Abstract
Propolis is a honeybee product with a very complex chemical composition and various pharmacological properties. This study was aimed to investigate antileishmanial activities of “Bursa” and “Hatay” propolis samples against Leishmania infantum and Leishmania tropica strains. Propolis samples were analysed with the gas chromatography-mass spectrometry technique. Promastigotes were incubated in Roswell Park Memorial Institute culture medium in the absence and presence of several concentrations (50, 100, 250, 500, 750, and 1,000 μg/mL) of each propolis sample. The viability and cell morphology of promastigotes in each concentration were examined after 24, 48, 72, and 96 h of incubation. The growth of leishmania parasites was significantly suppressed in the presence of 500, 750, and 1,000 μg/mL of Hatay propolis. Bursa propolis was found to be efficient in inhibiting the growth of leishmania promastigotes in culture media at these concentrations, 250, 500, 750, and 1,000 μg/mL. Thus, the in vitro results showed that the Hatay and Bursa propolis samples decreased significantly the proliferation of L. infantum and L. tropica parasites (p < 0.001); however, Bursa propolis was found to be more effective than Hatay propolis against leishmania promastigotes. These two natural products may be useful agents in the prevention of leishmanial infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmaniasis is considered by the World Health Organization as one of the major diseases caused by a protozoan (Murray et al. 2005).
Leishmaniasis is a group of infectious diseases caused by protozoan parasites that belong to the genus Leishmania and is transmitted by the bite of certain species of sand fly (subfamily Phlebotominae). Leishmaniasis, a significant cause of morbidity and mortality in several countries is still an increasing health problem in many parts of the world affecting people in 88 countries with about 350 million people living in areas of disease endemicity. Approximately 2 million new cases are reported each year (CDC 2009; WHO 2009).
Leishmaniasis is a widespread parasitic disease with a wide spectrum of clinical forms ranging from self-curing cutaneous leishmaniasis to fatal visceral leishmaniasis. The causative agents of disease or protozoans of the genus leishmania are transmitted by phlebotomine sand flies. Leishmania infantum (L. infantum) is the primary cause of visceral leishmaniasis in Turkey (Ok et al. 2002).
Leishmania tropica (L. tropica) causes cutaneous leishmaniasis that has been a serious health problem in various parts of our country. Cutaneous leishmaniasis, endemic to several regions of Turkey, is attributed almost exclusively to L. tropica (Ok et al. 2002).
Leishmaniasis is a public health problem in Turkey as well as in many countries. The disease occurs in various presentations, from the self-limiting and even self-healing cutaneous forms to a fatal systemic disease (Ok et al. 2002).
The treatment of leishmaniasis is a serious problem. This is primarily due to the unavailability of antileishmanial vaccines in the near future and because its chemotherapy still relies on the potentially toxic pentavalent antimonials that were developed many decades ago. The emergence of drug-resistant parasites presents an additional and major problem, and the appearance of resistance is limiting the drugs’ effectiveness (Croft and Coombs 2003).
Amphotericin B or pentamidine can be used as alternatives for resistant parasites (Croft and Coombs 2003); however, they are associated with toxic side effects and induction of parasite resistance. In addition, they require long-term treatment (Berman 2003). Given these reasons, the development of a new, cheap, safe, and easy-to-administer drug for the treatment of leishmanial diseases is absolutely necessary.
In the past few decades, there has been increasing concern in alternative therapies and the use of natural products, especially those derived from plants (Aksoy et al. 2007; Duran et al. 2008; Onlen et al. 2007a, b). Natural products play a highly significant role in new agents searching for the treatment of various diseases (Duran et al. 2006; Oksuz et al. 2005; Allahverdiyev et al. 2004).
Propolis has been used for a long time as a folk medicine to treat a lot of diseases (Ghisalberti 1979). It is a resinous substance collected by bees from the leaf buds or barks of trees and mixed with secreted beeswax and is a multifunctional material used by bees in the building, maintenance, and protection of their hives. Propolis is a useful substance in medicine. In the literature, propolis has various pharmacological activities such as antiparasitic (Duran et al. 2008), antibacterial (Onlen et al. 2007a, b; Duran et al. 2006; Oksuz et al. 2005; Onlen et al. 2007a, b), antifungal (Siqueira et al. 2009), antiviral (Shimizu et al. 2008), anti-inflammatory (McLennan et al. 2008), immunostimulatory (Cuesta et al. 2005), and anti-carcinogenetic activities (Diaz-Carballo et al. 2008).
