Abstract
The African penguin, Spheniscus demersus, the only penguin species that breeds in Africa, is endangered, and several diseases including avian malaria, babesiosis, and aspergillosis are common in some populations. From 2002 to 2010, spirochetes morphologically consistent with Borrelia were observed on thin blood smears from 115 of 8,343 (1.4%) African penguins admitted to rehabilitation centers in the Western Cape and Eastern Cape provinces of South Africa. Prevalence rates were significantly higher among chicks and juveniles compared with adults and for birds sampled during the summer months of October to February compared with winter months. The majority of infected birds were ultimately released, despite lack of antibiotic treatment; however, at least one bird is believed to have died of borreliosis based on characteristic gross and microscopic lesions. Analysis of partial flaB gene sequences indicated this was a relapsing fever Borrelia most similar to a Borrelia sp. detected in soft ticks from a seabird colony in Japan. This represents the fourth report of a relapsing fever Borrelia sp. in an avian species and highlights the need for additional studies of potentially pathogenic organisms infecting the African penguin in South Africa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The African penguin, Spheniscus demersus, is the only penguin species that is found in Africa. It breeds on 25 islands and four mainland localities across southwestern Africa (Kemper et al., 2007). In South Africa, penguins breed in the Western Cape and Eastern Cape provinces, separated by approximately 600km. The total population was estimated at over 1.45 million individuals at the start of the 20th century (Shannon and Crawford 1999) however numbers decreased to 141,000 breeding pairs in 1956 and subsequently to 26,000 breeding pairs in 2009 (Crawford et al., 2011). The IUCN conservation status of the species has been re-classified from Vulnerable to Endangered (BirdLife International 2010). Historical threats to the African penguin included guano and egg collection and current threats include oil spills, human disturbance, reduced food availability due to increased commercial fishing, and diseases such as aspergillosis and avian malaria (Westphal and Rowan 1970; Brossy 1992; Crawford et al. 1995; Graczyk et al. 1995; BirdLife International 2010).
Several vector-borne pathogens have been reported from African penguins including Babesia peircei, Plasmodium relictum spheniscidae, Plasmodium juxtanucleare, and Leucocytozoon tawaki (Bennett et al. 1992; Brossy 1992; Earle et al. 1993; Grim et al. 2003). Among free-ranging penguins, hemoparasite infections have only been reported from temperate penguin species from South Africa, Australia, New Zealand, and some regions of the South Atlantic. All surveys for hemoparasites in penguins from the sub-Antarctic and Antarctic have been negative, presumably because of a lack of appropriate vectors (Jones and Shellam 1999).
The emergence, establishment, or increased transmission of vector-borne pathogens is a complex relationship between the host, vector(s), and environment and can result from numerous changes such as the introduction of novel vectors or reservoir hosts, increased host densities that increase transmission, environmental changes that increase vector densities, or exposure of hosts to vectors. Additionally, stress due to oiling, lack of food, or degraded habitat can predispose penguins to develop clinical disease resulting from infection with a parasite that might not have been pathogenic in an immunocompetent healthy bird.
Spirochetes morphologically similar to Borrelia have been detected in blood smears of African penguins admitted to rehabilitation centers in South Africa. The current study was initiated to determine the prevalence of this spirochete in the African penguin and to characterize the organisms by sequence analysis of the flaB gene.
Materials and methods
From January 2002 to December 2010, blood smears were made from 8,343 African penguins admitted to the Southern African Foundation for the Conservation of Coastal Birds (SANCCOB) facility in Cape Town (Western Cape) (n = 7,693) and the Penguins- Eastern Cape (PEC) facility in Cape St. Francis (Eastern Cape) (n = 650). Smears were stained with either eosin–methylene blue or a modified Wright-Giemsa stain (Kyro-Quick stain, Kyron Laboratories, Benrose, South Africa) and examined for blood parasites. Upon admission, each penguin is given a full physical exam, and blood and fecal samples are collected for parasite analysis and birds are treated with a topical insecticide. Ectoparasites were observed on some birds but were not collected systematically. Soft ticks that were examined were identified as Carios sp. Chi-square analysis with Yates correction was used to detect differences in prevalence among month of sampling, season, and age.
