Abstract
Aedes aegypti transmits the viruses that cause yellow and dengue fevers. Vector control is essential, since a vaccine for dengue has not as yet been made available. This work reports on the larvicidal activity of Myracrodruon urundeuva leaf lectin (MuLL) against A. aegypti fourth-stage larvae (L4). Also, the resistance of MuLL to digestion by L4 gut proteases and the effects of MuLL on protease, trypsin-like and α-amylase activities from L4 gut were evaluated to determine if lectin remains active in A. aegypti gut and if insect enzyme activities can be modulated by MuLL. MuLL promoted mortality of L4 with LC50 of 0.202 mg/ml. Haemagglutinating activity of MuLL was detected even after incubation for 96 h with L4 gut preparation containing protease activity. MuLL affected the activity of gut enzymes, inhibiting protease and trypsin activities and stimulating α-amylase activity. The results suggest that MuLL may become a new biodegradable larvicidal agent for dengue control. Larvicidal activity of MuLL may be linked to its resistance to proteolysis by larval enzymes and interference in the activity of digestive larval enzymes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mosquito Aedes aegypti (Diptera, Culicidae) is the vector of the etiologic agents of yellow and dengue fevers. Yellow fever is an acute viral haemorrhagic disease endemic in tropical areas of Africa and Latin America. There are an estimated 200,000 cases each year and prevention strategies rely on a vaccine and antivirals (Monath 2008; World Health Organization 2009a). Dengue is a pandemic flu-like infection that affects approximately 50 million people every year and may turn into a potentially lethal complication called dengue haemorrhagic fever (World Health Organization 2009b). Cases of dengue are re-emerging in tropical and sub-tropical regions. Since there are no effective vaccines against serotypes of DEN virus, vector control remains as the sole form to minimize dengue incidence (Tauil 2002; World Health Organization 2009b).
Public health programs rely mainly on organophosphorous and pyrethroid insecticides to control A. aegypti larvae. However, on the one hand, this species’ larvae have become insecticide-resistant, and on the other hand, a genotoxic effect of the organophosphate temephos has been reported for concentrations normally used to control larvae (Aiub et al. 2002; Poupardin et al. 2008; Melo-Santos et al. 2010). Biological control using small mosquito-eating fishes and copepods as well as the entomopathogenic bacterium Bacillus thuringiensis serovar israelensis (Bti) have also been employed (Araújo et al. 2007; World Health Organization 2009b). Control using natural and biodegradable compounds (plant extracts, essential oils, saponins, flavonoids, lectins, and others) has been suggested as an alternative strategy to synthetic insecticides (e.g., Bagavan et al. 2008; Rajkumar and Jebanesan 2008; Autran et al. 2009; Coelho et al. 2009).
Lectins are carbohydrate-recognizing proteins able to interact with carbohydrates and glycoconjugates (Correia et al. 2008). Plant lectins have several biological properties, including a deleterious effect on the survival of insects from several orders (Lam and Ng 2011). In a study on chitin-binding lectin (WSMoL) isolated from Moringa oleifera seeds, a larvicidal effect on A. aegypti fourth-stage larvae (L4) was observed; the authors reported that WSMoL promoted morphological changes in larvae like hypertrophy of the segments, increased gut volume and absence of the epithelial layer delimiting the gut (Coelho et al. 2009). The peritrophic matrix present in the insect gut is a noncellular and semipermeable membrane which is important in the digestive processes and in protection against microorganisms and parasites (Tellam et al. 1999). Interaction of chitin-binding lectins with N-acetylglucosamine residues of chitin and glycosylated proteins present in peritrophic matrix may disrupt membrane integrity, possibly leading the insect to nutritional deprivation by reduction in permeability and restriction in the movement of nutrients and digestive enzymes across matrix pores (Czapla and Lang 1990; Eisemann et al. 1994; Macedo et al. 2003, 2007). It has also been suggested that mechanisms of insecticidal effect of lectins involve resistance to proteolysis, interaction with glycoconjugates along the digestive tract of insect, and binding to digestive enzymes (Zhu-Salzman et al. 1998; Carlini and Grossi-de-Sá 2002; Macedo et al 2003).