The antileishmanial effect of propolis against leishmania parasites has been shown in a few studies (Duran et al. 2008; Pontin et al. 2008; Ayres et al. 2007; Machado et al. 2007). Our previous study has shown that “Adana” propolis samples have significant antileishmanial activity against L. tropica strains (Duran et al. 2008).
The chemical composition of propolis is quite complicated. Its compounds and biological activities depend on many different factors. The chemical composition of propolis may differ depending on the geographical region, and its biological properties may vary according to different plant sources (Kumazawa et al. 2004; Bankova et al. 2002).
In the present study, an evaluation was made on the potential antileishmanial activity of two propolis samples collected from different regions (“Hatay” and “Bursa”) of Turkey against L. infantum and L. tropica strains. Moreover, it was aimed to investigate the relationship between the chemical composition of propolis and antileishmanial activity.
Material and method
Sample collection
Propolis samples were collected from the southeastern Mediterranean region of Anatolia (Hatay province) and south Marmara region (Bursa province) during 2007. Hand-collected propolis samples were kept in a dry and dark place and stored at 4°C until its processing.
Each of the propolis samples was sliced into small pieces after cooling at −40°C and then grated. The samples were dissolved in 96% ethanol (1:10, w/v) at 25°C for 14 days in clean dark brown glass bottles. Airtight bottles were shaken for 3 min at every 6 h for a period of 15 days. After the extraction period, the supernatant was filtered twice with Whatman no. 4 and no. 1 filter papers. Ethyl alcohol extract was then evaporated to dryness under a vacuum. Five micrograms of residue was mixed with 75 μL of dry pyridine and 50 μL bis(trimethylsilyl) trifluoroacetamide, heated, and the supernatant was analyzed by gas chromatography-mass spectrometry (GC-MS) (Sorkun et al. 2001).
Parasites
The L. infantum (MON-1) strain was obtained from Refik Saydam Central Institute of Hygiene, Ankara. The L. tropica (HOM/TR/94MG) strain was supplied from Mustafa Kemal University, Medical Faculty, the culture collection of parasitology. Leishmania parasites were cultured in a Novy, MacNeal, and Nicolle medium and subcultured in Roswell Park Memorial Institute (RPMI)-640 (Gibco-BRL) medium supplemented with 10% fetal calf serum (FCS) (Sigma, MI, USA), 100 IU/mL penicillin, and 100 μg/mL streptomycin (Gibco-BRL) at 26°C. The promastigotes of Leishmania strains were harvested by centrifugation at 3,000 rpm for 15 min at 4°C.
Effect of DMSO on the promastigotes
In order to test the effects of dimethyl sulfoxide (DMSO) against promastigotes, 1 × 105 parasites were inoculated into each well of 12-well plates containing RPMI-1640 culture medium, and parasite cells were allowed to grow for an additional 48 h in the presence of decreasing amounts of DMSO (8%, 4%, 2%, 1%, 0.5%). The non-toxic concentration was determined up to 2%. A concentration of 0.5% and 1% DMSO did not influence the growth of the parasites as determined microscopically. Therefore, it is considered non-toxic to Leishmania promastigotes (Fig. 1a). Furthermore, the morphology of the parasite cells exposed to DMSO (up to 1%) was not altered. Also, the high concentration (2%) of DMSO slightly suppressed the growth of L. tropica and L. infantum. In addition, the morphological changes were detected on the parasite cells exposed to DMSO (2%). In order to dissolve propolis, it was selected a concentration of DMSO lower than 2%. Therefore, propolis samples were dissolved in 1% DMSO.
