Abstract
The purpose of the study is to explore the efficacy of mefloquine administered orally at single, multiple doses, or in combination with artesuante, artemether, or praziquantel in mouse—Schistosoma japonicum model. A total of 205 mice were divided into 4 batches and each batch of mice was infected percutaneously with 40 S. japonicum cercariae for 35 days. The infected mice were treated orally with mefloquine at single doses, multiple daily doses, or combined with artesunate, artemether, or praziquantel, while infected but untreated mice served as control. All treated animals were killed 4 weeks post-treatment for assessment of effect. When infected mice were treated orally with mefloquine at single or multiple daily doses under the same total dose levels, the tendency to decrease the efficacy was seen. Particularly, when a lower single dose of 100 mg/kg was divided equally into five daily doses of 20 mg/kg, the efficacy decreased statistically significant (P < 0.05), i.e., the total worm and female worm reductions of 67.9% and 73.4% decreased to 31.3% and 30.3%, respectively. In infected mice treated with mefloquine or artesuante at a single dose of 100 mg/kg, a moderate effect against schistosomes was observed. No further significant reduction of total and female worm burdens was seen, when the two drugs combined together at the same dose level. On the other hand, administration of mefloquine combined with artesunate at single dose of 50 mg/kg, which exhibited no effect against schistosomes, resulted in significant reduction of total and female worm burdens in comparison with the groups treated with mefloquine and artesunate alone at the same dose level. Similar results were observed in treatment of infected mice with mefloquine in combination with artemether at the smaller dose of 50 mg/kg. The total worm burden was significantly lower than that of control and the female worm burden was also significant lower than that of groups treated with mefloquine and artemether alone. Interestingly, in administration of mefloquine 100 mg/kg combined with artemether 100 mg/kg to the infected mice, all female worms were killed and the total worm burden was also statistically significant lower than that of groups treated with either drug alone. Finally, when infected mice were treated with mefloquine combined with prazqiuatel at single dose of 50 mg/kg, no apparent improvement in efficacy was seen. Administration of mefloquine 100 mg/kg combined with praziquantel 100 mg/kg, only the difference of female worm burdens between praziquantel group and combined treatment group was statistically significant. The results indicate that under the same dose level of mefloquine, the efficacy of single dose is superior to that of multiple daily doses; mefloquine combined with artesunate or artemether at an invalid or moderate effective dose may show synergistic effect, especially the effect against female worms; no prominent synergistic effect is observed, when the similar dose level of mefloquine in combination with praziquantel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, praziquantel is the only drug of choice in the treatment of three major species of schistosomes infecting humans (Fenwick et al. 2006; Doenhoff et al. 2009). The important shortcoming of praziquantel is its much less efficacy against young developing stages of schistosomula (Yue et al. 1985; Sabah et al. 1986; Xiao et al. 1987) which might impact on the cure rate of the patients treated with praziquantel or reinfection of individuals after treatment with praziquantel in areas of heavy schistosomiasis transmission (Wu et al. 1993; Dabo et al. 2000; Danso-Appiah and de Vlas 2002; N’Goran et al. 2003). It is also worried by the public health society that heavy reliance on a single drug for schistosomiasis control may promote the selection and spread of drug-resistant parasites. Apart from praziquantel, artemether, and artesunate, the derivative of artemisinin have been developed as a prophylactic agent for schistosomiasis control through a series of experimental studies at the end of the last century (Xiao et al. 1995; Li et al. 1996; Xiao 2005; Utzinger et al. 2007). Although the two agents show promising prevention from schistosome infection, they are needed to be administered to the people, contacted infested water, every first or every other week during the whole transmission season of schistosomiasis which is inconvenient for the practical application and hinders their use in the endemic areas of schistosomiasis. Experimental studies indicate that artemether and artesunate exhibit potential effect against various stages of juvenile schistosomes and adult worms are less susceptible (Li et al. 1996; Xiao et al. 2000; Utzinger et al. 2007). Therefore, it is necessary to develop new type of antischistosomal drug. possessing effect on both juvenile and adult schistosomes.