Myracrodruon urundeuva (Anacardiaceae) is a tree widely distributed in Brazil, popularly known as “aroeira-do-sertão” and “urundel”. Its bark and/or wood have been proved to exert analgesic, antibacterial, antifungal, antioxidant, anti-inflammatory, anti-ulcer and termite repellent activities (Viana et al 2003; Souza et al. 2006; Sá et al. 2009a). Three chitin-binding lectins isolated from M. urundeuva bark (MuBL), heartwood (MuHL) and leaf (MuLL) showed insecticidal activity on termite Nasutitermes corniger, and it has been proposed that termiticidal activity may be due to resistance to proteolytic degradation by termite enzymes, chitin-binding property and antibacterial activity against symbiotic bacteria found in the termite gut (Sá et al. 2008; Napoleão et al. 2011). MuBL and MuHL showed larvicidal activity against A. aegypti L4, pointing to the interaction of lectins with larval peritrophic matrix (Sá et al. 2009b).
This work evaluated larvicidal activity of MuLL on A. aegypti L4, the effect of proteases from larval gut on MuLL haemagglutinating activity and the effect of MuLL on protease, trypsin-like and α-amylase activities from larvae gut.
Materials and methods
Plant materials
Leaves of M. urundeuva (Engl.) Fr. & All. (Division Magnoliophyta, Class Magnoliopsida, Subclass Rosidae, Order Sapindales, Family Anacardiaceae) were collected in the state of Maranhão, northeastern Brazil. A voucher specimen (identified by Mr. Gonçalo Mendes da Conceição) is deposited under number 054 at the Herbarium Aluisio Bittencourt, Centro de Estudos Superiores de Caxias, Universidade Estadual do Maranhão, Brazil. The leaves were air-dried, powdered, passed through 40 mesh screen, and stored at 28°C.
Haemagglutinating activity
Haemagglutinating assay was carried out according to Napoleão et al. (2011) in microtiter plates (Kartell, Italy) using suspension (2.5% v/v) of rabbit erythrocytes treated with glutaraldehyde (Bing et al. 1967). One haemagglutination unit (titer) was defined as the reciprocal of the highest dilution of sample promoting full agglutination of erythrocytes. Specific haemagglutinating activity was defined as the ratio between the titer and protein concentration (mg/ml). HA inhibitory assay was performed by incubation (45 min) of lectin sample with 0.5 mg/ml asialofetuin solution before erythrocyte suspension was added.
Purification of MuLL
MuLL was isolated according to Napoleão et al. (2011). Powdered leaves (10 g) were suspended in 0.15 M NaCl (100 ml), homogenized in a magnetic stirrer (16 h at 4°C), filtered through gauze and centrifuged (3,000 × g, 15 min). Next, the supernatant (leaf extract) was treated with ammonium sulphate (60–80% saturation) and the precipitated protein fraction was chromatographed on chitin column. MuLL was eluted with 1.0 M acetic acid. Protein concentrations in the leaf extract, precipitated fraction and MuLL were determined using bovine serum albumin (31–500 μg/ml) as standard (Lowry et al. 1951)
Larvicidal assay
A. aegypti eggs hatched in distilled water at a temperature range of 25–27°C and cat food (Whiskas®) was offered to larvae. When reaching the early fourth-stage (L4), larvae were collected and used in bioassays. Larvicidal activity was evaluated using an adaptation of the World Health Organization (1981) method described by Navarro et al. (2003). A stock solution of M. urundeuva leaf extract (protein concentration: 44.0 mg/ml) was used to provide a series of test solutions in the protein concentration range 8.0–14.0 mg/ml, obtained by dilution of the stock solution with distilled water. The final volume of each larvicidal assay was 20 ml of test solution or negative control (0.15 M NaCl) and contained 20–25 larvae in early L4 stage. Mortality rate (%) was determined after 24 h of incubation at 28 ± 2°C and 12/12 (light–dark) photoperiodism. Three independent experiments were run in triplicate. The same conditions were used in bioassays with purified MuLL (stock solution at 0.422 mg/ml; test concentration range: 0.1–0.3 mg/ml).
A. aegypti L4 gut extracts
Groups of fifty A. aegypti L4 were collected and immobilized by cooling at 4°C for 10 min. The gut of each larva was removed using a needle (8 mm in length; 0.3 mm caliber) and immediately homogenized with 1 ml acetate buffer (0.1 M sodium acetate pH 5.5 containing 0.02 M CaCl2 and 0.15 M NaCl) or Tris buffer (0.1 M Tris–HCl pH 8.0 containing 0.02 M CaCl2 and 0.15 M NaCl) using a 2-ml tissue grinder. The homogenates were centrifuged at 9,000×g at 4°C for 15 min and the supernatants (L4 gut extracts) were collected and evaluated for protein (Lowry et al. 1951) and carbohydrate (Dubois et al. 1956) concentrations as well as for haemagglutinating and enzyme activities.
