Abstract
Malaria transmission was assessed in two rural communities, Kona and Afamanaso in Sekyere South district, Ashanti Region, in the forest zone of Ghana to provide baseline data for ongoing clinical studies and the evaluation of the effect of interventions. Altogether, 3,479 Anopheles gambiae and 1,157 Anopheles funestus were caught by human landing catches. Sporozoite rates determined by either microscopy of salivary glands or enzyme-linked immunosorbent assay (ELISA) for Plasmodium falciparum in the two villages were 6.6% vs. 8.9% for the main vector A. gambiae and 3.2% vs. 6.3% for A. funestus. ELISA tests of dissected specimens compared to microscopy of salivary glands were 1.3 and 2.0 times more positive for A. gambiae and A. funestus, respectively. Plasmodium infections of 122 microscopically positive salivary glands of A. gambiae were identified by real-time PCR as 95 (77.9%) P. falciparum, 7 (5.7%) Plasmodium malariae, 7 (5.7%) Plasmodium ovale and 1 (0.8%) mixed infection of P. falciparum and P. malariae. Transmission in the area was found to be intense and perennial with some seasonal variations during the study period from Dec. 2003 to Aug. 2005. Although the two villages were only 10 km apart from each other, Annual Biting Rates (ABRs) and Annual Entomological Inoculation Rates (AEIRs) were much higher at Afamanaso (11,643 vs. 866) than at Kona (5,329 vs. 490). Most of the transmission (91.4%) occurred during bedtime hours from 21 to 6 h. It is important to note that there was still a substantial transmission before 21 h with AEIRs of 57.3 at Afamanso and 38.7 at Kona. The distribution of impregnated bednets alone, therefore, may not be sufficiently effective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malaria is the leading cause of mortality among children under 5 years old and pregnant women in Ghana and accounts for 40–60% outpatient attendance and for more income and workdays lost than any other disease (Asante et al. 2004). In the forest zone of the Ashanti Region, parasitaemia peaks at a prevalence of 93% in 11-year-old children and declines to a plateau of 20% in adults, as reported by Browne et al. (2000). Intensities of transmission, as defined by Entomological Inoculation Rates (EIRs), have so far been determined in the northern savanna areas (Appawu et al. 2004), in the coastal forest and coastal savannah (Appawu et al. 2001) and the forest-savanna transitional zone (Owusu-Agyei et al. 2009), but not in the main forest region of Ghana.
The present study aimed to describe malaria transmission in two communities in the forest zone by analysing monthly (MBR) and annual biting rates (ABRs) and EIRs by the two vectors Anopheles gambiae and Anopheles funestus. A clinical study conducted in parallel had shown that in this homogeneous forest environment malaria incidences of 3 to 15-month-old babies were highly heterogeneous between villages (Kreuels et al. 2008).
Materials and methods
Study sites
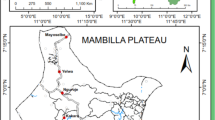
Studies were carried out in Afamanaso and Kona, two towns in the Afigya Sekyere District (714 km2, population 131,658, Ghana Statistical Service, 2000) in the north-eastern part of the Ashanti Region in the forest zone of Ghana. The district capital is Agona, located 27 km north of Kumasi (Fig. 1a). The area is characterised by semi-deciduous forest and farmland. The river Ofin with its tributaries meanders through the district including a forest reserve, providing pools of water and flooded marshy areas in the rainy season (Fig. 1b).
Afamanaso (6° 56′ N, 1° 30′ W), a rural village in the plain, 290 m above sea level and 2.5 km off the main road, is a typical farming village with a population of 2,508 (Ghana Statistical Service 2000). Most of the houses are made of mud with thatched roofs or covered with corrugated iron. Kona (6° 52′ N, 1° 30′ W) is a fast-developing town, 305–320 m above sea level, situated on a small mountain crest on the Kumasi–Ejura Road, with a population of 5,853, engaged in crafts and trading. Most of the houses are made of cement bricks. The peasant farmers of both villages mainly cultivate cocoa, plantain, maize, palm oil and fruit. Some farmers rear livestock, such as poultry, goats and sheep, but not cattle.