The effects of DMSO and Glucantime on the viability of L. tropica (a) and L. infantum (b) promastigotes compared with the control cells. L. tropica promastigotes were treated with DMSO and Glucantime. Negative control (containing only RPMI-1640 medium, not containing drug and DMSO): significantly increasing was observed in the number of L. tropica and L. infantum promastigotes after 24 h incubation. DMSO control (containing RPMI-1640 plus DMSO 1%): a significantly increasing number of L. tropica and L. infantum promastigotes was observed after 24 h of incubation. Positive control [containing RPMI-1640 plus Glucantime (150 μg/ml)]: a significantly decreasing number of L. tropica and L. infantum promastigotes was observed after 24 h of incubation. An important statistical decline was detected between the positive control group and the other groups (negative control and DMSO group) (p < 0.001). Each data point represents values from three independent experiments (n = 3). Represents significant results (p < 0.05) using the Kruskal–Wallis and Mann–Whitney U tests when the treated group was compared with the control
Cytotoxicity test
Preparation of the cell culture
To evaluate the cytotoxicity of propolis for human cells, the human larynx epidermoid carcinoma (HEp-2) cell line was used. The cells were cultured in RPMI-1640 medium with 10% (w/v) FCS. Incubation of the cells was made at 37°C in air with 5% carbon dioxide.
Propolis samples were dissolved in DMSO (Sigma, MI, USA). Stock solutions of propolis samples were prepared in DMSO at the concentration of 1%. The concentrations of tested samples were 50, 100, 250, 500, 750, and 1,000 μg/mL. Intact live promastigotes in the stationary growth phase were added to the microwell plate.
In order to test the effect of the propolis samples on HEp-2 cells, 1 × 105 cells were seeded into each well of 24-well plates (flat-bottomed), cultured for 6 h at 28°C, and the cells were allowed to grow for an additional 48 h. The propolis samples were diluted, whereupon decreasing amounts (3,200; 1,600; 800; 400; 200; 100; 75; 50; and 25 μg/mL) were placed per well. All experiments were performed in triplicate, and the results were expressed as log number cells per milliliter on the percentage of growth inhibition.
The cytotoxicity of the propolis samples was determined using a conventional hemocytometer and the trypan blue exclusion. The highest noncytocidal (on HEp-2 cells) concentration of the tested samples was determined to be 1,600 μg/mL (1.6 mg/mL). Therefore, propolis concentrations lower than 1.6 mg/mL were selected.
Antileishmanial activity
The promastigotes of L. tropica and L. infantum (105 parasites/mL) were incubated at 26°C for 96 h in RPMI-1640 culture medium supplemented with 10% FCS in the absence and presence of several concentrations (50, 100, 250, 500, 750, and 1,000 μg/mL) of each propolis samples in order to evaluate the viability of parasites. Cell growth was determined daily (24, 48, 72, and 96 h) by inverted microscopy.
Parasite viability was evaluated before and after the incubations by trypan blue exclusion with a hemocytometer. The viability of promastigotes was evaluated by mobility and lack of staining after trypan blue exclusion. Cell viability was determined as described previously by David et al. in 1997 (David et al. 1997).
The 50% inhibitory concentration (IC50) values were determined by logarithmic regression analysis of the data as described previously (David et al. 1997). Evaluation of cell morphology was carried out with fresh as well as Giemsa-stained preparations. The propolis samples were diluted in DMSO at 1% and then in RPMI. In all tests, 1% DMSO (the same concentration presented in the highest dose of the compounds) and medium alone were used as controls.
In the experiments, Glucantime (150 mg/mL) (Aventis, France) and 1% DMSO (without propolis) were used as positive and negative controls, respectively. The positive and negative controls were run using the same dilutions used for the propolis samples. All experiments were performed in triplicate.
Statistical analysis
The mean and standard deviation of at least three experiments were calculated. The Kruskal–Wallis and Mann–Whitney U tests were used to compare each group. P values less than 0.05 or equal were considered statistically significant. All statistical analyses in the present study were performed using SPSS for Windows, version 11.5.
Results
Chemical analysis
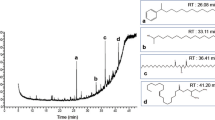
The percentage composition of identified compounds in Bursa and Hatay propolis samples are given in Table 1. The variability of the constituents of propolis in the two samples showed that they were collected from different plants depending on the geographic location.
