Abstract
Clinical, epidemiological, and pathological aspects of trypanosomiasis caused by Trypanosoma vivax in calves were reported for the first time in northeast Brazil. Clinical and epidemiological data, packed cell volumes (PCV), and parasitemia were assessed in 150 calves in May 2009 (rainy season—survey 1) and in 153 calves in November 2009 (dry season—survey 2) in three farms (A, B, and C). Prevalence of T. vivax in calves examined in the survey 1 was 63.3%, 65.0%, and 80.0% in farms A, B, and C, respectively. Morbidity varied from 63.3% to 80%, mortality from 15% to 30% and lethality from 23% to 37.5%. In survey 1, for all farms, high parasitemia (from 30.3 to 26.2 × 106 parasites/mL), fever (from 39.8 to 40.3°C), low PCV (from 15.7% to 18.1%), and body score (from 2.5 to 3.5) were detected. Calves showed depression, weight loss, pale mucous membranes, enlarged lymph nodes, edema of the dewlap, cough, coryza, and diarrhea. The animals from farms A and B were treated with diminazene aceturate. Six months after, in survey 2, non-treated calves from farm C showed values for prevalence (81.82), morbidity (81.82), mortality (12.73), and lethality (15.55) similar to those in survey 1 (P > 0.05). Also in survey 2, four calves aging merely 1–3 days old presented high parasitemia levels (from 32 × 106 to 74 × 106 parasites/mL), suggesting transplacental transmission. In conclusion, trypanosomiasis by T. vivax constitutes high prevalent disease for calves raised in Brazilian semiarid and may have transplacental transmission.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the trypanosomes that infect livestock, Trypanosoma vivax is one the most important pathogenic species for bovines and small ruminants. This parasite restricts the animal production, besides causing economic losses by the clinical signs of the infection such as restricted growth, abortion, anemia, treatment cost, and death of the affected animals (Jones and Dávila 2001; Desquesnes 2004; Gutierrez et al. 2006; Batista et al. 2006, 2008, 2009).
In Africa, T. vivax produces strong limitations to the ruminant production and can be transmitted by the biological vector, tsetse flies (Glossina spp.), or mechanically by other haematophagous flies. In Latin America, these flies do not exist, so the parasite had acquired another behavior to survive: the mechanical transmission by haematophagous Diptera such as Stomoxys sp. and Tabanus sp. (Jones and Dávila 2001; Desquesnes 2004).
T. vivax was introduced into Latin America with cattle imported from Africa, probably in the late nineteenth century, and nowadays is a widespread parasite in Central and South America (Jones and Dávila 2001; Cortez et al. 2006, 2009). The transportation of cattle herds had been the mean of dispersion for T. vivax related to many countries such as French Guyana, Colombia, Panama, Bolivia, Venezuela, and Costa Rica (Silva et al. 1998; Desquesnes 2004; García et al. 2006; Oliveira et al. 2009). In Brazil, T. vivax was initially reported in Pará State, where the parasite was detected in a water buffalo showing fever and emaciation (Shaw and Lainson 1972). Ever since, T. vivax has already been related in cattle herds of several Brazilian states from northern to southern regions (Serra Freire 1981; Silva et al. 1996, 2009; Paiva et al. 2000a; Linhares et al. 2006a, b; Guerra et al. 2008; Batista et al. 2007, 2009; Cuglovici et al. 2010).
In previous outbreaks reported in Brazilian semiarid, cattle, sheep, and goats showed characteristic clinical condition in the acute phase: high parasitemia associated with fever, weakness, lethargy, and a decrease in productivity caused by weight loss, drop in milk production, abortion, and perinatal mortality (Batista et al. 2007, 2009). In contrast to those severe outbreaks caused by T. vivax, in the Brazilian endemic regions such as Pantanal (central region) and Amazonia (northern region), infection is generally cryptic and asymptomatic. Apparently, abortion and neonate infections caused by T. vivax are not important in the endemic regions (Osório et al. 2008; Paiva et al. 2000a).
Data suggested that the Brazilian semiarid region is non-endemic for trypanosomiasis, probably because the environmental conditions (long dry periods and high temperatures) are not favorable for the vector development during most parts of the year. Thus, the bovines do not develop active immunity, and when the population of the mechanical vectors increases in the rainy season, outbreaks occur, causing high parasitemia and hence mortality and economic losses (Batista et al. 2007, 2008).