Protease activity
Protease activity was determined using azocasein (Sigma-Aldrich, USA) as substrate according to Azeez et al. (2007). L4 gut extract in Tris buffer (150 μg protein) was mixed with 300 μl of 0.1 M sodium phosphate pH 7.5 containing 50 μl of 0.6% (w/v) azocasein. The mixture was supplemented with 100 μl of 0.1% (v/v) Triton X-100 and incubated at 37°C for 3 h. The reaction was stopped by adding 200 μl of 10% (v/v) trichloroacetic acid and the assay was incubated at 4°C for 30 min. Afterwards, centrifugation (9,000 × g for 10 min) was performed and the absorbance of the supernatant at 366 nm was determined. One unit of protease activity was defined as the amount of enzyme that gave an increase of 0.01 in absorbance.
Trypsin-like activity
Trypsin activity was determined by incubating (30 min, 37°C) L4 gut extract in Tris buffer (35 μg of protein) with 8 mM N-benzoyl-dl-arginyl-p-nitroanilide (BApNA, 5 μl) in Tris–HCl 0.1 M pH 8.0 (160 μl). Trypsin activity was followed by measurement of absorbance at 405 nm (Kakade et al. 1969). One unit of trypsin activity was defined as the amount of enzyme that hydrolyzes 1 μmol BApNA/min. Substrate hydrolysis was controlled by incubating (60 min, 37°C) bovine trypsin (5 μg; Sigma-Aldrich, USA) with 8 mM BApNA (5 μl).
α-amylase activity
The assay was carried out based on the method described by Bernfeld (1955). L4 gut extract in acetate buffer (100 μl; 170 μg of protein) was incubated at 50°C for 10 min with 400 μl of a 1% (w/v) soluble starch (Merck, Germany) solution in 0.1 M sodium acetate pH 5.5 containing 0.02 M CaCl2 and 0.15 M NaCl. The reaction was stopped by adding 500 μl of 3,5-dinitrosalicylic acid (DNS). Next, the assays were heated at 100°C in boiling water for 6 min and immediately cooled on ice for 15 min. Then, absorbance was measured at 540 nm. The amount of reducing sugars was determined using a standard curve of the reaction of different glucose concentrations with DNS (Y = 0.1261X–0.0157, where Y is the absorbance at 540 nm and X is the glucose concentration in mg/ml). One unit of enzyme activity was defined as the amount of enzyme required to generate 1 μmol glucose/min. As positive control, the same procedure was carried out with 1.0 mg/ml α-amylase from hog pancreas (Sigma-Aldrich, USA). Reaction blanks were performed without starch.
Zymography for proteases
Zymography was carried out according to the method described by Garcia-Carreño et al. (1993). L4 gut extract (20 μg of protein) incubated in Tris buffer (30 min) was submitted to SDS-PAGE using a 12% (w/v) gel (Laemmli 1970) at 4°C. After electrophoresis, the gel was immersed in 2.5% Triton X-100 in 0.1 M Tris–HCl pH 8.0 to remove SDS and incubated (30 min, 4°C) with 3% casein (w/v) in 0.1 M Tris–HCl pH 8.0. Temperature was then raised to 37°C and maintained at this value for 90 min to allow the digestion of casein by L4 gut proteases. Finally, the gel was stained for protein with 0.02% (v/v) Coomassie Brilliant Blue in 10% (v/v) acetic acid and washed with destaining solution (40% methanol, 10% acetic acid, and 50% distilled water). Light bands against the dark background indicated proteolytic activity.
Effect of L4 gut extract on MuLL haemagglutinating activity
L4 gut extract in Tris buffer (150 μg of protein) was incubated with MuLL (150 μg) for 60 min as well as for 24, 48, 72, 96 and 120 h at 37°C. After each incubation period, the enzyme reaction was stopped by heating the mixtures at 100°C for 20 min. Subsequently, haemagglutinating and protease activities were evaluated. Controls of enzyme activity were performed by incubating L4 gut extract (150 μg of protein) with distilled water for the same time periods and at the same temperature as the experimental samples. Haemagglutinating activity was controlled by incubating MuLL (150 μg of protein) with Tris buffer at 37°C for the same incubation periods.