To molecularly characterize the spirochete observed in blood smears, we conducted polymerase chain reaction (PCR) for Borrelia spp. on blood (∼20 μl) from two penguins from 2009. Blood was spotted on glass slides, allowed to dry, and fixed with ethanol. The dried blood was prepared for DNA extraction by scraping the blood from the slide and mixing with 50 μl of phosphate-buffered saline. DNA was extracted from the solution using QIAGEN DNA extraction kit (QIAGEN Inc., Valencia, CA).
A 330-bp region of the flagellin gene (flaB) was targeted using the external primers FLALL and FLARL and the internal primers FLALS and FLARS in a nested PCR assay previously described (Barbour et al. 1996). Stringent protocols and controls were utilized in all PCR assays to prevent and detect contamination. DNA extraction, primary amplification, secondary amplification, and product analysis were performed in separate dedicated laboratory areas. A negative water control was included in each set of DNA extractions, and one water control was included in each set of primary and secondary PCRs.
Sequences obtained from this study and from other Borrelia spp. stored in GenBank were aligned using the multisequence alignment ClustalX program (Thompson et al. 1994). Phylogenetic analyses were conducted using Molecular Evolutionary Genetics Analysis program version 2.1 (Kumar et al. 2004) using the neighbor-joining algorithm using the Kimura 2-parameter model and maximum parsimony using a heuristic search. The GenBank accession number for the flaB gene sequence of the Borrelia sp. from the African penguins is JN402326.
Results
Spirochetes morphologically compatible with Borrelia (Fig. 1) were observed in blood smears from 115 of 8,343 (1.4%) African penguins. Significant differences in prevalence rates were noted by age with higher prevalence rates being noted in chicks (p < 0.0001) and juveniles (p < 0.0004) compared with adult penguins (Table 1). Of the four infected adult penguins detected, at least three were believed to be immunocompromised because two were oiled and one was molting prior to submission. The fourth adult penguin was admitted with neurologic signs probably related to clinical borreliosis (see below). A significantly higher prevalence was noted in summer months (Oct–Feb) compared with winter months (March–Sept) (Table 1). Among the summer months, penguins sampled in November (5.1%) and December (4.8%) had significantly (p < 0.0001) higher prevalence rates compared to all other months and accounted for 84 of the 115 (73%) total positives. At SANCCOB, prevalence rates have increased significantly since 2002 (χ 2 = 37.17, df = 6, p < 0.00001) with prevalence rates in 2002 and 2003 being significantly less than in 2004, 2006–2008, and 2010 (all p < 0.007) (Fig. 2). No differences in prevalence between years were noted at PEC (Fig. 2). Interestingly, not all penguins were blood smear positive immediately upon admission. At SANCCOB, 55 (50%) penguins were negative at admission but were first noted to be positive >6 days post admission with 10 penguins only being noted as positive after being in the facility for >30 days.
At SANCCOB, the majority of Borrelia-infected penguins (n = 82, 75%) did not receive antibiotic treatment; however, the majority of these infected birds (n = 60, 73%) were ultimately released. Numerous causes of death were determined for 33 Borrelia-infected penguins including pneumonia, airsacculitis, aspergillosis, babesiosis, gastroenteritis, hematoma, blindness, malaria, and one possible case of borreliosis. Cause of death was not determined for the remainder because post mortem examinations were not conducted. At PEC, one penguin was admitted with a history of unsteady gait, circling, and torticollis. The penguin was given supportive care and 15 mg enrofloxacin daily per os (Baytril, Bayer) but died 4 days after admission. Grossly, the penguin had splenomegaly and possibly hepatomegaly. Histologically, splenic reticuloendothelial hyperplasia with hemosiderosis; edema in the lungs; and moderate, subacute, lymphocytic meningoencephalitis were noted. In response to this death, the other Borrelia-positive penguins at PEC received 25–50 mg/kg doxycycline daily per os for at least 10 days.