Neonate disease and mortality and abortion are the most important reasons for economic losses in livestock productivity. T. vivax is considered a major cause of reproductive failure in African cattle and goat. In general, some trypanotolerant breeds have normal gestation and parturition when infected with T. vivax. However, susceptible breeds of cattle and goat infected with African T. vivax have a high probability to abort or premature delivery in comparison to uninfected animals. Transplacental T. vivax transmission was proved by parasite detection in fetuses and neonates. After born, calves from infected animals often present low weights and premature death in Africa (Ogwu et al. 1986; Okech et al. 1996a, b).
In Brazil, the effects of trypanosomiasis caused by T. vivax in pregnancy influencing the neonates' health were not specifically related. This study aimed to relate the clinical and pathological signs of the natural infection by T. vivax in calves, as well as to evaluate some epidemiological aspects such as prevalence, mortality, and lethality rates in infected calves from a region in Brazilian semiarid where severe outbreaks due to T. vivax have been reported before.
Material and methods
Studied area and outbreaks period
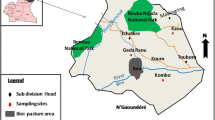
The study was performed in three farms where we previously reported trypanosomiasis by T. vivax outbreaks in dairy cattle (Batista et al. 2007, 2008). We examined three farms (A, B, and C) of cattle in the municipality of Belém do Brejo do Cruz in Paraíba State (northeastern Brazil) located at 6°20′38″ west latitude and at 37°44′48″ south longitude, respectively. According to the Köppen climate classification, the area is classified as hot and has semiarid climate (Bsh) with average annual rainfall of ∼500 mm, average annual temperature of 27°C, and average annual relative humidity of 50%. There are two established seasons in the year: a 3–5-month rainy season (generally between February and May), regionally known as winter, and a long dry season, known as summer, that lasts from 7 to 9 months (generally between June and January). Investigation was carried out in randomly selected animals of farms A, B, and C during the rainy season, in May 2009 (survey 1) and during the dry season, in November 2009 (survey 2).
Experimental animals
The investigated bovines in all farms were characterized by mixed breeding (Holstein and Brown Swiss). Twice in a day, the calves were gathered with their mothers in a collective corral to be fed with sorghum silage and concentrate. In survey 1, from a total of 300 calves (120 in farm A, 80 in farm B, and 100 in farm C), and in survey 2, from a total of 306 (104 in farm A, 92 in farm B, and 110 in farm C), 50% of calves were randomly chosen to be assessed. The animals were selected regarding the sex, weight, and body condition score as described before (Freitas Júnior et al. 2008).
Clinical exams, blood samples collection, and T. vivax diagnosis
Clinical data and blood samples from the calves were collected once in survey 1 and once in survey 2. The selected animals were examined to assess rectal temperature, and status of mucous membrane and external lymph nodes. The body score was determined using a scale of 0 for very thin to 5 for fat animals.
Blood samples were collected by jugular vein puncture, and the diagnosis of trypanosomiasis was performed by examination of Giemsa-stained smears of leukocyte layers in the buffy coat technique (BCT). In survey 1, we also examined by BCT 36 cows from farm 1, 62 cows from farm 2, and 60 cows from farm 3. Packed cell volume (PCV) values and parasitemia were determined as reported previously (Batista et al. 2006). The parasitological diagnosis of animals was confirmed using a T. vivax-specific polymerase chain reaction (PCR) assay as described before (Rodrigues et al. 2010).
Treatment of the infected calves using diminazene aceturate
Immediately after the confirmation of the T. vivax diagnosis on survey 1, the calves from farms A and B, proven infected by T. vivax, were treated with 5 mg/kg diminazene aceturate via intramuscular. The animals from farm C did not receive any treatment. The infection control was evaluated by clinical examinations and by the BCT diagnosis, as previously described by Batista et al. (2007).
Epidemiological aspects of T. vivax infection
Data about disease history, hygiene practices, management of newborn calves, number of calves born alive or dead, and the possible risk factors for the occurrence of trypanosomiasis by T. vivax were collected from each farm. We also established the prevalence, morbidity, mortality, and lethality coefficients for the calves.
