4):H975–H984. doi:10.1152/ajpheart.00154.2010
Gupta S, Wen JJ, Garg NJ (2009) Oxidative stress in Chagas disease. Interdiscip Perspect Infect Dis 2009:190354. doi:10.1155/2009/190354
Gutierrez FR, Lalu MM, Mariano FS, Milanezi CM, Cena J, Gerlach RF, Santos JE, Torres-Dueñas D, Cunha FQ, Schulz R, Silva JS (2008) Increased activities of cardiac matrix metalloproteinases matrix metalloproteinase (MMP)-2 and MMP-9 are associated with mortality during the acute phase of experimental Trypanosoma cruzi infection. J Infect Dis 197(10):1468–1476
Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, Steinhoff HJ, Goedecke A, Schrader J, Gladwin MT, Kelm M, Rassaf T (2008) Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia–reperfusion injury. Proc Natl Acad Sci U S A 105(29):10256–10261. doi:10.1073/pnas.0801336105
Heussler V, Sturm A, Langsley G (2006) Regulation of host cell survival by intracellular Plasmodium and Theileria parasites. Parasitology 132(Suppl):S49–S60. doi:10.1017/S0031182006000850
Hong J, Bose M, Ju J, Ryu JH, Chen X, Sang S, Lee MJ, Yang CS (2004) Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis 25(9):1671–1679. doi:10.1093/carcin/bgh165
Kang Q, Chen A (2009a) Curcumin inhibits srebp-2 expression in activated hepatic stellate cells in vitro by reducing the activity of specificity protein-1. Endocrinology 150(12):5384–5394. doi:10.1210/en.2009-0517
Kang Q, Chen A (2009b) Curcumin suppresses expression of low-density lipoprotein (LDL) receptor, leading to the inhibition of LDL-induced activation of hepatic stellate cells. Br J Pharmacol 157:1354–1367
Kuttan G, Kumar KB, Guruvayoorappan C, Kuttan R (2007) Antitumor, anti-invasion, and antimetastatic effects of curcumin. Adv Exp Med Biol 595:173–184
Li L, Ahmed B, Mehta K, Kurzrock R (2007) Liposomal curcumin with and without oxaliplatin: effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol Cancer Ther 6(4):1276–1282. doi:10.1158/1535-7163.MCT-06-0556
Machado FS, Martins GA, Aliberti JC, Mestriner FL, Cunha FQ, Silva JS (2000) Trypanosoma cruzi-infected cardiomyocytes produce chemokines and cytokines that trigger potent nitric oxide-dependent trypanocidal activity. Circulation 102(24):3003–3008
Medina FA, Cohen AW, de Almeida CJ, Nagajyothi F, Braunstein VL, Teixeira MM, Tanowitz HB, Lisanti MP (2007) Immune dysfunction in caveolin-1 null mice following infection with Trypanosoma cruzi (Tulahuen strain). Microbes Infect 9(3):325–333. doi:10.1016/j.micinf.2006.12.011
Melo RC (2009) Acute heart inflammation: ultrastructural and functional aspects of macrophages elicited by Trypanosoma cruzi infection. J Cell Mol Med 13(2):279–294. doi:10.1111/j.1582-4934.2008.00388.x
Nagajyothi F, Desruisseaux M, Bouzahzah B, Weiss LM, Andrade Ddos S, Factor SM, Scherer PE, Albanese C, Lisanti MP, Tanowitz HB (2006) Cyclin and caveolin expression in an acute model of murine Chagasic myocarditis. Cell Cycle 5(1):107–112
Nagajyothi F, Desruisseaux MS, Thiruvur N, Weiss LM, Braunstein VL, Albanese C, Teixeira MM, de Almeida CJ, Lisanti MP, Scherer PE, Tanowitz HB (2008) Trypanosoma cruzi infection of cultured adipocytes results in an inflammatory phenotype. Obesity (Silver Spring) 16(9):1992–1997
Nagajyothi F, Zhao D, Machado FS, Weiss LM, Schwartz GJ, Desruisseaux MS, Zhao Y, Factor SM, Huang H, Albanese C, Teixeira MM, Scherer PE, Chua SC Jr, Tanowitz HB (2010) Crucial role of the central leptin receptor in murine Trypanosoma cruzi (Brazil strain) infection. J Infect Dis 202(7):1104–1113. doi:10.1086/656189
Nagajyothi F, Weiss LM, Silver DL, Desruisseaux MS, Scherer PE, Herz J, Tanowitz HB (2011) Trypanosoma cruzi utilizes the host low density lipoprotein receptor in invasion. PLoS Negl Trop Dis 5(2):e953. doi:10.1371/journal.pntd.0000953
Reddy RC, Vatsala PG, Keshamouni VG, Padmanaban G, Rangarajan PN (2005) Curcumin for malaria therapy. Biochem Biophys Res Commun 326(2):472–474
Romero EL, Morilla MJ (2010) Nanotechnological approaches against Chagas disease. Adv Drug Deliv Rev 62(4–5):576–588
Rowin KS, Tanowitz HB, Wittner M, Nguyen HT, Nadal-Ginard B (1983) Inhibition of muscle differentiation by trypanosoma cruzi. Proc Natl Acad Sci U S A 80(20):6390–6394
Tyler KM, Engman DM (2000) Flagellar elongation induced by glucose limitation is preadaptive for Trypanosoma cruzi differentiation. Cell Motil Cytoskeleton 46:269–278
Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, Brenner DE (2008) Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev 17(6):1411–1417. doi:10.1158/1055-9965.EPI-07-2693
Wang Z, Nakayama T (2010) Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm 2010:535918. doi:10.1155/2010/535918
Yuan HY, Kuang SY, Zheng X, Ling HY, Yang YB, Yan PK, Li K, Liao DF (2008) Curcumin inhibits cellular cholesterol accumulation by regulating SREBP-1/caveolin-1 signaling pathway in vascular smooth muscle cells. Acta Pharmacol Sin 29(5):555–563. doi:10.1111/j.1745-7254.2008.00783.x
Zhang L, Tarleton RL (1996) Persistent production of inflammatory and anti-inflammatory cytokines and associated MHC and adhesion molecule expression at the site of infection and disease in experimental Trypanosoma cruzi infections. Exp Parasitol 84(2):203–213
Acknowledgments
The study was supported in part by the United States National Institutes of Health (NIH) grants AI-076248-01 (HBT) and the Einstein Diabetes Center (Point grant to HBT). We would like to thank Vicki Braunstein for her expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagajyothi, F., Zhao, D., Weiss, L.M. et al. Curcumin treatment provides protection against Trypanosoma cruzi infection. Parasitol Res 110, 2491–2499 (2012). https://doi.org/10.1007/s00436-011-2790-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2790-9