In 2009, antimalarial drug mefloquine, an amino alcohol compound, has been found to be effective against Schistosoma mansoni and S. japonicum (Keiser et al. 2009; Xiao et al. 2009a, b, c). The feature of antischistosomal properties of mefloquine is that the compound possesses similar potential effect against juvenile stages and adult schistosomes, when the drug is administered to the infected mice at a single dose of 200 or 400 mg/kg (Keiser et al. 2009; Xiao et al. 2009a). While a lower dose of mefloquine (150 mg/kg) may only reduce the egg production in S, manoni-infected mice significantly (Van Nassauw et al. 2008). Further morphological, histopathological, and ultrastructural observation indicate that mefloquine exhibits strong and fast lethal action on both juvenile and adult S. japonicum which reveals in extensive and severe damage to the worm tegument, musculature, parenchymal tissues, digestive system, and reproductive system (Xiao et al. 2009b, 2010a, b; Zhang et al. 2009).
Mefloquine has been widely used in treatment and prevention of malaria and it is effective under the oral administration of doses in the range of 0.5–1.5 g as a single dose. Meanwhile, the side effects of mefloquine are dose-related. At the doses below 1 g, they are mild to moderate, overdoses of mefloquine may cause severe side effects including neuropsychiatric toxicity (UNDP/World Bank/WHO Update 1983; Alkadi 2007; Tooveys 2009). Experimental study indicates that the ED50 and ED90 of mefloquine against the Plasmodium berghei N strain in mice are 1.5 and 3.8 mg/kg (Peters et al. 1977), respectively, which is much lower than the mefloquine dose of 200–400 mg/kg used to eliminate 90–100% of female schistosomes in infected mice. Meanwhile, in healthy volunteers, a single mefloquine dose of 1 g produces a maximum concentration of about 1 μg/ml (Desjardins et al. 1979; Schwartz et al. 1982) which is 9-fold lower than the minimal effective concentration against schistosomes in vitro (Xiao et al. 2009c). According to these data, one may suggest that administration of the regular dose of mefloquine used in treatment of malaria to the patients with schistosomiasis may results in poor efficacy. In recent year, artemisinin-based combination therapy has been recommended by WHO in order to retard the development of resistance and synergism, and mefloquine combined with artesuante has been used extensively in treatment of malaria. Since mefloquine, artesunate and aretmether display broad-spectrum antischistosomal activities and act against various stages including adult schistosomes, it is interesting to observe the efficacy of mefloquine combined with artesunate in treatment of patients co-infected with malaria and schistosomiasis. Recently, a report indicated that in endemic area, where malaria and schistosomiasis co-exist, a single dose of mefloquine 25 mg/kg administered to the schoolchildren infected with Schistosoma haematoboum exhibited a cure rate of 21%, but significantly lower egg reduction of 74% was seen. Nevertheless, in the schoolchildren co-infected with S. mansoni, poor effect assessed by cure rate and egg reduction rate was observed. But when aforementioned patients were treated with mefloquine in combination with artesunate resulted in higher cure rate and egg reduction rate (Keiser et al. 2010). In this paper we report the efficacy of mefloquine administered orally at single, multiple doses or in combination with artesuante, artemether, or praziquantel in mouse model infected with S. japonicum.
Materials and methods
Parasites, mice, and experimental infection
S. japonicum cercariae (Anhui isolate) freshly shed from infected intermediate host snail Oncomelania hupensis were provided by the Department of Vector Biology of our institute.
Two hundred and five female Kunming strain mice, weighing 20–22 g, were purchased from Shanghai Experimental Animal Center, Chinese Academy of Sciences (Shanghai, China). Animals were kept at animal facilities and had free access to rodent food and tap water throughout the study. After 1 week acclimatization, each mouse was infected with 40 S. japonicum cercariae via shaved abdominal skin. Mice were used for experimental treatment 35 days post-infection.
Drugs
Mefloquine hydrochloride was provided by Libang Pharmaceutical Co. Ltd (Xian, China); praziquantel was purchased from Shanghai Sixth Pharmaceutical Factory; artemether was the gift of Yunnan Kunming Pharmaceutical Corporation (Kunming, China) and artesunate was obtained from Guangxi Guilin Pharmaceutical Corporation (Guilin, China). The aforementioned drugs were suspended in 7% Tween-80 and 3% alcohol at final concentrations of 2, 5, 10, or 20 g/l. The volume of each dose given to mice was 10 mL/kg.
Experimental treatment
Single and multiple administration
Forty-two infected mice were divided into six groups. Three groups of five to seven mice were treated orally with mefloquine 200 mg/kg by the following dose schedule, i.e., the drug was given at a single dose of 200 mg/kg, 100 mg/kg daily for two consecutive days or 50 mg/kg daily for four consecutive days. Other two groups of seven mice were either treated orally with mefloquine at a single dose of 100 mg/kg or a daily dose of 20 mg/kg for five consecutive days. The remaining group of ten infected but untreated mice served as control.