Effect of DMSO
In the DMSO group, the cell cultures were incubated with DMSO alone (without any supplement). Effects of DMSO on the viability of promastigotes were compared to control cells. The DMSO control showed no toxic effect at 1% (v/v) for the promastigotes. Selected concentration of DMSO (containing 1% DMSO) did not inhibit the growing of parasites cells. At the end of 96 h, there were no statistically significant difference in the cell number between the control and the DMSO-containing groups (p > 0.05). Also, no cytopathological changes were observed compared with the control group (Fig. 1a, b).
Morphologic change of parasites
In order to assess the morphological changes on promastigotes due to propolis, damaged parasites were examined using an inverted microscope and compared with intact leishmania parasites (Fig. 2a). We found that some components in the parasite cytoplasm appeared to be follicle-like structures. In addition, it was determined there were some cytopathological changes on the cells, such as granulation, loss of flagellum, and rounding of the parasites (Fig. 2b).
Leishmania promastigotes with normal morphology (a), cytopathological changes due to propolis on leishmania promastigotes (b) (objective 1,000×). a Leishmania promastigotes display a typical morphology with short, narrow cell body, and an elongated flagellum. b Leishmania promastigotes after propolis treatment. The morphological changes on promastigotes (damaged parasites) due to propolis are seen. Some cytopathological changes on leishmania promastigotes such as granulation, loss of flagellum, and rounding of the parasites. Red arrows show cytopathological changes on cells such as granulation, loss of flagellum, and rounding of the parasites. Each experiment was performed in triplicate (n = 3) and generated similar morphological features
Typical promastigote morphology was observed at low propolis concentrations (for Bursa propolis, 50 and 100 μg/mL; for Hatay propolis, 50, 100, and 250 μg/mL), atypical parasite cells were not shown in these propolis-treated cultures (Fig. 2b).
Growth inhibitory effects in vitro
It was determined that propolis samples exhibited the antileishmanial effect in vitro in a dose- and time-dependent manner. The cell viability and morphological alterations of promastigotes were evaluated in the presence of different concentrations of Bursa propolis samples against both L. infantum (HOM/TR/00/OG-VL) and L. tropica (HOM/TR/94MG) strains. No morphological changes (cytopathological effects) of the parasites were seen in the presence of up to 100 μg/mL of propolis (include 100 μg/mL). There were no nuclear or cytoplasmic changes on parasite cells. Furthermore, an increase in the number of parasites was determined in the samples taken from culture at 24, 48, 72, and 96 h of incubation (p > 0.05). The low concentrations of the Bursa propolis samples such as 50 and 100 μg/mL did not cause any cytopathological changes on the parasite cells while the high concentrations of the same propolis samples such as 250, 500, 750, and 1,000 μg/mL triggered cytopathological effects on the parasite cells. Bursa propolis with lower concentration than 250 μg/mL did not affect the viability of promastigotes. The present results indicated that Bursa propolis with a minimum concentration value of 250 μg/mL significantly reduced cell viability compared to the control group (p < 0.001) (Fig. 3a, b).
Antileishmanial activity of Bursa propolis against L. infantum (a) and L. tropica (b) promastigotes compared with the control groups. L. infantum and L. tropica promastigotes were treated with Bursa propolis at various concentrations of propolis (50, 100, 250, 500, 750, and 1,000 μg/ml). Negative control (containing only RPMI-1640 medium, not containing Bursa propolis): a significantly increasing number of leishmania promastigotes was observed depending on incubation time. DMSO control (containing DMSO 1% plus Bursa propolis): a significantly increasing number of leishmania promastigotes was observed depending on incubation time. Treated groups (containing 50 and 100 μg/ml of Bursa propolis): a significantly increasing number of L. infantum and L. tropica promastigotes was observed depending on incubation time. Treated groups (containing 250, 500, 750, and 1,000 μg/ml of Bursa propolis): a significantly decreasing number of L. infantum and L. tropica promastigotes was observed depending on incubation time. Each data point represents values from three independent experiments (n = 3). Represents significant results (p < 0.05) using the Kruskal–Wallis and Mann–Whitney U tests when the treated group was compared with the control
Unlike the Bursa propolis, in studies on Hatay propolis with concentrations of 50, 100, and 250 μg/mL, leishmania promastigotes showed the typical morphology after 24, 48, 72, and 96 h of incubation. Cytopathological changes on the parasite cells were detected at 500 μg/mL and at higher values than this concentration. The growth of leishmania parasites was significantly suppressed in the presence of 500, 750, and 1,000 μg/mL of Hatay propolis. Hatay propolis with a minimum concentration value of 500 μg/mL significantly reduced cell viabilities of leishmania cells compared to the control group (p < 0.001). It was determined that concentrations lower than 500 μg/mL such as 50, 100, and 250 μg/mL did not suppress the parasite growth (Fig. 4a, b).