Three seasons can be distinguished: a minor rainy season from Mar to Jun, a major rainy season from July to Oct, and a dry season from Nov to Feb (Meteorological Service, Kumasi). Monthly rainfall was measured in Kona from Mar 2004 to Aug 2005 (Figs. 2, 3). Total rainfall for 1 year (Sept 2004 to Aug 2005) was 2,124 mm, with a minimum of 25 mm in Jan 2005 and a maximum of 480 mm in May 2005. The average monthly rainfall was 177 mm. It was exceptional that the minor rainy season in 2005 had more rainfall than the previous major rainy season of 2004.
Mosquito collection
Human landing catches (HLC) were performed twice a month at three sites in each town beginning Dec 2003 in Kona and Mar 2004 in Afamanaso until the end of Aug 2005. One mosquito collector caught from 1800 h to midnight and a second one from midnight to 0600 h. Collectors rotated from site to site to compensate for possible differences in individual attraction to mosquitoes. The mosquitoes collected were kept in cool boxes and transported to the laboratory for further processing the next morning.
Identification and processing of mosquitoes
Mosquitoes were sorted into Anopheles species and Culicinae. The former were further identified using the keys of Gillies and Coetzee (1987); culicines were counted and discarded. From A. gambiae s.l., legs and wings were removed before dissection and retained for species identification by polymerase chain reaction (PCR), following the protocol of Scott et al. (1993). Anopheles females were dissected under a stereomicroscope, their midgut and ovaries were removed, and the latter were examined under a compound microscope to determine parity by inspection of the ovarian tracheoles (Detinova 1962). Salivary glands were examined for sporozoites, using a compound microscope. Sporozoite positive salivary glands were stored at −80°C for later determination of Plasmodium species by real-time PCR (Mangold et al. 2005). Head and thorax of all Anopheles females were examined for the presence of circumsporozoite (CS) P. falciparum antigen, using the enzyme-linked immunosorbent assay (ELISA) (Wirtz 1987). Head and thorax of nulliparous females were used as negative control. A mosquito was considered infective if it was found positive by salivary gland dissection and/or ELISA.
Statistics
Statistica for Windows, 1993, StatSoft Inc., Tulsa, OK, USA, was used for the statistical analysis of the results. Differences between percentages and chi2 values were analysed with the Quick Probability Calculator of this programme.
Results
Vector collection
Altogether, 4,636 Anopheles mosquitoes were collected during 217 full-night HLCs in the two villages, 63.6% (2,948) in Afamanaso and 36.4% (1,688) in Kona. Morphologically, 75% (3,479) were identified as A. gambiae s.l. and 25% (1,157) as A. funestus. One hundred thirty-five A. gambiae were classified by PCR as A. gambiae s.s.; DNA of three specimens did not amplify.
Parous rates
Parous rates of the Anopheles females were high throughout. Mean parous rates of A. gambiae and A. funestus were 85.8% vs. 85.2% at Afamanaso and 84.0% vs. 82.2% at Kona. Parous rates of A. gambiae were significantly higher (P = 0.0029) in the dry season (90%) than in the major rainy season (83%). Parous rates of A. funestus of the dry and the major rainy season did not vary significantly at 85.3% vs. 84.3%, respectively (P = 0.71).
Annual and seasonal biting activities
Anopheles biting activities were perennial but varied seasonally. When MBRs of months with more or less than 100 mm rainfall were compared, means of MBRs were always lower in months with less rain. Differences, however, were only significant for A. gambiae in Kona (P = 0.024, Mann–Whitney U test). A. funestus contributed 36% of the bites in Afamanaso but only played a minor role of 8.9% of bites in Kona (Figs. 2 and 3, Table 1). A person passing a night at Afamanaso received an average 31.9 bites per night (b/p/n), more than twice as much as a person in Kona at 14.6 b/p/n.