Mefloquine combined with artesunate
Two tests were undertaken. In the first test, 41 infected mice were divided into seven groups. Four groups of five to seven mice were treated orally with mefloquine or artesuante at a single dose of 50 mg/kg or 100 mg/kg, respectively, other two groups received mefloquine 50 mg/kg in combination with artesunate 50 mg/kg or mefloquine 100 mg/kg combined with artesunate 100 mg/kg. The remaining group of ten untreated but infected mice served as control. In the second test, 51 infected mice were divided into four groups. Three groups of ten to 11 mice were administered orally with mefloquine and artesunate at a single dose of 50 mg/kg, or mefloquine 50 mg/kg combined with artesunate 50 mg/kg. The remaining 20 infected but untreated mice served as control.
Mefloquine combined with artemether
Two tests were continued. In the first test 27 infected mice were divided into four groups. Three groups of five to six mice received oral single mefloquine 50 mg/kg, artemether 50 mg/kg, or mefloquine 50 mg/kg in combination with artemether 50 mg/kg. The remaining ten untreated but infected mice served as control. In the second test, 30 infected mice were equally divided into six groups. Three groups were treated orally with single mefloquine 100 mg/kg, artemether 50 or 100 mg/kg. Two groups were treated with mefloquine combined with artemether according to the following dose schedules, i.e., mefloquine 100 mg/kg plus artemether 50 mg/kg and mefloquine 100 mg/kg plus artemether 100 mg/kg. The remaining five infected but untreated mice served as control.
Mefloquine combined with praziquantel
Two tests were carried out. In the first test, 26 infected mice were divided into four groups. Three groups of five to six mice were treated with single oral mefloquine 50 mg/kg, praziquantel 50 mg/kg or mefloquine 50 mg/kg in combination with praziquantel 50 mg/kg. The remaining ten infected but untreated mice served as control. In the second test, 30 infected mice were divided equally into six groups. Five groups were administered orally with mefloquine at a single dose of 50 and 100 mg/kg, praziquantel 100 mg/kg, mefloquine 50 mg/kg plus praziquantel 100 mg/kg or meflloquine 100 mg/kg plus praziquantel 100 mg/kg. The remaining five untreated but infected mice served as control.
Dissection and collection of schistosomes
All treated and control groups of mice were sacrificed 4 weeks post-treatment by bloodletting and schistosomes were collected from hepatic and portomesenteric veins using a perfusion technique (Yolles et al. 1947). Schistosomes recovered from each mouse were sexed and counted.
Statistical analysis
Statistical Package for the Social Sciences (version 13.0) software was applied to calculate the total and female worm burdens for each treated and control group, while total and female worm reductions for each treated group were then calculated in comparison with the corresponding control. The difference of total worm burden or female worm burden between each treated group and the corresponding control group was analyzed by using nonparametric method (Mann–Whitney test).
Results
Effect of single and multiple administration of mefloquine
When mice infected with adult schistosome were treated orally with mefloquine at a single dose of 200 mg/kg, the total and female worm burdens were significantly lower than that of control (P < 0.01) with total and female worm reductions of 80.7% and 92.7%, respectively (Table 1). Under the same total dose of 200 mg/kg, administration of mefloquine at a daily dose of 100 mg/kg for two consecutive days or 50 mg/kg for four consecutive days, the total and female worm burdens were also significantly lower than that of control (P < 0.01). Although the total worm burdens of these two groups were higher than that of the group received mefloquine at a single dose of 200 mg/kg, the difference of the total worm burdens between each multiple administration group and single administration group was not statistically significant (P > 0.05). However, in the group received mefloquine 50 mg/kg daily for 4 days the female worm burden was significantly higher than that of group treated with single mefloquine 200 mg/kg (P < 0.05). In two groups treated orally with mefloquine at a single dose of 100 mg/kg or a daily dose of 20 mg/kg for five consecutive days, the total worm burdens of them were significantly lower than that of control group (P < 0.01 or P < 0.05; Table 1) with total worm reductions of 67.9% and 31.3%, respectively. The difference of female worm burdens between the group treated with mefloquine 100 mg/kg group and control group was also statistically significant (P < 0.01). But no such difference was seen when the female worm burden, revealed in the group treated with mefloquine 20 mg/kg daily for five consecutive days, was compared to the control (P > 0.05). Interestingly, in comparison of total and female worm burdens, the differences between the two groups treated with mefloquine at a single dose (100 mg/kg) and five daily doses (20 mg/kg) were statistically significant (P < 0.01 or P < 0.05).