Necropsy examination
Five naturally dead calves previously diagnosed as positive for T. vivax by BCT were submitted to a necropsy examination. Fragments from organs by thoracic and abdominal cavities and central nervous system were collected for histopathological exams. Tissue samples were fixed using 10% buffered formalin and then embedded in paraffin. Sections of 5.0 μm were cut using a microtome, and the tissue samples were stained using classical hematoxylin–eosin method (Bancroft and Cook 1984).
Diagnosis of T. vivax infection through PCR was performed in samples from the cardiac muscle for three animals that showed severe histological lesions, according to methodology described by Bezerra et al. (2008).
Statistical analysis
The statistical analysis of prevalence, morbidity, mortality, and lethality coefficients was made as described by Thrusfield (2004). Tukey's test was used to compare data of parasitemia, PCV, temperature, and body score from each observation time using the SAS statistical software package. Results were considered to be significant at the P < 0.05 level.
Results
Diagnosis and clinical signs of calves infected with T. vivax
The calves positive for T. vivax by BCT showed clinical signs of depression, weight loss, pale mucous membranes, enlarged lymph nodes, edema of the dewlap, cough, coryza, diarrhea, and low body score. In hematological examinations, we detected a high number of trypanosomes in the BCT. Calves were confirmed as infected with T. vivax by the PCR amplification of a fragment of cathepsin L-like gene specific for this species. The animals with highest number of trypanosomes found in peripheral blood also presented the highest rectal temperature. In parasitological negative animals, the PCV values was higher than those that had parasites detected by BCT. The PCV decreased with the increasing parasitemia. The average PCV values of the parasitemic calves were lower than the limit of normality for bovines (Table 1).
In survey 1, 9 T. vivax-infected calves from farm A, 6 from farm B, and 15 from farm C presented a worsening. The acute clinical signs observed were muscle weakness, ataxia, dyspnea, and external decubitus, culminating with spontaneous death after 6 days (Table 2).
In survey 2, 6 months after the outbreak beginning, calves from farms A and B showed no parasitemia, normal temperature, and PCV values since they were treated after diagnosis performed in survey 1. After treatment, the calves presented a gradual recovering on corporal weight to a medium body score (Table 1). For calves raised in farm C, and hence, non-treated, symptoms and pathogenic features did not differ (P > 0.05) statistically between the two surveys.
In farm C, calves born after survey 1 and aging up to 3 months presented high parasitemia associated with an increased rate of the rectal temperature and low values of PCV as shown in Table 1. It is important to emphasize that among them, a 1-day-old calf and three 3-day-old calves presented a very high parasitemia level ranging from 32 × 106 up to 74 × 106 parasites/mL On the other hand, the weaned calves aging 4 to 5 months presented normal rectal temperature, in spite of the low body score, anemia, and very low parasitemia, in many cases only detectable by PCR. Seven calves with low PCV, body score, and parasitemia presented a critical infection culminating with death, similar to the observed in survey 1.
T. vivax detection in dairy cattle
T. vivax infection was assessed in 158 dairy cows and diagnosed by BCT in animals of all the three farms. In the first survey carried out during the rainy season, overall prevalence in farms A, B, and C was 41.6%, 13.3%, and 46.6%, respectively. Despite high overall prevalence of 33.8%, in the same period, mortality of cows never exceeded 6.5%.
Epidemiological aspects
The introduction of animals from herds where T. vivax had been previously reported was probably the main factor leading to new outbreaks in nearby farms. An increase in the rain frequency in survey 1 favored the increase in the population of vector insects as tabanids and Stomoxys sp., thus facilitating mechanical transmission and justifying all outbreaks that occurred in the rainy season. In addition, we did not observe the adoption of any specific control procedures for the vectors during all the investigation. In general, calf management in all farms consisted basically animal identification, umbilicus disinfection, anthelmintic treatment, and vaccination against aftosa fever, rabies, brucellosis, and clostridiosis.
In survey 1, which was carried out in rainy season during the outbreaks, the prevalence of T. vivax infection in calves examined in farms A, B, and C was 63.3%, 65.0%, and 80.0%, respectively. The morbidity varied from 63.3% to 80.0%, whereas the mortality varied from 15% to 30% and the lethality from 23.1% to 37.5%.