Effect of MuLL on protease, trypsin-like and α-amylase activities from L4 gut extracts
The effect of MuLL on protease activity was evaluated by incubating (30 min at 37°C) L4 gut extract in Tris buffer (150 μg of protein) with lectin (8.75–70 μg; 0.82–4.48 μM) before determination of protease activity as described above. Control assay was performed by submitting preparation of MuLL (8.75–70 μg) to the same reaction steps. The activity of L4 gut extract (20 μg of protein) incubated (20 min, 37°C) with MuLL (30 μg) was also evaluated by zymography for proteases as described above.
The activity of trypsin from L4 gut extract in Tris buffer (35 μg protein) was determined after incubation (30 min, 37°C) with MuLL (5.0–71.4 μg; 3.14–25.15 μM) in Tris buffer. Next, 4 or 8 mM BApNA (5 μl) was added and the assay was incubated for 60 min at 37°C. Inhibition curves were plotted and a Dixon plot analysis was employed to determine the constant of inhibition (Segel 1975). Dixon plots were generated using the reciprocal velocity (1/v) versus lectin concentration. Intersection of the regression lines for each BApNA concentration yielded the inhibition constant K i.
The effect of MuLL on α-amylase activity was evaluated by incubating (30 min at 27°C) L4 gut extract in acetate buffer (170 μg of protein) with MuLL (3.5–35 μg; 0.24–2.0 μM) before determination of α-amylase activity. Control assay was performed by submitting MuLL (3.5–35 μg) in acetate buffer to the same reaction steps.
Statistical analysis
Standard deviations (SD) were calculated using GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA, USA) and data were expressed as a mean of replicates ± SD. Significant differences between treatment groups were analysed by Student’s t-test (significance at p < 0.05) using Origin 6.0 program. The lethal concentrations required to kill 16% (LC16), 50% (LC50) and 84% (LC84) of larvae in 24 h were calculated by probit analysis with a reliability interval of 95% using the computer software StatPlus® 2006 (AnalystSoft, Canada).
Results and discussion
Dengue incidence has increased around the world. Some 2.5 billion people are now at risk from the disease and the expanding of A. aegypti populations may bring greater numbers of people into contact with this vector. In this scenario, the application of appropriate insecticides to larval habitats, especially those found in households, prevents mosquito breeding (World Health Organization 2009b). Plant lectins have been evaluated as new biodegradable compounds for use in A. aegypti control (Coelho et al 2009; Sá et al. 2009b).
M. urundeuva leaf extract in 0.15 M NaCl showed high protein concentration (44.0 mg/ml) and specific haemagglutinating activity of 11,915. Soluble proteins from leaf extract were precipitated using ammonium sulphate. The 60–80% precipitate showed protein concentration of 23.1 mg/ml and specific haemagglutinating activity (16,934) higher than that of leaf extract. MuLL was isolated by chromatography of 60–80% precipitate on a chitin column, according to a protocol previously described by Napoleão et al. (2011). MuLL showed specific haemagglutinating activity of 23,405, which was neutralized by asialofetuin.
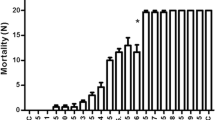
M. urundeuva leaf extract showed larvicidal activity on A. aegypti L4 (Fig. 1a), and the lethal concentrations required to kill 16% (LC16), 50% (LC50) and 84% (LC84) of larvae in 24 h were 8.1, 10.9 and 13.7 mg/ml of protein, respectively. MuLL also promoted mortality of larvae (Fig. 1b) with LC16, LC50 and LC84 of 0.140, 0.202 and 0.264 mg/ml, respectively. The purification process resulted in increment of insecticidal effect and indicates that MuLL may be the active principle of the extract.