Mefloquine combined with artesunate
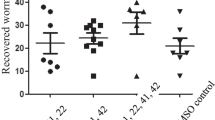
In infected mice treated orally with mefloquine or artesunate at a single dose of 50 mg/kg, the total worm burdens and female worm burdens were somewhat lower than that of control, but the differences between the group treated with each drug and control group were not statistically significant (P > 0.05) with worm burden and female worm burden reductions of 20.5% and 13.8% as well as 11.6% and 6.4%, respectively (Table 2). When the dose of mefloquine and artesunate was increased to 100 mg/kg, their total worm burdens and female worm burdens were significantly lower than that of control (P < 0.05 or P < 0.01) with total worm reductions and female worm reductions of 67.9% and 73.4% as well as 59.8% and 74.3%, respectively (Table 2). In the group treated with mefloquine 50 mg/kg combined with artesunate 50 mg/kg, the total worm burden was lower than that of control group or two groups treated with the same dose of mefloquine and artesunate alone, but the difference between the combined treatment group and the control group or each group treated with mefloquine or artesunate alone was not statistically significant (P > 0.05). On the other hand, the female worm burden revealed in the combined treatment group was significantly lower than that of control group and the group treated with mefloquine or artesunate alone (P < 0.05). In the group treated with mefloquine 100 mg/kg in combination with artesunate 100 mg/kg, the total worm burden and female worm burden were significantly lower than that of control group (P < 0.01; Table 2) with total and female worm reductions of 76.7% and 87.2%, respectively. In comparison of total worm burden and female worm burden obtained from this combined treatment group and the corresponding groups treated with mefloquine or artesunate alone at a single dose of 100 mg/kg, no differences were seen between the combined treatment group and the group treated with mefloquine or artesunate alone (P > 0.05).
In a further test, infected mice treated orally with mefloquine or artesuante at a single dose of 50 mg/kg resulted in similar total worm burdens and female worm burdens compared to the control group (P > 0.05; Table 2), While in the group treated with mefloquine 50 mg/kg in combination with artesuante 50 mg/kg, the total worm burden and female worm burden were not only significantly lower than that of control (P < 0.01) with total and female worm reductionss of 43.2% and 65.5% (Table 2), respectively, but also shown significant differences between combined treatment group and each group treated with mefloquine and artesuante alone either in total worm burdens or in female worm burdens (P < 0.01).
Mefloquine combined with artemether
When mefloquine or artemether was administered orally to the infected mice at a single dose of 50 mg/kg. Their total worm burdens were similar to that of control (P > 0.05; Table 3), while significant difference of female worm burdens was only seen between artemether-treated group and control group (P < 0.05). In the group treated with mefloquine 50 mg/kg in combination with artemether 50 mg/kg, the total and female worm burdens were significantly lower than that of control (P < 0.01) with total worm and female worm reductions of 53.5% and 86.7% (Table 3), respectively. Meanwhile, in combined treatment group, the total worm burden was lower than that obtained from the groups treated with mefloquine or artemether alone, but the difference between combined treatment group and each mefloquine or artemether-treated group was not statistically significant (P > 0.05). Nevertheless, the female worm burden revealed in combined treatment group was significant lower than that of each group treated with mefloquine or artemether alone (P < 0.05 or P < 0.01).
Further test showed that in infected mice treated orally with artemether at a single dose of 50 mg/kg, the total worm burden and female worm burden were lower than that of control, but the differences between treated group and control group was not statistically significant (P > 0.05; Table 3). When atemether or mefloquine was administered to the infected mice at a single dose of 100 mg/kg, their total worm burdens and female worm burdens were significantly lower than that of the control (P < 0.05) with total and female worm reductions of 48.9% and 77.8% (mefloquine) as well as 55.6% and 69.4% (artemether). After administration of mefloquine 100 mg/kg combined with artemether 50 mg/kg, the total worm burden was significantly lower than that of control group (P < 0.05), while the differences of total worm burdens between the combined treatment group and each group treated with mefloquine 100 mg/kg or artemether 50 mg/kg alone were not statistically significant (P > 0.05). But in combined treatment group, all female worms were eliminated which was superior to that of control group and two groups treated with mefloquine or artemether alone. (P < 0.05 or 0.01). Similar results were seen when the infected mice were treated with mefloquine 100 mg/kg in combination with artemether 100 mg/kg, but its total worm burden was also significantly lower than that of two groups treated with mefloquine or artemether alone (P < 0.05).