After 6 months, during the dry season, we reported survey 2 in farm C. In this case we did not verify for calves of farm C, a significant change in the values for prevalence of T. vivax infection determined by BCT, morbidity, mortality, and lethality like we did obtain in survey 1 (Table 3).
Anatomopathological and histopathological studies
The most common anatomopathological findings in the five T. vivax-infected and naturally dead calves submitted for necropsy were pale carcass, enlarged lymph nodes, fat atrophy, ascites, hydrothorax, hydropericardium, visceral congestion especially in the liver and lung, splenomegaly, ecchymosis, and petechial hemorrhages on epicardium.
Histopathological exam revealed hyperplasia on lymphoid follicles of spleen and lymph nodes with a high plasmoblast number (Fig. 1). A chronic multifocal interstitial nephritis associated with vacuolized hepatocytes in centrolobular liver was detected in two calves. A severe myocarditis was found in three calves and it was characterized by a high lymphocyte and plasmocyte number with macrophage foci in the interstitium (Fig. 2). Other evaluated organs were normal. All samples of the heart muscle tested by T. vivax using PCR assay based on cathepsin L-like gene were positive.
Discussion
The trypanosomiasis by T. vivax is a disease of African origin that can cause deaths and economic losses in livestock in Brazil and has been recently described in cattle, sheep, and goats in the semiarid region (Batista et al. 2007, 2008, 2009). Cattle mortality is often reported in the region where we previously described T. vivax outbreaks in bovines (Batista et al. 2007, 2008). In spite of being an emergent disease in Brazil, the most part of the farmers is not capable to recognize the clinical and pathological signs of T. vivax infection. Oftentimes, the mortality of calves is empirically attributed to intestinal parasites and nutritional deficiencies by the farmers. In fact the region has been described as enzootic to T. vivax and cattle, sheep, and goat suffer mild to severe infections (Batista et al. 2008, 2009). Abortion rates and the mortality of offspring up to 3 months are highest during rainy season. Then, we hypothesize that there is a higher mortality level than the actual records for calves at this region caused by this parasite, and probably, data have not been recorded by the absence of the specific diagnosis and also of the knowledge of the disease.
To our knowledge, this is the first report of the epidemiological situation of natural T. vivax infection in calves raised at the state of Paraíba, northeast Brazil. The most important clinical signs found in T. vivax-infected calves were apathy, lethargy, weight loss, pale mucous membranes, enlarged lymph nodes, and low body score. These clinical signs associated with high rectal temperatures and low PCV in the high parasitemic animals allow us to suggest that the animals with positive diagnosis in the rainy season developed acute disease. This clinical condition of T. vivax-infected animals has been associated to recent parasite entry in areas of enzootic instability, where the animals did not establish a previous contact with the parasite and, hence, do not develop a protective immunity against T. vivax infections (Crowe et al. 1983; Desquesnes 2004).
A higher prevalence (63.3–80%) of T. vivax infection in calves in survey 1 in comparison to the indexes for cows (13.3–46.6%) was detected, which suggests that the cryptic chronic infections of more resistant cows were not detectable by BCT. The high prevalence of infected calves is probably associated to two forms of transmission: transplacental and mechanical transmission through multiple bites of haematophagous Diptera in rainy season. Also, the mortality rate of T. vivax-infected calves ranged between 15% and 30%. In the same period, mortality of adult cows never exceeded 6.5%. In every farm during survey 1, the prevalence of trypanosomiasis and levels of parasitemia in calves were higher than those previously demonstrated by our team in dairy cows raised at the same Brazilian region (Batista et al. 2008).
In spite of the habitual relates of calf mortality in the herds infected by T. vivax, there is a lack of studies regarding the real consequences of the parasite infection in young animals. Our findings have demonstrated that calves are more susceptible to trypanosomiasis by T. vivax, showing more accentuated clinical manifestations and higher morbidity and mortality than adult cows of the same herds. Trypanosome infection increased calf mortality significantly especially up to 3 months. Therefore, it is clear that the animal age affects the prevalence and severity of T. vivax infection.