Infection rates
Altogether, 4,634 Anopheles females were tested for the presence of P. falciparum CS protein. Salivary glands were removed from 2,858 mosquitoes and were examined microscopically for the presence of sporozoites; 6.6% of 2,280 A. gambiae and 3.2% of 570 A. funestus turned out to be positive. Of the non-dissected mosquitoes (N = 1174), 8.9% of A. gambiae and 6.3% A. funestus were positive in ELISA, indicating that the ELISA was 1.3 times more sensitive for A. gambiae and 2.0 times more sensitive for A. funestus. Differences were significant (P = 0.00084 vs. P = 0.023). When ELISA results for mosquitoes with and without salivary glands were compared, differences were only significant for A. funestus (Table 2). When microscopically negative mosquitoes were retested with ELISA, 3.1% of 2,154 A. gambiae and 1.9% of 536 A. funestus became positive. For the calculation of infection rates and EIRs, mosquitoes, either positive in microscopy and or ELISA, were used (A. gambiae 9.1%, A. funestus 5.8%). Infection rates of A. funestus were always lower than those of A. gambiae (Table 1).
Entomological inoculation rates (EIR)
Annual entomological inoculation rate (AEIR) was 866 in Afamanaso and 490 in Kona. The contribution of A. funestus reached 35.7% in Afamanao but only 7.2% in Kona (Table 1).
Hourly biting activities and risk of transmission
Biting activities of both A. gambiae and A. funestus started as early as 1800 h, peaked between 2300 and 0200 h, and persisted until 0600 h in the morning (Fig. 4). There were no significant differences between the two species (chi2 test of homogeneity by Brandt–Snedecor, Sachs 1999, P = 0.75). The percentage of infected A. gambiae and A. funestus caught before, during bedtime and in the early morning hours were assessed to calculate the risk to be bitten by mosquitoes or to pick up an infection during different time intervals (Table 3). In both communities, 85% of all bites and infected bites occurred during bedtime hours between 2100 h in the evening and 0400 h in the morning, and about 91% between 2100 and 0600 h in the morning. Infection rates of both species did not change significantly (chi2 = 1.91, P = 0.38 for A. gambiae, chi2 = 0.71, P = 0.38 for A. funestus) during the three time intervals (Table 3). It is important to note that high transmission rates occurred before bedtime from 1800 to 2100 h with an EIR of 57.3 at Afamanaso and 38.7 at Kona.
Identification of Plasmodium species
Plasmodium infections in 121 of 139 microscopically positive salivary glands (110 A. gambiae, 11 A. funestus, 18 did not amplify) could be identified by real-time PCR (Table 4); the distribution of Plasmodium species was 87.6% P. falciparum, 5.8% P. malariae and 5.8% P. ovale. As expected, no P. vivax infection was detected. One A. gambiae contained a mixed infection of P. falciparum with P. malariae (Table 4).
The kdr gene in Anopheles gambiae in the study area
The knock down resistance (kdr) gene was highly prevalent in the A. gambiae populations of the two study villages and only absent in 3 of the 109 successfully amplified specimens (Table 5).
Discussion
Altogether, 3,479 A. gambiae and 1,157 A. funestus, which had been caught by HLCs in the two study villages Kona and Afamanaso, were examined. Samples of A. gambiae from both villages were identified as A. gambiae sensu stricto, which agrees with the results of Tuno et al. (2010), who furthermore recorded a high human blood ratio and strong endophilic behaviour of this species. The genotype of A. gambiae was not determined. It can be assumed that it was the S form which predominates in the forest region of Ghana and is positively associated with malaria (De Souza et al. 2010). All 52 specimens collected at Kumasi were identified as S form and carried the kdr mutation. (Yawson et al. 2004). This was in accordance with the high kdr (89.9% homozygous) detected in our material from Kona and Afamanaso.