Antileishmanial activity of Hatay propolis against L. infantum (a) and L. tropica (b) promastigotes compared with the control groups. L. infantum and L. tropica promastigotes were treated with Hatay propolis at various concentrations (50, 100, 250, 500, 750, and 1,000 μg/ml). Negative control (containing only RPMI-1640 medium, not containing Hatay propolis): a significantly increasing number of L. infantum and L. tropica promastigotes was observed depending on incubation time. DMSO control (containing DMSO 1% plus Hatay propolis): a significantly increasing number of L. infantum and L. tropica promastigotes was observed depending on incubation time. Treated groups (containing 50, 100, and 250 μg/ml of Hatay propolis): a significantly increasing number of L. infantum and L. tropica promastigotes was observed depending on incubation time. Treated groups (containing 500, 750, and 1,000 μg/ml Hatay propolis): a significantly decreasing number of L. infantum promastigotes was observed depending on incubation time. Each data point represents values from three independent experiments (n = 3). Represents significant results (p < 0.05) using the Kruskal–Wallis and Mann–Whitney U tests when the treated group was compared with the control
Glucantime was used as a positive control. In the drug-control group, parasites cultured with 150 μg/mL of Glucantime arrested parasite growth during 24 h (Fig. 1a, b).
When the negative control (without propolis) is compared with the positive control (with Glucantime, 150 μg/mL), an important statistical decline in the drug group at the end of the 24 h incubation was found (p < 0.001). This decline in the cell counts was more prominent following the 48-h incubation. In addition, all of the parasites strains died after incubation period of 96 h.
IC50 values
The effects of Bursa and Hatay propolis on the viability of L. infantum and L. tropica were tested. The IC50s for L. infantum promastigotes were 125 μg/mL for Bursa propolis and 325 μg/mL for Hatay propolis, and the IC50s for L. tropica promastigotes were 175 μg/mL for Bursa propolis and 350 μg/mL for Hatay propolis (Fig. 5). Figure 5 shows the viabilities of leishmania promastigotes in the absence and presence of the propolis samples during time. Bursa propolis samples at 250 μg/mL were able to kill 100% of the L. infantum and L. tropica promastigotes in 96 h; however, Hatay propolis samples were unaffected at 250 μg/mL.
IC50 values for Bursa and Hatay propolis against L. infantum and L. tropica promastigotes. Leishmania promastigotes were treated with Hatay and Bursa propolis at various concentrations. The IC50 value for L. infantum promastigotes were 125 μg/ml for Bursa propolis and 325 μg/ml for Hatay propolis. The IC50 value for L. tropica promastigotes were 175 μg/ml for Bursa propolis and 350 μg/ml for Hatay propolis
Discussion
Pentavalent antimonial compounds have been the first-line therapeutic option for the antileishmanial therapy since years. Because of their potential toxicity and acquired drug resistance, the use of these drugs is limited (Croft and Coombs 2003). Microbial resistance due to the irrational use of pharmaceutical drugs has been an increasing problem for years. Natural products have played a major role in drug discovery because the spreading of antimicrobial resistance and some limitations of new drugs have led to the consideration of natural therapy for the treatment of various diseases. Among natural compounds, propolis has been considered to be the most promising compound, which has various pharmacological properties (Newton et al. 2002).