The larvicidal activity of MuLL was lower than the larvicidal activities of MuBL (LC50 of 0.125 mg/ml) and MuHL (LC50 of 0.04 mg/ml) from M. urundeuva (Sá et al. 2009b). Additionally, an advantage of the use of MuLL is the ease to obtain the leaves and the conservation of plant integrity. The differences in larvicidal effects of the M. urundeuva lectins are probably due to their distinct physical–chemical characteristics. MuBL, MuHL and MuLL differ from each other with respect to carbohydrate content, interaction with monosaccharide and glycoproteins as well as behavior in different pH values (Napoleão et al. 2011)
A. aegypti L4 gut extract in Tris showed higher protein (3.5 mg/ml) and carbohydrate (0.3 mg/ml) contents than the extract in acetate buffer, which contained 3.4 and 0.28 mg/ml of protein and carbohydrate, respectively. Neither extract presented haemagglutinating activity. Evaluation of digestive enzyme activities from A. aegypti L4 gut showed high protease activity (540 U/mg), and zymography for proteases revealed multiple polypeptide bands (Fig. 2a, inset 1). Trypsin-like endopeptidase (0.98 U/mg) and α-amylase (0.13 U/mg) activities were revealed by detection of hydrolysis of BApNA and soluble starch, respectively. Trypsin-like and α-amylase activities have been described in midgut of A. aegypti aquatic stages and highest activities were detected in fourth stage larvae (Yang and Davies 1971; McGeachin et al. 1972; Borovsk and Meola 2004).
Effect of MuLL on A. aegypti L4 digestive enzymes. a Protease activity from L4 gut extract in the presence of MuLL; inset: zymography for proteases of L4 gut extract proteases (1) and of L4 gut extract incubated with lectin (2). Arrows indicate the polypeptides bands with reduced intensity of the lytic zone after incubation of L4 gut extract with MuLL. The 100% activity of L4 gut protease corresponded to absorbance of 1.019 ± 0.036. b Trypsin-like activity from gut extract towards 8 mM BApNA in the presence of MuLL. The 100% activity of L4 gut trypsin corresponded to absorbance of 0.235 ± 0.014. c Effect of MuLL on the activity of α-amylase from L4 gut extract
The effect of L4 gut extract in Tris buffer on MuLL haemagglutinating activity was determined for investigating whether MuLL is active after contact with gut extract. Heating of incubation mixtures neutralized protease activity from L4 gut and did not interfere in MuLL haemagglutinating activity due to its thermo-stability. Table 1 shows that MuLL without gut extract treatment showed higher haemagglutinating activity than that incubated with gut extract, and that haemagglutinating activity was reduced after incubation for 60 min, 24, 48 and 96 h and neutralized after 120 h.
The reduction of haemagglutinating activity can be due to inhibition of MuLL by carbohydrates from L4 gut extract and partial proteolysis by larval enzymes. The absence of haemagglutinating activity reveals that MuLL was inactivated only after longer incubation times. Similarly to MuLL, the insecticidal lectins from M. oleifera seeds, Talisia esculenta seeds and Bauhinia monandra leaf were resistant to digestion by insect proteases for 12, 15 and 48 h, respectively (Macedo et al. 2004, 2007; Oliveira et al. 2011).
Lectins with insecticidal activity require an appropriate level of resistance against proteolysis in the insect gut to be able to exert their effects (Coelho et al. 2007). When active in insect gut, lectin may interact with glycosylated enzymes and glycoconjugates along the digestive tract (Zhu-Salzman et al. 1998; Fitches et al. 2008). Napoleão et al. (2011) showed that haemagglutinating activities of MuBL, MuHL and MuLL were resistant to digestion by N. corniger gut preparation containing trypsin-like activity; the authors suggested that resistance of MuLL to proteolysis by termite enzymes is linked to the glycoprotein nature of lectin and stability over wide pH and temperature ranges. It has been suggested that binding of lectins to glycoconjugates in insect gut may offer protection against proteolytic activity (Zhu-Salzman et al. 1998; Lagarda-Diaz et al. 2009).
The resistance of MuLL to L4 gut proteases led us to evaluate the effect of lectin on protease, trypsin-like and amylase activities in gut. Inhibition or stimulation of these digestive enzymes may result in a metabolic imbalance, growth impairment and mortality of insect larvae (Applebaum et al. 1961; Borovsk and Meola 2004; Bhattacharyya et al. 2007; Macedo et al. 2007; Nanasahe et al. 2008; Babu and Subrahmanyam 2010).