The high parasitemia in four neonate calves up to 3 days old and born by chronically infected cows in the farm C in survey 2 strongly suggests the transplacental transmission. We highlight that the prepatent period, known as the period between infection of the host and the earliest time at which the parasite can be detected, ranges between 4 and 7 days for T. vivax infection in bovine (Stephen 1986; Bezerra and Batista 2008). Thus, for the reported neonates, there was no time enough for a mechanical transmission, which in fact, it was dramatically reduced since the period when survey 2 occurrence was dry with high temperatures. These environmental characteristics did not favor the development of haematophagous vectors.
Recent studies involving the histopathologic lesions and isolation of protozoans by organs, tissues, and liquids from fetuses have confirmed the role played by these microorganisms in the transplacental transmission of many domestic animals species (Peters et al. 2000; Williams et al. 2005; Razmi et al. 2007; Dubey 2009; Chryssafidis et al. 2011; Wiengcharoen et al. 2011). The congenital transmission of T. vivax in Brazilian cattle still requires confirmatory studies, but other studies confirmed the transplacental transmission of the parasite in Africa and South America (Losos and Ikede 1972; Ogwu et al. 1986; Mendéndez and Willian 1993). The maternal infection by T. vivax can affect the growing and the maturity of infected fetuses, what can culminate with a premature delivery, perinatal losses, and a low weight of the neonate (Okech et al. 1996a). In the present study, weak calves were born from infected cows. Probably the severity of clinical signs of trypanosomiasis in calves aging up to 3 months is related to their infection vulnerability caused by the immature immunological system. Therefore, it is clear that the animal age affects the prevalence of T. vivax infection.
The morbidity and mortality in calves are directly involved with the economic losses in livestock, because these epidemiologic indicators are directly associated with the farm productivity (Schmidek et al. 2004). In Brazilian northeast, a value between 3.6% and 12% for calf mortality is considered normal. Therefore, mortality rates between 15% and 30% found for our team are above the normal ones. As consequence, T. vivax infection represents negative economic impact by the reduced PCV levels, depressed weight gains, and higher calf mortality and could be a limitation to dairy cattle production.
The high lethality showed in calves infected by T. vivax in survey 1 is generally associated with severe anemia, the most frequent hematological finding of natural or experimental infection by T. vivax (Gardiner et al. 1989; Espinosa et al. 2000; Silva and Dávila 2001; Cortez et al. 2006; Bezerra et al. 2008; Batista et al. 2007, 2009; Chamond et al. 2010).
The extension of the tissue damage caused by T. vivax infection was evaluated by anatomopathological and histopathological study. Lesions observed in lymph nodes, spleen, liver, kidney, and heart corroborated that the trypanosomiasis caused by T. vivax is an infection with systemic consequences (Paiva et al. 2000b; Batista et al. 2006, 2008). Some studies also related renal and hepatic damages in trypanosomiasis by T. vivax (Paiva et al. 2000b; Batista et al. 2006, 2008; Chamond et al. 2010).
The multifocal mononuclear myocarditis found for naturally infected calves was similar to that we described before in sheep experimentally infected with a strain of T. vivax isolated from a bovine in the first outbreak in the Brazilian semiarid (Batista et al. 2006). Apathy and lethargy are generally associated with cardiac lesions caused by parasites, and the serious myocardial injuries probably generated deterioration on heart function, which can contribute to the high lethality rates observed in the acute phase of the infection. The presence of T. vivax in the cardiac tissue, confirmed in previous study by PCR, corroborated the extravascular migration of the parasite and suggest that it may be directly associated with cardiac lesions, contributing to the histopathological lesions reported in cattle and mice infected with T. vivax (Masake 1980; Kimeto et al. 1990; Chamond et al. 2010).
The control of the disease was performed by treatment with diminazene aceturate in farms A and B. The success of treatment was confirmed by BCT, normal temperature and PCV, and the recovery of body score of the calves. The infection by T. vivax, if not treated, can advance to the chronic form of the disease. This fact plays an important role to the epidemiology of the disease, since the animals remain as asymptomatic carriers, consisting in an important source of infection to the rest of the herd. That condition can be worse in the rainy season, when the number of haematophagous flies increases. In areas where there is no cyclic transmission of T. vivax by tsetse flies, the early treatment of infected herds promotes the fast stop in the mechanical transmission (Stephen 1986). Otherwise, it is important to emphasize that the treatment of all the animals in the herd is unnecessary because this procedure is expensive and inefficient for the control of the disease. Moreover, the large use of trypanocidal drugs can induce resistance, and drug administration should be restricted only to infected animals showing clinical disturbances (Vargas and Arellano 1997). T. vivax in Brazilian semiarid is already widespread in herds of cattle, goat, and sheep occurring, apparently, most times as cryptic or oligosymptomatic infection (Batista et al. 2009).