Mefloquine combined with praziquantel
In infected mice treated orally with mefloquine or praziquantel at a single dose of 50 mg/kg, the total worm burdens and female worm burdens of them were lower than that of control group, but only the differences between praziquantel-treated group and control group was statistically significant (P < 0.01) In the group treated with mefloquine 50 mg/kg combined with praziquantel 50 mg/kg, the total worm burden was the same as praziquanel 50 mg/kg group, but the difference of total worm burdens between combined treatment group and control group was not statistically significant (P > 0.05). While the female worm burden of combined treatment group was significantly lower than that of control group and mefloquine 50 mg/kg group (P < 0.05; Table 4). No difference of female worm burdens between combined treatment group and the group treated with praziquantel alone was seen (P > 0.05).
In a further test, groups of infected mice treated orally with mefloquine at a single dose of 50, 100 mg/kg, or praziquantel 100 mg/kg, their total and female worm burdens were lower than that of control group, but only the difference of total worm burdens and female worm burdens between the group treated with mefloquine 100 mg/kg and control group were statistically significant (P < 0.05). When infected mice were treated orally with mefloquine 50 mg/kg in combination with praziquantel 100 mg/kg, the total worm burden and female worm burden were significantly lower than that of control group (P < 0.05, P < 0.01; Table 4). Meanwhile, only the difference of female worm burdens between combined treatment group and the group treated with mefloquine 50 mg/kg alone was statistically significant (P < 0.05). In administration of mefloquijne 100 mg/kg combined with praziquantel 100 mg/kg to the infected mice, the total worm burden and female worm burden were significantly lower than that of control (P < 0.01; Table 4). In comparison with the two groups treated with the same dose of mefloquine and praziquantel alone, only the difference of female worm burdens between praziquantel group and combined treatment group was statistically significant (P < 0.05).
Discussion
In previous papers (Keiser et al. 2009; Xiao et al. 2009a, b, c, 2010a, b; Zhang et al. 2009), we reported the feature of antischistosomal properties of mefloquine, including the susceptibility of various stages of schsotosomes to the drug, relationship between the dose and efficacy, the impact of infection intensity on the efficacy, histopathological and ultrastructural alterations of schistosomes induced by the drug. But the influence of mefloquine, administered at single or multiple doses under the same total dose, on the efficacy is still not known which might be beneficial in optimization of treatment schedule. Our results indicate that under the same total dose of mefloquine, multiple daily doses show the tendency to reduce the efficacy of the drug against schistosomes. In particular, when a lower single dose of 100 mg/kg is divided equally into five daily doses, the efficacy of mefloquine against schistosomes declines significantly. Since in vitro a higher minimal effective concentration of mefloquine (10 μg/ml) is necessary to kill the schistosomes (Xiao et al. 2009c), it is suggested that sufficient higher drug concentration in the blood is rather important in killing the worms, i.e., administration of a small daily dose of mefloquine to the infected mice, the blood concentration could not reach the level sufficient to kill the worms, although the half-life of mefloquine is longer, i.e., 17 h, in mice (Rozman et al. 1978).
When infected mice are treated with mefloquine or artesuante at a single dose of 100 mg/kg, a moderate efficacy is observed. But the two drugs administered together do not show significant reduction of total and female worm burdens in comparison with the two drugs used alone at the same dose level. On the other hand, administration of mefloquine combined with artesunate at a single dose of 50 mg/kg, which exhibits no effect against schistosomes, results in significant reduction of female worm burden. But the reduction of total worm burden is not significant, because of small number of mouse used in each group. Therefore, we repeat the test and increase the number of mouse in each group. The results indicate that administration of mefloquine in combination with artesunate at an invalid dose level reveal synergistic effect against schistosomes. This may explain the promising efficacy in clinical treatment of the patients with schistosomiasis using the regimen of mefloquine combined with artesuante against malaria (Keiser et al. 2010) and which encourages us to do more clinical observation.