Coinfections with other vector-borne parasites were noted on blood smear analysis at both rehabilitation centers with 5 and 40 penguins being infected with Plasmodium spp. and B. peircei, respectively, at SANCCOB and three of six penguins from PEC being infected with B. peircei (Fig. 1). Only 1 of the 650 penguins from PEC was positive for Plasmodium spp.
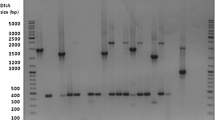
A 327-bp amplicon was amplified and sequenced from two Borrelia blood smear-positive penguins. The two sequences were identical and were most similar (99%) to Borrelia sp. K64 previously detected in Carios sawaii ticks removed from seabirds in Japan (Takano et al. 2009) followed by numerous Borrelia turicatae sequences (97.9%). Phylogenetic analyses indicated that the penguin Borrelia sp. was included in a well-supported clade with Borrelia sp. K64 and B. turicatae (Fig. 3).
Discussion
Several different species of Borrelia have been previously reported from numerous avian species including Borrelia anserina, the causative agent of fowl spirochetosis, and Borrelia burgdorferi sensu lato (including B. burgdorferi sensu stricto, Borrelia garinii, and Borrelia valaisiana, each causative agents of Lyme disease). B. anserina is found worldwide and was once a significant pathogen of domestic poultry (McNeil et al. 1949). Changes in poultry rearing have resulted in the elimination of soft tick infestations, but borreliosis can still be a problem in small or backyard flocks (Ataliba et al. 2007). Worldwide, passerines are common hosts for different genospecies of B. burgdorferi sensu lato (Richter et al. 2000; Michalik et al. 2008). Enzootic cycles of B. garinii are maintained in numerous species of seabirds throughout the world by Ixodes uriae, a common tick species infesting seabirds (Olsén et al. 1993; Gylfe et al. 1999). Exposure of seabirds appears to be variable by host species and by locality which may be related to differential behavior of hosts (nesting density), habitat suitability for ticks, or host reaction to Borrelia infections (Staszewski et al. 2008).
Among penguins, only a single study has reported antibodies to a Borrelia species (B. burgdorferi) in king penguins (Aptenodytes patagonicus) from the Crozet Archipelago in the southern Indian Ocean (Gauthier-Clerc et al. 1999). Because no spirochetes were isolated and characterized, the species of Borrelia infecting the king penguins is unknown. Based on our phylogenetic studies, the Borrelia sp. we detected in the African penguins from eastern South Africa was clearly a relapsing fever Borrelia (RFB) and was distinct from other RFB including B. anserina. This finding represents only the fourth report of a RFB in an avian species. The first report was in a northern spotted owl (Strix occidentalis caurina) that died from a systemic RFB infection caused by Borrelia hermsii, a zoonotic RFB that utilizes rodents as natural hosts (Thomas et al. 2002; Bunikis et al. 2004; Fischer et al. 2009). Domestic chickens are naturally infected with two RFBs, B. anserina and Borrelia duttonii. Although B. anserina can be a significant pathogen, B. duttonii has not been shown to cause clinical disease in experimental or naturally infected chickens (Kervan 1947; McCall et al. 2007).
Tick-borne relapsing fever of humans is a zoonotic infection caused by 15 species of RFB and is associated with recurrent periods of pyrexia and spirochetemia. Each species of RFB is associated with a specific species of soft tick (Ornithodoros sp.) vector. In many parts of Africa, diagnosis is complicated because of the high prevalence of malaria that has a similar clinical presentation (Nordstrand et al. 2007). In western Africa, Borrelia crocidurae, which utilizes rodents and shrews as reservoir hosts, is most often associated with human disease (Godeluck et al. 1994). B. duttonii is common in eastern and central Africa and is rarely found in western Africa (Fukunaga et al. 2001; Nordstrand et al. 2007). In Tanzania, natural infections with B. duttonii have been reported in pigs and chickens (McCall et al. 2007), but no wildlife reservoir has been identified. In addition, molecular characterization of Borrelia from human RFB cases and ticks in Tanzania indicates that another undescribed species is endemic (Kisinza et al. 2003; Mitani et al. 2004). Although rare human RFB cases have been reported in South Africa since 1912, the Borrelia species has not been determined (Rosenthal 1982). Two other RFBs, Borrelia graingeri and Borrelia tillae, have been isolated from Ornithodoros spp. in southern Africa, but these species have not been associated with human infections (Zumpt and Organ 1961) nor have they been genetically characterized. Thus, we do not know the relatedness of the penguin RFB to the RFB agent in South African humans, B. graingeri or B. tillae. We detected Carios (formerly Ornithodoros) sp. ticks on some penguins but none were tested for Borrelia because they were blood-fed. Future studies should investigate the vector potential of this Carios sp. for the penguin Borrelia sp.