In conclusion, trypanosomiasis by T. vivax constitutes a high prevalent and important health problem to both calves and cows raised in the Brazilian semiarid and may have transplacental transmission. Clinical signs have varied with the age of calves, and more severe disease was found in calves aging up to 3 months, while the weaned calves presented milder infections despite anemia and low body score, similar to those observed in dairy cows (Batista et al. 2008). In addition, further studies should be performed to confirm the transplacental transmission in Brazilian calves.
References
Bancroft JD, Cook HC (1984) Manual of histological techniques. Churchill Livingstone, Edinburgh
Batista JS, Correa FR, Barbosa RC, Guerra JL (2006) Experimental infection by Trypanosoma vivax in sheep. Pesqui Vet Bras 26:31–37
Batista JS, Riet-Correa F, Teixeira MMG, Madruga CR, Simões SDV, Maia TF (2007) Trypanosomosis by Trypanosoma vivax in cattle in the Brazilian semiarid: description of an outbreak and lesions in the nervous system. Vet Parasitol 143:174–181
Batista JS, Bezerra FSB, Lira RA, Carvalho JRG, Rosado Neto AM, Petri AA, Teixeira MMG (2008) Clinical, epidemiological and pathological signs of natural infection in cattle by Trypanosoma vivax in Paraiba, Brazil. Pesqui Vet Bras 28:63–69
Batista JS, Oliveira AF, Rodrigues CMF, Damasceno CAR, Oliveira IRS, Alves HM, Paiva ES, Brito PD, Medeiros JMF, Rodrigues AC, Teixeira MMG (2009) Infection by Trypanosoma vivax in goats and sheep in the Brazilian semiarid region: from acute disease outbreak to chronic cryptic infection. Vet Parasitol 165:131–135
Bezerra FSB, Batista JS (2008) Effects of Trypanosoma vivax on reproduction—a review. Acta Vet Bras 2:61–66
Bezerra FSB, García HA, Alves HM, Oliveira IRS, Silva AE, Teixeira MMG, Batista JS (2008) Trypanosoma vivax in testicular and epidydimal tissues of experimentally infected sheep. Pesqui Vet Bras 28:575–582
Chamond N, Cosson A, Blom-Potar MC, Jouvion G, D'Archivio S, Medina M, Droin-Bergère S, Huerre M, Goyard S, Minoprio P (2010) Trypanosoma vivax infections: pushing ahead with mouse models for the study of Nagana. I. Parasitological, hematological and pathological parameters. PLoS Negl Trop Dis 4:1–10
Chryssafidis AL, Soares RM, Rodrigues AAR, Carvalho NAT, Gennari SM (2011) Evidence of congenital transmission of Neospora caninum in naturally infected water buffalo (Bubalus bubalis) fetus from Brazil. Parasitol Res 108:741–743
Cortez AP, Ventura RM, Rodrigues AC, Batista JS, Paiva F, Añez N, Machado RZ, Gibson WC, Teixeira MMG (2006) The taxonomic and phylogenetic relationships of Trypanosoma vivax from South América and Africa. Parasitology 133:159–169
Cortez AP, Rodrigues AC, Garcia HA, Neves L, Batista JS, Bengaly Z, Paiva F, Teixeira MM (2009) Cathepsin L-like genes of Trypanosoma vivax from Africa and South America—characterisation, relationships and diagnostic implications. Mol Cell Probes 23:44–51
Crowe JS, Barry JD, Luckins AG, Ross CA, Vickernun K (1983) All metacyclic variable antigen types of Trypanosoma congolense identified using monoclonal antibodies. Nature 306:389–391
Cuglovici DA, Bartholomeu DC, Reis-Cunha JL, Carvalho AU, Ribeiro MF (2010) Epidemiologic aspects of an outbreak of Trypanosoma vivax in a dairy cattle herd in Minas Gerais state, Brazil. Vet Parasitol 169:320–326