The sporozoite rates for A. gambiae were always higher than those of A. funestus which corroborates with the findings of Owusu-Agyei et al. (2009) from the forest transitional zone in Brong Ahafo, north of Kumasi. This is in contrast to the coastal forest, the coastal savannah and also to the northern savannah of Ghana where infection rates of A. funestus were higher than those of A. gambiae (Appawu et al. 2001, 2003; Okoye et al. 2005).
The malaria transmission in both study villages was perennial and intensive with annual EIRs of 866 for Afamanaso and 490 for Kona. This was comparable with the transmission in the Sudan savannah (418) of northern Ghana (Appawu et al. 2004) and higher than that measured in the forest savannah transitional zone around Kintampo (269) and the coastal forest at Dodowa (21.9) (Owusu-Agyei et al. 2009; Appawu et al. 2001).
Biting rates and transmission varied in both villages with rainfall. A. gambiae was the main vector contributing 81% of the transmission. A. funestus was the secondary vector. However, the contribution of A. funestus differed in the two villages, 36% in Afamanaso but only 7.7% in Kona. Similarly A. funestus was found to be the secondary vector also in Dodowa (Appawu et al. 2001), the area around Kintampo (Owusu-Agyei et al. 2009) and in the Kassena Nankana District of the northern savannah (Appawu et al. 2004). Differences in the amount of transmission in Kona and Afamanaso were in parallel with the number of malaria episodes per year of small children of 2.2 and 1.1, respectively (Kreuels et al. 2008). The larger population in Kona (5,853, Afamanaso 2,508) might lead to a dilution of man vector contact and a main reason for the difference in biting rates and EIRs determined in both villages. Differences of transmission and species composition of the vector populations are further influenced by the distinct topographies of the two study sites. Afamanaso is a village in the plain surrounded by the Ofin river and swampy areas, while Kona is located on a ridge with only small streams in the valley.
The hourly biting activities were similar for both vectors with peaks from 23.00-02.00 h as described by Gillies and de Meillon (1968). It was important to note that essential biting and transmission occurred before bedtime from 1800–2100 h in both villages with AEIRs of 57.3 in Afamanaso and 38.7 in Kona. These AEIRs were even higher than the AEIR of 22 measured for whole nights at Dodowa in the coastal forest (Appawu et al. 2004), but malaria prevalences in the human population of 42.2% in April and 51.3% in August (Afari et al. 1995) were comparable with 50.7% determined in Ashanti Region (Browne et al. 2000). It is to be feared that the use of impregnated long-lasting bednets may not be effective in preventing transmission in areas with such high early-evening transmission rates: i.e. before bedtime. Children are only sufficiently protected by bednets in areas with no transmission in the early evening hours, e.g. Dodowa of the coastal forest (Appawu et al. 2001). It is another problem that in Afamanaso 15.9% of women and 37% of men sleep outside (Tuno et al. 2010).
References
Afari EA, Appawu M, Dunho S, Baffoe-Wilmot A (1995) Malaria infection, morbidity and transmission in two ecological zones Southern Ghana. Afr J Health Sci 2:312–315
Appawu MA, Baffoe-Wilmot A, Afari EA, Dunyo S, Koram KA, Nkrumah FK (2001) Malaria vector studies in two ecological zones in southern Ghana. Afr Entomol 9:59–65
Appawu MA, Bosompem KM, Dadzie S, McKakpo US, Anim-Baidoo I, Dykstra E, Szumlas DE, Rogers WO, Koram K, Fryauff DJ (2003) Detection of malaria sporozoites by standard ELISA and VecTestTM dipstick assay in field-collected anopheline mosquitoes from a malaria endemic site in Ghana. Trop Med Int Health 8:1012–1017
Appawu M, Owusu-Agyei S, Dadzie S, Asoala V, Anto F, Koram K, Rogers W, Nkrumah F, Hoffman SL, Fryauff DJ (2004) Malaria transmission dynamics at a site in northern Ghana proposed for testing malaria vaccines. Trop Med Int Health 9:164–170
Asante FA, Asenso-Okyere K, d’Almeida S, Mwabu G, Okorosobo T (2004) Economic burden of malaria in the African region: evidence from Ghana. Commun Dis Bull Afr Region 2(4):1–4
Browne EN, Frimpong E, Sievertsen J, Hagen J, Hamelmann C, Dietz K, Horstmann RD, Burchard GD (2000) Malariometric update for the rainforest and savanna of Ashanti region, Ghana. Ann Trop Med Parasitol 94:15–22
De Souza D, Kelly-Hope l, Lawson B, Wilson M, Boakye D (2010) Environmental factors associated with the distribution of Anopheles gambiae s.s. in Ghana; an important vector of lymphatic filariasis and malaria. PLoS One 5(3):e9927
Detinova TS (1962) Age grouping methods in Diptera of medical importance with special reference to some vectors of malaria. Monogr Ser World Health Organ 47:13–191
Ghana Statistical Service (2000) Ghana living standards survey. Report of the fourth round (GLSS4) 1–192. Accra.