Protease activity was significantly (p < 0.05) reduced in the presence of MuLL (Fig. 2a) with a highest inhibition by 30%. Zymography of L4 gut extract after incubation with MuLL revealed reduced intensity of the lytic zone observed for two polypeptides of approximately 14.2 and 16.9 kDa (Fig. 2a, inset 2). MuLL also showed inhibitory effects on trypsin, a serine proteinase, and calculation provided a K i value of 2.8 μM (Fig. 2b). Trypsin inhibitory activity was also reported for lectins from Annona coriacea seed, a larvicidal agent against Anagasta kuehniella (Coelho et al. 2007). Lectins are able to block enzyme by binding to the sugar moiety in case of glycosylated enzymes or by binding to other sites than the substrate binding site in case of non-glycosylated enzymes (Macedo et al. 2007).
MuLL increased activity of α-amylase from L4 gut extract (Fig. 2c). Similarly, the insecticidal lectin isolated from Bauhinia monandra leaf showed an in vitro stimulatory effect on α-amylase activity from midgut homogenates of Callosobruchus maculatus, probably by increasing affinity of the enzyme to its substrate (Macedo et al. 2007).
The results described here indicate that MuLL killed A. aegypti larvae due to resistance to proteolysis by gut enzymes and interference in enzyme activities in gut, properties common to insecticide lectins. The larvicidal activity of MuLL points out the possibility of using this lectin to control the spreading of dengue fever by impairment of the biological cycle of the viral vector.
References
Aiub CAF, Coelho ECA, Sodré E, Pinto LFR, Felzenszwalb I (2002) Genotoxic evaluation of the organophosphorous pesticide temephos. Genet Mol Res 1:159–166
Applebaum SW, Jankovic M, Birk Y (1961) Studies on the midgut amylase activity of Tenebrio molitor L. larvae. J Insect Physiol 7:100–108
Araújo AP, Melo-Santos MAV, Carlos SO, Rios EMMM, Regis L (2007) Evaluation of an experimental product based on Bacillus thuringiensis sorovar. israelensis against Aedes aegypti larvae (Diptera:Culicidae). Biol Control 41:339–347
Autran ES, Neves IA, da Silva CSB, Santos GKN, da Câmara CAG, Navarro DMAF (2009) Chemical composition, oviposition deterrent and larvicidal activities against Aedes aegypti of essential oils from Piper marginatum Jacq. (Piperaceae). Bioresour Technol 100:2284–2288
Azeez A, Sane AP, Bhatnagar D, Nath P (2007) Enhanced expression of serine proteases during floral senescence in Gladiolus. Phytochemistry 68:1352–1357
Babu SR, Subrahmanyam B (2010) Bio-potency of serine proteinase inhibitors from Acacia senegal seeds on digestive proteinases, larval growth and development of Helicoverpa armigera (Hübner). Pest Biochem Physiol 98:349–358
Bagavan A, Rahuman AA, Kamaraj C, Geetha K (2008) Larvicidal activity of saponin from Achyranthes aspera against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 103:223–229
Bernfeld P (1955) Amylases, α and β. Methods Enzymol 1:149–158
Bhattacharyya A, Leighton SM, Babu CR (2007) Bioinsecticidal activity of Archidendron ellipticum trypsin inhibitor on growth and serine digestive enzymes during larval development of Spodoptera litura. Comp Biochem Physiol C 145:669–677
Bing DH, Weyand JG, Stavinsky AB (1967) Hemagglutination with aldehyde-fixed erythrocytes for assay of antigens and antibodies. Proc Soc Exp Biol Med 124:1166–1170
Borovsk D, Meola SM (2004) Biochemical and cytoimmunological evidence for the control of Aedes aegypti larval trypsin with Aea-TMOF. Arch Insect Biochem Phys 55:24–139
Carlini CR, Grossi-de-Sá MF (2002) Plant toxic proteins with insecticidal properties. A review on their potential as bioinsecticides. Toxicon 40:1515–1539
Coelho MB, Marangoni S, Macedo MLR (2007) Insecticidal action of Annona coriacea lectin against the flour moth Anagasta kuehniella and the rice moth Corcyra cephalonica (Lepidoptera: Pyralidae). Comp Biochem Physiol C 146:406–414