Desquesnes M (2004) Livestock trypanosomoses and their vectors in Latin America. OIE, Paris
Dubey JP (2009) Toxoplasmosis in pigs—the last 20 years. Vet Parasitol 164:89–103
Espinosa E, Sandoval E, Mavare M, Gonzalez N, Rangel L (2000) Comparación de la serie eritrocítica y leucocítica en ovejas y cabras infectadas con Trypanosoma vivax. Vet Trop 1:29–39
Freitas Júnior JE, Rocha Júnior VR, RennóFP MelloMTP, Carvalho AP, Caldeira LA (2008) Efeito da condição corporal ao parto sobre o desempenho produtivo de vacas mestiças Holandês × Zebu. Rev Bras Zootec 37:116–121
García H, García ME, Pérez G, Bethencourt A, Zerpa E, Pérez H, Mendoza-León A (2006) Trypanosomiasis in Venezuelan water buffaloes: association of packed-cell volumes with seroprevalence and current trypanosome infection. Ann Trop Med Parasitol 100:297–305
Gardiner PR, Assoku RKG, Whitelaw DD, Murray M (1989) Haemorrhagic lesions resulting from Trypanosoma vivax infection in Ayrshire cattle. Vet Parasitol 31:187–197
Guerra RMSNC, Feitosa Júnior AB, Santos HP, Abreu-Silva AL, Santos ACG (2008) Biometry of Trypanosoma vivax found in a calf in the state of Maranhão, Brazil. Cienc Rural 38:833–835
Gutierrez C, Corbera JA, Morales M, Büscher P (2006) Trypanosomosis in goats: currents status. Ann N Y Acad Sci 108:300–310
Jones TW, Dávila AMR (2001) Trypanosoma vivax out of Africa. Trends Parasitol 17:99–101
Kimeto BA, Mugera GM, Nyaga PN (1990) Haemorrhagic pancarditis in cattle infected with Trypanosoma vivax. Vet Parasitol 34:295–301
Linhares GFC, Dias-Filho FC, Fernandes PR, Duarte SC (2006) Bovine trypanosomiasis in the municipality of Formoso do Araguaia County, Tocantins, Brazil (case report). Cienc Anim 7:455–460
Losos GJ, Ikede BO (1972) Review of pathology of diseases in domestic and laboratory animals caused by Trypanosoma congolense, T. vivax, T. brucei, T. rhodesiense, and T. gambiense. Vet Pathol 9:1–71
Masake RA (1980) The pathogenesis of infection with Trypanosoma vivax in goats and cattle. Vet Rec 107:551–557
Meléndez MF, William F (1993) Perinatal infection with Trypanosoma vivax in a calf in Venezuela. J Parasitol 79:293–294
Ogwu D, Osori DIK, Njoku CO, Ezeokoli CD, Kumi-Diaka J (1986) Effects of the reproductive status in Zebu heifers on the immunoglobulin M and G levels in bovine Trypanosoma vivax infection. Anim Reprod Sci 12:179–187
Okech G, Watson ED, Luckins AG, Makawiti DW (1996a) Effect of Trypanosoma vivax infection on late pregnancy and postpartum return to cyclicity in Boran cattle. Theriogenology 46:859–869
Okech G, Watson ED, Luckins AG, Makawiti DW (1996b) The effect of experimental infection of Boran cattle in early and mid-pregnancy with Trypanosoma vivax. Br Vet J 152:441–451b
Oliveira JB, Hernández-Gamboa J, Jiménez-Alfaro C, Zeledón R, Blandón M, Urbina A (2009) First report of Trypanosoma vivax infection in dairy cattle from Costa Rica. Vet Parasitol 163:136–139
Osório ALAR, Madruga CR, Desquesnes M, Soares CO, Ribeiro LRR, Costa SCG (2008) Trypanosoma (Duttonella) vivax: its biology, epidemiology, pathogenesis, and introduction in the new world—a review. Mem Inst Oswaldo Cruz 103:1–13
Paiva F, Lemos RAA, Nakasato L, Mori AE, Brum KB, Bernardo KC (2000a) Ocorrência de Trypanosoma vivax em bovinos do Estado de Mato Grosso do Sul, Brasil. I—Acompanhamento clínico, laboratorial e anatomopatológico de rebanhos infectados. Rev Bras Parasitol Vet 9:135–141a