In the literature, antileishmanial effects of propolis which were gathered from the different geographic zones have been shown (Salatino et al. 2005; Teixeira et al. 2008). Although a limited number of studies have been made in this field, these kinds of studies support the possibility that propolis has potential antileishmanial activity against the leishmania species (Duran et al. 2008; Pontin et al. 2008; Ayres et al. 2007; Machado et al. 2007). Propolis may be promising as a potential antiprotozoal agent against the leishmania species; however, it has not been explored widely yet. It is known that the chemical composition and antimicrobial activity of propolis can change from region to region depending on the diversity of plants (Salatino et al. 2005; Teixeira et al. 2008). Due to its geographical position, Turkey has a rich natural plant habitat with approximately 10,000 species, many of which being native to the country and not found anywhere else in the world (http://www.allaboutturkey.com). According to our previous study, Adana propolis has been found to be quite active against L. tropica (Duran et al. 2008). Therefore, the in vitro antileishmanial activities of two propolis samples which were collected from two different regions of Turkey were investigated against the L. infantum and L. tropica promastigotes.
None of these samples at the selected concentrations were toxic to mammalian cells. Both of the tested propolis samples had significant antileishmanial activity on cultured promastigotes of leishmania strains. These studies were consistent with the results of previous studies (Duran et al. 2008; Pontin et al. 2008; Ayres et al. 2007; Machado et al. 2007).
One of the aims of this study was to compare differences among the antileishmanial activities of the propolis samples obtained from Bursa and Hatay geographic areas. In our study, Bursa propolis for leishmania strains was found more effective than Hatay propolis. We think that it might be due to a diverse geographic location and plant diversity. Propolis is collected by bees from numerous different plant species, depending on the geographic area and the local flora. Therefore, significant variations may occur in the chemical composition, and the impact spectrum of propolis depends directly on the local flora. It was reported that the quality and quantity of the constituents in propolis and its biological activity may vary widely according to the geographic location and to the different plant sources (Uzel et al. 2005). Our findings confirm the results of Uzel et al.’s study.
The propolis samples tested in this study showed a strong activity against two different species of leishmania. Propolis samples succeeded in inhibiting the growth of leishmania strains in varying concentrations. There were significant statistical differences in terms of cell count between the propolis-treated groups and the control groups (p < 0.01). Comparing propolis samples tested, Bursa propolis samples possessed higher antileishmanial activity against both L. infantum and L. tropica than the Hatay propolis samples. Although remarkable results were found in the present study with respect to antileishmanial activity, both Bursa and Hatay propolis samples were less active than Glucantime.
As reported in the literature, a wide variety of biologically active compounds of propolis can play an important role for this antileishmanial activity. Some compounds of propolis have been reported to possess antileishmanial activity (Duran et al. 2008; Pontin et al. 2008; Ayres et al. 2007; Machado et al. 2007). It was shown that propolis samples were quite effective against L. tropica and L. infantum. The chemical compositions of propolis samples were analysed by high-resolution GC-MS. The present study emphasized that ethanolic extracts of the two types of Turkish propolis reduced the proliferation of L. infantum and L. tropica promastigotes in vitro. Bursa propolis samples contain a high concentration of cinnamyl cinnamate and ethyl oleate and aromatic acid esters such as benzyl cinnamate, benzenedicarboxylic acid. Hatay propolis samples contain high concentration of aromatic esters and flavanols such as chrysin and benzopyran (Table 1). We would rather think that the compounds such as aromatic acids, aromatic acid esters, flavanols, and cinnamic acid esters may be responsible for the antileishmanial effects.
It was reported that the antimicrobial activity of propolis was associated with the presence of flavonoids and derivatives of caffeic acid (Salomao et al. 2008; Benkovic et al. 2009; Marcucci et al. 2000; Prytzyk et al. 2003). The composition percentages of the two propolis samples were found to be similar. Both Bursa and Hatay propolis have aromatic acids, aromatic acid esters, flavanols, and cinnamic acid esters.