Similar results are also observed when infected mice are treated with mefloquine in combination with artemether at a single invalid dose or a lower effective dose, i.e., 50 mg/kg. The characteristic of such combined treatment is also killing more female worms which is beneficial for treatment of schistosomiasi, because the eggs laying by female schistosomes are the pathogen of the disease (Warren 1982). Interestingly, in infected mice treated with mefloquine 50 or 100 mg/kg in combination with artemether 100 mg/kg results in elimination of all female worms, especially in the group treated together with the two drugs at a dose of 100 mg/kg, the total worm burden is also significantly lower than that of group treated with each drug alone, demonstrating that when a higher dose of mefloquine and artemether is applied, the synergistic effect may further elevated. The results also suggested that in treatment of schistosomiasis mefloquine combined artemetehr can be considered in the future study.
Since mefloquine and prazqiantel are different type of antischistosomal drugs, it is suggested that combined treatment of the two drugs may increase the efficacy. Actually, no apparent improvement in efficacy is seen, when infected mice are treated with mefloquine at an ineffective dose of 50 mg/kg combined with the same dose of praziquantel. When the dose of mefloquine and praziquantel used in combined treatment is increased to 100 mg/kg, the efficacy is significantly superior to that of control. While in comparison with mefloquine administered alone, no significant difference is seen, although the female worm burden in combined treatment group is significantly lower than that of the group treated with praziquantle alone. Our experimental studies indicate that when smaller dose of mefloquine administered simultaneously with smaller dose of artesunate or artemether, synergistic effect is seen. It is deserved to proceed further observation on mefloquine combined with artesuante or artemether in treatment of schistosomiasis in endemic areas where malaria and schistosomiasis co-exist.
References
Alkadi HO (2007) Antimalarial drug toxicity: a review. Chemotherapy 53:385–391
Dabo A, Doucoure B, Koita O, Diallo M, Kouriba B, Klinkert MQ, Doumbia S, Doumbo O (2000) Reinfection by Schistosoma haematobium and S mansoni despite repeated praziquantel treatment in office du Niger (Mali). Med Trop 60:351–355, Mars
Danso-Appiah A, de Vlas SJ (2002) Interpreting low praziquantel cure rates of Schistosoma mansoni infections in Senegal. Trends Parasitol 18:125–129
Desjardins RF, Pamplin C III, Bredow J, von Barry KG, Canfield CJ (1979) Kinetics of a new antimalarial, mefloquine. Clin Pharmacol Ther 26:372–379
Doenhoff MJ, Hagan P, Cioli D, Southgate V, Pica-Mattoccia L, Botros S, Coles G, Tchuem Tchuenté LA, Mbaye A, Engels D (2009) Praziquantel: its use in control of schistosomiasis in sub-Saharan Africa and current research needs. Parasitology 136:1825–1835
Fenwick A, Rollinson D, Southgate V (2006) Implementation of human schistosomiasis control: challenges and prospects. Adv Parasitol 61:567–622
Keiser J, Chollet J, Xiao SH, Mei JY, Jiao PY, Utzinger J, Tanner M (2009) Mefloquine—an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl Trop Dis 3:e350
Keiser J, N’Guessan NA, Adoubryn KD, Silue KD, Vounatson P, Hartz C, Utzinger J, N’Goran EK (2010) Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate, and praziquantel against Schistosoma heamatobium: randomized, exploratory open-label trial. Clin Infect Dis 50:1205–1213
Li SW, Wu LJ, Liu ZD, Hu LS, Xu YX, Liu YM, Liu X, Fang JT (1996) Studies on prophylactic effect of artesunate on Schistosomiasis japonica. Chin Med J 109:848–853
N’Goran EK, Utzinger J, Gnaka HN, Yapi A, N’Guessan NA, Kigbafori SD, Lengeler C, Chollet J, Shuhua X, Tanner M (2003) Randomized, double-blind, placebo-controlled trial of oral artemether for the prevention of patent Schistosoma haematobium infections. Am J Trop Med Hyg 68:24–32
Peters W, Howells RE, POrtus J, Robinson BL, Thomas S, Warhurst DC (1977) The c chemotherapy of rodent malaria, XXVII. Studies on mefloquine (WR 142, 490). Ann Trop Med Parasitol 71:407–418