Paiva F, Lemos RAA, Nakazato L, Brum KB, Bernardo KC, Madruga CR, Schenk MA (2000b) Trypanosoma vivax em bovinos no pantanal do estado do Mato Grosso do Sul, Brasil: II—Inoculação experimental. Rev Bras Parasitol Vet 9:143–148b
Peters M, Wagner F, Schares G (2000) Canine neosporosis: clinical and pathological findings and first isolation of Neospora caninum in Germany. Parasitol Res 86:1–7
Razmi GR, Maleki M, Farzaneh N, Garoussi MT, Fallah AH (2007) First report of Neospora caninum-associated bovine abortion in Mashhad area, Iran. Parasitol Res 100:755–757
Rodrigues AC, Garcia HA, Batista JS, Minervino AHH, Góes-Cavalcante G, Silva FM, Ferreira C, Campaner M, Paiva F, Teixeira MMG (2010) Characterization of spliced leader genes of Trypanosoma (Megatrypanum) theileri: phylogeographical analysis of Brazilian isolates from cattle supports spatial clustering of genotypes and parity with ribosomal markers. Parasitology 137:111–122
Schmidek A, Costa PMJR, Toledo LM et al. (2004) Mortalidade até a desmama em bovinos das raças Nelore e Guzerá: efeitos de raça, comportamento e morfologia de tetos. In: XXII Encontro Anual de Etologia, Campo Grande
Serra Freire NM (1981) Oiapoque—outro foco de Trypanosoma vivax no Brasil. Rev Bras Med Vet 4:30–31
Shaw JJ, Lainson R (1972) Trypanosoma vivax in Brazil. Ann Trop Med Parasitol 66:25–32
Silva RAMS, Dávila AMR (2001) Bovine trypanosomosis due to Trypanosoma vivax in the German Bush province, Bolivia. Parasitology 25:92–95
Silva RAMS, Silva JA, Schneider RC, Freitas J, Mesquita D, Mesquita TC, Ramirez L, Dávila AMR, Pereira EB (1996) Outbreak of trypanosomiasis due to Trypanosoma vivax (Ziemann, 1905) in bovine of the Pantanal, Brasil. Mem Inst Oswaldo Cruz 5:561–562
Silva RAMS, Morales G, Eulert E, Montenegro A, Ybañez R (1998) Outbreaks of trypanosomosis due to Trypanosoma vivax in cattle in Bolivia. Vet Parasitol 76:153–157
Silva AS, Costa MM, Polenz MF, Polenz CH, Teixeira MMG, Lopes STA, Monteiro SG (2009) First report of Trypanosoma vixax in bovines in the State of Rio Grande do Sul, Brazil. Cienc Rural 39:2550–2554
Stephen LE (1986) Trypanosomisis: a veterinary perspectiva. Pergamon Press, New York
Thrusfield M (2004) Epidemiologia veterinária. Roca, São Paulo
Vargas TM, Arellano SC (1997) La tripanosomiasis bovina en América Latina y el Caribe. Vet Montividel 33:17–21
Wiengcharoen J, Thompson RCA, Nakthong C, Rattanakorn P, Sukthana Y (2011) Transplacental transmission in cattle: is Toxoplasma gondii less potent than Neospora caninum? Parasitol Res 108:1235–1241
Williams RH, Morley EK, Hughes JM, Duncanson P, Terry RS, Smith JE, Hide G (2005) High levels of congenital transmission of Toxoplasma gondii in longitudinal and cross-sectional studies on sheep farms provides evidence of vertical transmission in ovine hosts. Parasitology 130:301–307
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Batista, J.S., Rodrigues, C.M.F., Olinda, R.G. et al. Highly debilitating natural Trypanosoma vivax infections in Brazilian calves: epidemiology, pathology, and probable transplacental transmission. Parasitol Res 110, 73–80 (2012). https://doi.org/10.1007/s00436-011-2452-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2452-y