The zoonotic potential of the RFB detected in African penguins is unknown, but many RFB species are zoonotic. The Borrelia sp. from the African penguin was most closely related to a RFB detected in soft ticks (C. sawaii) from a colony of Swinhoe's storm petrels (Oceanodroma monorhis) and streaked shearwaters (Calonectris leucomelas) in Japan (Takano et al. 2009). Ticks from this bird colony have been associated with a disease consistent with RFB in humans (Tsurumi et al. 2002).
Currently, little is known about this Borrelia species; for example, is the African penguin the natural host, is it emerging in penguins, or is it a possible pathogen for penguins or humans? Although infections of penguins were noted at one facility annually since 2002, there was a general trend of increasing prevalence at both rehabilitation centers. The pathogenicity of this RFB for penguins is unknown, but it is likely not highly pathogenic to immunocompetent penguins as the majority of infected penguins were released without any antibiotic treatment. In general, immunocompromised or naive individuals are more likely to become infected, and in this study, we found a higher prevalence in chicks and in adults with concurrent issues. One of the Borrelia-positive penguins that died did have neurologic signs and lesions similar to those reported in the fatal infection of an owl with B. hermsii and in domestic fowl with B. anserina infections (McNeil et al. 1949; Dickie and Barrera 1964; Thomas et al. 2002; Bunikis et al. 2004). Similarly, a fatal case of RFB infection in a Pipistrellus bat from southern England had anemia, splenomegaly, hepatomegaly, and blood-tinged pleural fluid (Evans et al. 2009). Further studies are needed to determine the other possible hosts (avian or mammalian), vector, distribution, pathogenicity, and prevalence of this Borrelia sp. in African penguins throughout their range. Because populations are declining significantly, the emergence of any potential pathogen represents an important threat to the long-term recovery of the endangered African penguin population.
References
Ataliba AC, Resende JS, Yoshinari N, Labruna MB (2007) Isolation and molecular characterization of a Brazilian strain of Borrelia anserina, the agent of fowl spirochaetosis. Res Vet Sci 83:145–149
Barbour AG, Maupin GO, Teltow GJ, Carter CJ, Piesman J (1996) Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J Infect Dis 173:403–409
Bennett GF, Earl RA, Peirce MA (1992) The Leucocutozoidae of South African birds: Caprimulgidae, Columbidae, Gruidae, and Spheniscidae. Onderspoort J Vet Res 59:229–234
BirdLife International (2010) Species factsheet: Spheniscus demersus. Available at http://www.birdlife.org. Accessed 14 Aug 2011
Brossy JJ (1992) Malaria in wild and captive Jackass penguins Spheniscus demersus along the southern African coast. Ostrich 63:10–12
Bunikis J, Tsao J, Garpmo U, Berglund J, Fish D, Barbour AG (2004) Typing of Borrelia relapsing fever group strains. Emerg Infect Dis 10:1661–1664
Crawford RJM, Williams AJ, Hofmeyer JH, Klages NTW, Randall RM, Cooper J, Dyer BM, Chesselet Y (1995) Trends of African penguin Spheniscus demersus populations in the 20th century. S Afr J Mar Sci 16:101–118
Crawford RJM, Altwegg R, Barham BJ, Barham PJ, Durant JM, Dyer BM, Geldenhuys D, Makhado AB, Pichegru L, Ryan PG, Underhill LG, Upfold L, Visagie J, Waller LJ, Whittington PA (2011) Collapse of South Africa’s penguins in the early 21st century. Afr J Mar Sci 33:139–156