Bursa propolis samples had more effect than the Hatay propolis samples against L. infantum and L. tropica promastigotes. The ratio of the aromatic acids, aromatic acid esters, fatty acid ester, and cinnamic acid esters were found to be remarkable higher than those of the Hatay propolis samples. The observed significant antileishmanial activity of propolis could be attributed to its some compounds such as cinnamic acid esters, aromatic acids, and aromatic acid esters.
The potential antiprotozoal activity of propolis for the treatment of some parasitic diseases, e.g., malaria, had already been suggested in previous studies (Ayres et al. 2007; Freitas et al. 2006).
While low propolis concentrations (up to 100 μg/mL) did not affect the growth of leishmania strains, higher propolis concentrations inhibited the growth of both L. tropica and L. infantum and caused the degenerative changes on leishmania parasites; however, no toxic effects on the HEp-2 cells were noticed. Dimethyl sulfoxide alone with the used concentration in experiments did not affect the growth of the parasites; thus, the inhibition of cell growth was due only to the propolis effects. The study indicated that the ethanolic extracts (250, 500,750, and 1,000 μg/mL) of two different propolis samples caused growth inhibition of L. infantum and L. tropica promastigotes at the IC50 < 350 μg/mL (Fig. 5). Experiments showed that Bursa propolis was effective against the leishmania species in vitro with IC50 values of 125 μg/mL for L. infantum and 175 μg/mL for L. tropica, whereas IC50 values for Hatay propolis were 325 μg/mL for L. infantum and 350 μg/mL for L. tropica.
In conclusion, we have clearly demonstrated the efficacy of the propolis samples against L. infantum and L. tropica in vitro. The propolis samples used in this study, especially Bursa propolis, presents promising activity. Natural products have been the major sources for drug discovery and the development of novel antileishmanial agents. In the present study, the results confirmed that propolis is a potential source of new and selective drugs for the treatment of leishmaniasis. Further studies are needed in order to understand its antiprotozoal mechanism.
References
Aksoy A, Duran N, Toroglu S, Koksal F (2007) Short-term effect of mastic gum on salivary concentrations of cariogenic bacteria in orthodontic patients. Angle Orthod 77:124–128
Allaboutturkey (2009) http://www.allaboutturkey.com/millipark.htm. Accessed 11 Jan 2009
Allahverdiyev A, Duran N, Ozguven M, Koltas S (2004) Antiviral activity of the volatile oils of Melissa officinalis L. against herpes simplex virus type-2. Phytomedicine 11:657–661
Ayres DC, Marcucci MC, Giorgio S (2007) Effects of Brazilian propolis on Leishmania amazonensis. Mem Inst Oswaldo Cruz 102:215–220
Bankova V, Popova M, Bogdanov S, Sabatini A (2002) Chemical composition of European propolis: expected and unexcepted results. Z Naturforsch C 57:530–533
Benkovic V, Knezevic AH, Orsolic N et al (2009) Evaluation of radioprotective effects of propolis and its flavonoid constituents: in vitro study on human white blood cells. Phytother Res 23:1159–1168
Berman J (2003) Current treatment approaches to leishmaniasis. Curr Opin Infect Dis 16:397–401
CDC (2009) http://www.cdc.gov/ncidod/dpd/parasites/leishmania/factsht_leishmania.htm#common. Accessed 11 Jan 2009
Croft SL, Coombs GH (2003) Leishmaniasis—current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol 19:502–508
Cuesta A, Rodriguez A, Esteban MA, Meseguer J (2005) In vivo effects of propolis, a honeybee product, on gilthead seabream innate immune responses. Fish Shellfish Immunol 18:71–80
David J, Satoskar AR, Brombacher F, Shoemaker CB, Titus RG, Boza M (1997) Immunomodulatory properties of maxadilan, a peptide derived from sand fly saliva. Acta Parasitol Turcica 21:174