Coelho JS, Santos NDL, Napoleão TH, Gomes FS, Ferreira RS, Zingali RB, Coelho LCBB, Leite SP, Navarro DMAF, Paiva PMG (2009) Effect of Moringa oleifera lectin on development and mortality of Aedes aegypti. Chemosphere 77:934–938
Correia MTS, Coelho LCBB, Paiva PMG (2008) Lectins, carbohydrate recognition molecules: are they toxic? In: Siddique YH (ed) Recent trends in toxicology, vol. 37. Transworld Research Network, Kerala, pp 47–59
Czapla TH, Lang BA (1990) Effect of plant lectins on the larval development of European corn borer (Lepidoptera: Pyralidae) and southern corn rootworm (Coleoptera: Chrysomelidae). J Econ Entomol 83:2480–2485
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugar and related substances. Anal Chem 28:350–356
Eisemann CH, Donaldson RA, Pearson RD, Cadogan LC, Vacuolo T, Tellman RL (1994) Larvicidal activity of lectins on Lucilia cuprina: mechanism of action. Entomol Exp Appl 72:1–11
Fitches E, Philip J, Hinchliffe G, Vercruysse L, Chougule N, Gatehouse JA (2008) An evaluation of garlic lectin as an alternative carrier domain for insecticidal fusion proteins. Insect Sci 15:483–495
García-Carreño FL, Dimes LE, Haard NF (1993) Substrate gel electrophoresis for composition and molecular weight of proteinases and proteinaceous proteinase inhibitors. Anal Biochem 214:65–69
Kakade ML, Simons N, Liener IE (1969) An evaluation of natural vs. synthetic substrates for measuring the antitryptic activity of soybean samples. Cereal Chem 46:518–526
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lagarda-Diaz I, Guzman-Partida AM, Urbano-Hernandez G, Ortega-Nieblas MM, Robles-Burgeño MR, Winzerling J, Vazquez-Moreno L (2009) Insecticidal action of PF2 lectin from Olneya tesota (Palo Fierro) against Zabrotes subfasciatus larvae and midgut glycoconjugate binding. J Agric Food Chem 57:689–694
Lam SK, Ng TB (2011) Lectins: production and practical applications. Appl Microbiol Biotechnol 89:45–55
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Macedo MLR, Damico DCS, Freire MGM, Toyama MH, Marangoni S, Novello JC (2003) Purification and characterization of an N-acetylglucosamine-binding lectin from Koelreuteria paniculata seeds and its effect on the larval development of Callosobruchus maculatus (Coleoptera: Bruchidae) and Anagasta kuehniella (Lepidoptera: Pyralidae). J Agric Food Chem 51:2980–2986
Macedo MLR, Castro MM, Freire MGM (2004) Mechanisms of the insecticidal action of TEL (Talisia esculenta lectin) against Callosobruchus maculatus (Coleoptera: Bruchidae). Arch Insect Biochem Physiol 56:84–96
Macedo MLR, Freire MGM, Silva MBR, Coelho LCBB (2007) Insecticidal action of Bauhinia monandra leaf lectin (BmoLL) against Anagasta kuehniella (Lepidoptera: Pyralidae), Zabrotes subfasciatus and Callosobruchus maculatus (Coleoptera: Bruchidae). Comp Biochem Physiol A 146:486–498
McGeachin RL, Willis TG, Roulston EF, Lang CA (1972) Variation in alpha-amylase during the life span of the mosquito. Comp Biochem Physiol B 43:185–191
Melo-Santos MAV, Varjal-Melo JJM, Araújo AP, Gomes TCS, Paiva MHS, Regis LN, Furtado AF, Magalhães T, Macoris MLG, Andrighetti MTM, Ayres CFJ (2010) Resistance to the organophosphate temephos: mechanisms, evolution and reversion in an Aedes aegypti laboratory strain from Brazil. Acta Trop 113:180–189
Monath TP (2008) Treatment of yellow fever. Antiviral Res 78:116–124
Nanasahe PC, Doyle E, Fitches E, Gatehouse JA (2008) Biochemical characterization of midgut digestive proteases from Mamestra brassicae (cabbage moth; Lepidoptera: Noctuidae) and effect of soybean Kunitz inhibitor (SKTI) in feeding assays. J Insect Physiol 54:563–572
Napoleão TH, Gomes FS, Lima TA, Santos NDL, Sá RA, Albuquerque AC, Coelho LCBB, Paiva PMG (2011) Termiticidal activity of lectins from Myracrodruon urundeuva against Nasutitermes corniger and its mechanisms. Int Biodeter Biodegr 65:52–59