Gillies MT, de Meillon B (1968) The Anophelinae of Africa, South of the Sahara. Publ S Afr Inst Med Res 54:1–343
Gillies MT, Coetzee M (1987) A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical Region). South Africa Institute for Medical Research 55:1–143
Kreuels B, Kobbe R, Adjei S, Kreuzberg C, von Reden C, Bäter K, Klug S, Busch W, Adjei O, May J (2008) Spatial variation of malaria incidence in young children from a geographically homogeneous area with high endemicity. J Infect Dis 197:85–93
Mangold KA, Manson RU, Koay ESC, Stephens L, Regner MA, Thomas RB, Peterson LR, Kaul KL (2005) Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol 43:2435–2440
Okoye PN, Wilson MD, Boaky DA, Brown CA (2005) Impact of the Okyereko irrigation project in Ghana on the risk of human malaria infection by Anopheles species (Diptera: Culicidae). Afr Entomol 13:249–253
Owusu-Agyei S, Asante KP, Adjuik M, Adjei G, Awini E, Adams M, Newton S, Dosoo D, Dery D, Agyeman-Budu A, Gyapong J, Brian Greenwood B, Chandramohan D (2009) Epidemiology of malaria in the forest-savanna transitional zone of Ghana. Malar J 8:220
Sachs L (1999) Angewandte Statistik. Anwendung statistischer Methoden. Springer, Berlin
Scott JA, Brogdon WG, Collins FH (1993) Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg 49:520–529
Tuno N, Kjaerandsen J, Badu K, Kruppa T (2010) Blood-feeding behavior of Anopheles gambiae and Anopheles melas in Ghana, western Africa. J Med Entomol 47:28–31
Wirtz R (1987) Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ 1987(65):39–45
Yawson AE, McCall PJ, Wilson MD, Donnelly MJ (2004) Species abundance and insecticide resistance of Anopheles gambiae in selected areas of Ghana and Burkina Faso. Med Vet Entomol 18:372–377
Acknowledgements
The study was partially funded by the Bundesministerium für Bildung und Forschung (grant 01KA0202). We are indebted to the vector collectors and the people in Kona and Afamanaso for continuous cooperation. We appreciate the support of the staff of Kumasi Centre for Collaborative Research in Tropical Medicine (KCCR). In particular we acknowledge the assistance of Dr. Christof Berberich, head of KCCR laboratories. Visits of Rolf Garms in Ghana were made possible by support of the German Senior Experten Service in 2003, 2005 and 2006. Ayimbire Abonuusum thanks the Bernhard Nocht Institute for Tropical Medicine for a 1-year scholarship and research stay in Hamburg, Germany and the Kwame Nkrumah University of Science and Technology, Kumasi, Ghana for a PhD grant. We thank Dr. Jean Pierre Lin for proofreading of the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abonuusum, A., Owusu-Daako, K., Tannich, E. et al. Malaria transmission in two rural communities in the forest zone of Ghana. Parasitol Res 108, 1465–1471 (2011). https://doi.org/10.1007/s00436-010-2195-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2195-1