Rozman RS, Molek NA, Koby R (1978) The absorption, distribution, and excretion in mice of the antimalarial mefloquine, erythro-2, 8-bis(trifluoromethyl)-alpha- (2-piperidyl)-4-quinolinemethanol hydrochloride. Drug Metab Dispos 6:654–658
Sabah AA, Fletcher C, Webbe G, Doenhoff MJ (1986) Schistosoma mansoni: chemotherapy of infections of different ages. Exp Parasitol 61:294–303
Schwartz DE, Eckert G, Hartmann D, Weber B, Richard-Lenoble D, Ekue JM, Gemtilini M (1982) Single dose kinetics of mefloquine in man. Plasma levels of the unchanged drug and of one of its metabolites. Chemotherapy 28:70–84
Tooveys S (2009) Meflooquine neurotoxicity: a literature review. Travel Med Infect Dis 7:2–6
UNDP/World Bank/WHO Update (1983) Development of mefloquine as an antimalarial drug. Bull WHO 61:169–178
Utzinger J, Xiao SH, Tanner M, Keiser J (2007) Artemisinins for schistosomiasis and beyond. Curr Opin Investig Drugs 8:105–116
Van Nassauw L, Toovey S, Op V, den Bosch J, Timmermans JP, Vercruysse J (2008) Schistosomicidal activity of the antimalarial drug, mefloquine, in Schistosoma mansoni-infected mice. Travel Med Infect Dis 6:253–258
Warren KS (1982) Schistosomiasis: host-pathogen biology. Rev Infect Dis 4:771–775
Wu Z, Bum K, Yuan L, Yang G, Zhu J, Liu Q (1993) Factors contributing to reinfection with Schistosomiasis japonica aftertreatment in the lake region of China. Acta Trop 54:83–88
Xiao SH (2005) Development of antischistosomal drugs in China, with particular consideration to praziquantel and the artemisinins. Acta Trop 96:153–167
Xiao SH, Yue WJ, Yang YQ, You JQ (1987) Susceptibility of Schistosoma japonicum of different developmental stages to praziquantel. Chin Med J 100:759–768
Xiao SH, You JQ, Yang YQ, Wang CZ (1995) Experimental studies on early treatment of schistosomal infection with artemether. Southeast Asia J Trop Med Public Health 26:306–318
Xiao SH, Booth M, Tanner M (2000) The prophylactic effects of artemether against Schistosoma japonicum infections. Parasitol Today 16:122–126
Xiao Sh, Mei JY, Jian PY (2009a) Further study on mefloquine concerning several aspects in experimental treatment of mice and hamsters infected with Schistosoma japonicum. Parasitol Res 106:131–138
Xiao SH, Chollet J, Utzinger J, Mei JY, Jiao PY, Keiser J, Tanner M (2009b) Effect of single-dose oral mefloquine on the morphology of adult Schistosoma japonicum in mice. Parasitol Res 105:853–861
Xiao SH, May JY, Jiao PY (2009c) The in vitro effect of mefloquine and praziquantel against juvenile and adult Schistosoma japonicum. Parasitol Res 106:237–246
Xiao SH, Xue J, Shen BG (2010a) Tegumental alterations of adult Schuistosoma japonicum harbored in mice treated with a single oral dose of mefloquine. Chin J Parasitol Parasitic Dis 28:1–7
Xiao Sh, Xue J, Shen BG (2010b) Transmission electron microscopic observation on ultrastructural alterations in Schistosoma japonicum caused by mefloquine. Parasitol Res 106:1179–1187
Yolles TK, Morre DV, DeGusti DL, Ripsom CA, Meleney MS (1947) A technique for the perfusion of laboratory animals for the recovery of schistasomes. J Parasitol 33:419–426
Yue WJ, You JQ, Mei JY (1985) Prophylactic activity of praziquantel in animals infected with Schistosoma japonicum. Acta Pharmacol Sin 6:186–188
Zhang CW, Xiao SH, Utzinger J, Chollet J, Keiser J, Tanner M (2009) Histopathological changes in adult Schistosoma japonicum harbored in mice treated with a single dose of mefloquine. Parasitol Res 104:1407–1416
Acknowledgment
The study is supported by Major National Science and Technology Projects, 2009ZX10004-302, The Platform of Basic Research for the Important Parasitic Diseases Control Technology Based on Functional Genomics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, Sh., Mei, Jy. & Jiao, Py. Effect of mefloquine administered orally at single, multiple, or combined with artemether, artesunate, or praziquantel in treatment of mice infected with Schistosoma japonicum . Parasitol Res 108, 399–406 (2011). https://doi.org/10.1007/s00436-010-2080-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2080-y