Dickie CW, Barrera J (1964) A study of the carrier state of avian spirochetosis in the chicken. Avian Dis 8:191–195
Earle RA, Huchzermeyer FW, Bennett GF, Brossy JJ (1993) Babesia peircei sp. nov. from the jackass penguin. S Afr J Zool 28:88–90
Evans NJ, Bown K, Timofte D, Simpson VR, Birtles RJ (2009) Fatal borreliosis in bat caused by relapsing fever spirochete, United Kingdom. Emerg Infect Dis 15:1331–1333
Fischer RJ, Johnson TL, Raffel SJ, Schwan RG (2009) Identical strains of Borrelia hermsii in mammal and bird. Emerg Infect Dis 15:2064–2066
Fukunaga M, Ushijima Y, Aoki LY, Talbert A (2001) Detection of Borrelia duttonii, a tick-borne relapsing fever agent in central Tanzania, within ticks by flagellin gene-based nested polymerase chain reaction. Vector Borne Zoonotic Dis 1:331–338
Gauthier-Clerc M, Jaulhac B, Frenot Y, Bachelard C, Monteil H, Le Maho Y, Handrich Y (1999) Prevalence of Borrelia burgdorferi (the Lyme disease agent) antibodies in king penguin Aptenodytes patagonicus in Crozet Archipelago. Polar Biology 22:141–143
Godeluck B, Duplantier JM, Ba K, Trape JF (1994) A longitudinal survey of Borrelia crocidurae prevalence in rodents and insectivores in Senegal. Am J Trop Med Hyg 50:165–168
Grim KC, Van der Merwe E, Sullivan M, Parsons N, McCutchan TF, Cranfield M (2003) Plasmodium juxtanucleare associated with mortality in black-footed penguins (Spheniscus demersus) admitted to a rehabilitation center. J Zoo Wildl Med 34:250–255
Graczyk TK, Brossy JJ, Plös A, Stoskopf MK (1995) Avian malaria seroprevalence in jackass penguins (Spheniscus demersus) in South Africa. J Parasitol 81:703–707
Gylfe Ǻ, Olsen B, Straševičius D, Marti Ras N, Weihe P, Noppa L, Östberg Y, Baranton G, Bergström S (1999) Isolation of Lyme disease Borrelia from puffins (Fratercula arctica) and seabird ticks (Ixodes uriae) on the Faeroe Islands. J Clin Microbiol 37:890–896
Jones HI, Shellam GR (1999) Blood parasites in penguins, and their potential impact on conservation. Mar Ornithol 27:181–184
Kemper J, Underhill LG, Crawford RJM, Kirkman SP (2007) Revision of the conservation status of seabirds and seals breeding in the Benguela Ecosystem. In: Kirkman SP (ed) Final report of the BCLME (Benguela Current Large Marine Ecosystem) Project on Top Predators as Biological Indicators of Ecosystem Change in the BCLME. Avian Demography Unit, Cape Town
Kervan P (1947) Recherches sur la sensibilite' du poulet a Spirochaeta duttonii. Absence d'immunite' contre Spirochaeta gallinarum. Bulletin Societe' du Pathologie Exotique 40:152–155
Kisinza WN, McCall PJ, Mitani H, Talbert A, Fukunaga M (2003) A newly identified tick-borne Borrelia species and relapsing fever in Tanzania. Lancet 362:1283–1284
Kumar S, Tamura K, Nei M (2004) MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
McCall PJ, Hume JC, Motshegwa K, Pignatelli P, Talbert A, Kisinza W (2007) Does tick-borne relapsing fever have an animal reservoir in East Africa? Vector Borne Zoonotic Dis 7:659–666
McNeil E, Ninshaw WR, Kissling RE (1949) A study of Borrelia anserina infection (spirochetosis) in turkeys. J Bacteriol 57:191–206
Michalik J, Wodecka B, Skoracki M, Sikora B, Stanczak J (2008) Prevalence of avian-associated Borrelia burgdorferi s.l. genospecies in Ixodes ricinus ticks collected from blackbirds (Turdus merula) and song thrushes (T. philomelos). Int J Med Microbiol 298S1:129–138