Navarro DMAF, Oliveira PES, Potting RJP, Brito AC, Fital SJF, Sant’Ana AEG (2003) The potential attractant or repellent effects of different water types on oviposition in Aedes aegypti L. (Dipt., Culicidae). J Appl Entomol 127:46–50
Oliveira CFR, Luz LA, Paiva PMG, Coelho LCBB, Marangoni S, Macedo MLR (2011) Evaluation of seed coagulant Moringa oleifera lectin (cMoL) as a bioinsecticidal tool with potential for the control of insects. Process Biochem 46:498–504
Poupardin R, Reynaud S, Strode C, Ranson H, Vontas J, David JP (2008) Cross-induction of detoxification genes by environmental xenobiotics and insecticides in the mosquito Aedes aegypti: impact on larval tolerance to chemical insecticides. Insect Biochem Mol Biol 38:540–551
Rajkumar S, Jebanesan A (2008) Bioactivity of flavonoid compounds from Poncirus trifoliate L. (Family: Rutaceae) against the dengue vector, Aedes aegypti L. (Diptera: Culicidae). Parasitol Res 104:19–25
Sá RA, Napoleão TH, Santos NDL, Gomes FS, Albuquerque AC, Xavier HS, Coelho LCBB, Bieber LW, Paiva PMG (2008) Induction of mortality on Nasutitermes corniger (Isoptera, Termitidae) by Myracrodruon urundeuva heartwood lectin. Int Biodeter Biodegr 62:460–464
Sá RA, Argolo ACC, Napoleão TH, Gomes FS, Santos NDL, Melo CML, Albuquerque AC, Xavier HS, Coelho LCBB, Bieber LW, Paiva PMG (2009a) Antioxidant, Fusarium growth inhibition and Nasutitermes corniger repellent activities of secondary metabolites from Myracrodruon urundeuva heartwood. Int Biodeter Biodegr 63:470–477
Sá RA, Santos NDL, da Silva CSB, Napoleão TH, Gomes FS, Cavada BS, Coelho LCBB, Navarro DMAF, Bieber LW, Paiva PMG (2009b) Larvicidal activity of Myracrodruon urundeuva lectins on Aedes aegypti. Comp Biochem Physiol C 149:300–306
Segel IH (1975) Enzyme kinetics: Behavior and analysis of rapid equilibrium and steady state enzyme systems. Wiley-Interscience, New York, 984
Souza SMC, Aquino LCM, Milach AC Jr, Bandeira MAM, Nobre MEP, Viana GSB (2006) Antiinflammatory and antiulcer properties of tannins from Myracrodruon urundeuva Allemão (Anacardiaceae) in rodents. Phytother Res 21:220–225
Tauil PL (2002) Aspectos clínicos do controle do dengue no Brasil. Cad Saude Publica 18:867–871
Tellam RL, Wijffels G, Wiladsen P (1999) Peritrophic matrix proteins. Insect Biochem Mol Biol 29:87–101
Viana GSB, Bandeira MAM, Matos FJA (2003) Analgesic and antiinflammatory effects of chalcones isolated from Myracrodruon urundeuva Allemão. Phytomedicine 10:189–195
World Health Organization (1981) Instructions for determining the susceptibility or resistance of mosquito larvae to insecticides. WHO/VBC/81.807, pp. 1–6
World Health Organization (2009a) Yellow Fever. Fact sheet 100
World Health Organization (2009b) Dengue and dengue haemorrhagic fever. Fact sheet 117
Yang YJ, Davies DM (1971) Trypsin and chymotrypsin during metamorphosis in Aedes aegypti and properties of the chymotrypsin. J Insect Physiol 17:117–131
Zhu-Salzman K, Shade RE, Koiwa H, Salzman RA, Narasimhan M, Bressan RA, Hasegawa PM, Murdock LL (1998) Carbohydrate binding and resistance to proteolysis control insecticidal activity of Griffonia simplicifolia lectin II. Proc Natl Acad Sci USA 95:15123–15128
Acknowledgments
This study was supported by research grants and fellowships (LCBBC, PMGP) from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and financial support from the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We thank Maria Barbosa Reis da Silva for technical assistance and Felix Nonnenmacher for English editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Napoleão, T.H., Pontual, E.V., de Albuquerque Lima, T. et al. Effect of Myracrodruon urundeuva leaf lectin on survival and digestive enzymes of Aedes aegypti larvae. Parasitol Res 110, 609–616 (2012). https://doi.org/10.1007/s00436-011-2529-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2529-7