Mitani H, Talbert A, Fukunaga M (2004) New World relapsing fever Borrelia found in Ornithodoros porcinus ticks in central Tanzania. Microbiol Immunol 48:501–505
Nordstrand A, Bunikis I, Larsson C, Tsogbe K, Schwan TG, Nilsson M, Bergström S (2007) Tickborne relapsing fever diagnosis obscured by malaria, Togo. Emerg Infect Dis 13:117–123
Olsén B, Jaenson TGT, Noppa L, Bunikis J, Bergström S (1993) A Lyme borreliosis cycle in seabirds and Ixodes uriae ticks. Nature 362:340–342
Richter D, Spielman A, Komar N, Matuschka FR (2000) Competence of American robins as reservoir hosts for Lyme disease spirochetes. Emerg Infect Dis 6:133–138
Rosenthal E (1982) Relapsing fever in Cape Town. A case report. S Afr Med J 61:801–802
Shannon LJ, Crawford RJM (1999) Management of the African penguin Spheniscus demersus – insights from modeling. Marine Ornithology 27:119–128.
Staszewski V, McCoy KD, Boulinier T (2008) Variable exposure and immunological response to Lyme disease Borrelia among North Atlantic seabird species. Proc Biol Sci 275:2101–2109
Takano A, Muto M, Sakata A, Ogasawara Y, Ando S, Hanaoka N, Tsurumi M, Sato F, Nakamura N, Fujita H, Watanabe H, Kawabata H (2009) Relapsing fever spirochete in seabird tick, Japan. Emerg Infect Dis 15:1528–1530
Thomas NJ, Bunikis J, Barbour AG, Wolcott MJ (2002) Fatal spirochetosis due to a relapsing fever-like Borrelia sp. in a northern spotted owl. J Wildl Dis 38:187–193
Thompson JD, Higgins DG, Giboson TJ, Clustal W (1994) Improving and sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tsurumi M, Kawabata H, Sato F (2002) Present status and epidemiological investigation of Carios (Ornithodoros) capensis in the colony of the black-footed albatross Diomedea nigripes on Tori-shima, Izu Islands, Japan. J Yamashina Inst Ornithol 10:250–256
Westphal A, Rowan MK (1970) Some observation on the effects of oil pollution on the jackass penguin. Ostrich 41S:521–526
Zumpt F, Organ D (1961) Strains of spirochaetes isolated from Ornithodoros zumpti Heisch and Guggisberg and from wild rats in the Cape province. A preliminary note. S Afr J Lab Clin Med 7:31–35
Acknowledgments
The authors thank the many staff and volunteers at SANCCOB and PEC, especially B. Bousfield (PEC), who provided clinical assistance. We also thank T. Gous (SANCCOB and State Veterinary Laboratory, Department of Agriculture) for histological examination of tissues from one penguin. We also thank M. Peirce for assistance and helpful comments. SANCCOB is supported by a wide range of donors, including the International Fund for Animal Welfare (IFAW), Hans Hoheisen Charitable Trust and the National Lottery Distribution Trust Fund (NLDTF). This research is supported by the Sea Research Foundation (Mystic Aquarium) and the Georgia Aquarium. NJP also acknowledges support from the National Research Foundation (SEACHANGE programme, Earthwatch Institute and the University of Cape Town Research Committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yabsley, M.J., Parsons, N.J., Horne, E.C. et al. Novel relapsing fever Borrelia detected in African penguins (Spheniscus demersus) admitted to two rehabilitation centers in South Africa. Parasitol Res 110, 1125–1130 (2012). https://doi.org/10.1007/s00436-011-2602-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2602-2