Diaz-Carballo D, Malak S, Bardenheuer W, Freistuehler M, Peter Reusch H (2008) The contribution of plukenetione A to the anti-tumoral activity of Cuban propolis. Bioorg Med Chem 16:9635–9643
Duran G, Duran N, Culha G, Ozcan B, Oztas H, Ozer B (2008) In vitro antileishmanial activity of Adana propolis samples on Leishmania tropica: a preliminary study. Parasitol Res 102:1217–1225
Duran N, Koc A, Oksuz H et al (2006) The protective role of topical propolis on experimental keratitis via nitric oxide levels in rabbits. Mol Cell Biochem 281:153–161
Freitas SF, Shinohara L, Sforcin JM, Guimaraes S (2006) In vitro effects of propolis on Giardia duodenalis trophozoites. Phytomedicine 13:170–175
Ghisalberti EL (1979) Propolis: a review. Bee World 60:59–84
Kumazawa S, Hamasaka T, Nakayama T (2004) Antioxidant activity of propolis of various geographic origins. Food Chem 84:329–339
Machado GM, Leon LL, De Castro SL (2007) Activity of Brazilian and Bulgarian propolis against different species of Leishmania. Mem Inst Oswaldo Cruz 102:73–77
Marcucci MC, Ferreres F, Custodio AR et al (2000) Evaluation of phenolic compounds in Brazilian propolis from different geographic regions. Z Naturforsch C 55:76–81
McLennan SV, Bonner J, Milne S et al (2008) The antiinflammatory agent propolis improves wound healing in a rodent model of experimental diabetes. Wound Repair Regen 16:706–713
Murray HW, Berman JD, Davies CR, Saravia NG (2005) Advance in leishmaniasis. Lancet 366:1561–1577
Newton SM, Lau C, Gurcha SS, Besra GS, Wright CW (2002) The evaluation of forty-three plant species for in vitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria canadensis. J Ethnopharmacol 79:57–67
Ok UZ, Balcioglu IC, Ozkan AT, Ozensoy S, Ozbel Y (2002) Leishmaniasis in Turkey. Acta Trop 84: 43–48
Oksuz H, Duran N, Tamer C, Cetin M, Silici S (2005) Effect of propolis in the treatment of experimental Staphylococcus aureus keratitis in rabbits. Ophthalmic Res 37:328–334
Onlen Y, Duran N, Atik E et al (2007a) Antibacterial activity of propolis against MRSA and synergism with topical mupirocin. J Altern Complement Med 13:713–718
Onlen Y, Tamer C, Oksuz H, Duran N, Altug ME, Yakan S (2007b) Comparative trial of different anti-bacterial combinations with propolis and ciprofloxacin on Pseudomonas keratitis in rabbits. Microbiol Res 162:62–68
Pontin K, Da Silva Filho AA, Santos FF et al (2008) In vitro and in vivo antileishmanial activities of a Brazilian green propolis extract. Parasitol Res 103:487–492
Prytzyk E, Dantas AP, Salomao K et al (2003) Flavonoids and trypanocidal activity of Bulgarian propolis. J Ethnopharmacol 88:189–193
Salatino A, Teixeira EW, Negri G, Message D (2005) Origin and chemical variation of Brazilian propolis. Evid Based Complement Alternat Med 2:33–38
Salomao K, Pereira PR, Campos LC et al (2008) Brazilian propolis: correlation between chemical composition and antimicrobial activity. Evid Based Complement Alternat Med 5:317–324
Shimizu T, Hino A, Tsutsumi A, Park YK, Watanabe W, Kurokawa M (2008) Anti-influenza virus activity of propolis in vitro and its efficacy against influenza infection in mice. Antivir Chem Chemother 19:7–13
Siqueira AB, Gomes BS, Cambuim I et al (2009) Trichophyton species susceptibility to green and red propolis from Brazil. Lett Appl Microbiol 48:90–96
Sorkun K, Suer B, Salih B (2001) Determination of chemical composition of Turkish propolis. Z Naturforsch C 56:666–668
Teixeira EW, Message D, Negri G, Salatino A, Stringheta PC (2008) Seasonal variation, chemical composition and antioxidant activity of Brazilian propolis samples. Evid Based Complementary Alternat Med (in press)
Uzel A, Sorkun K, Oncag O, Cogulu D, Gencay O, Salih B (2005) Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiology Res 160:189–195
WHO (2009) http://www.who.int/leishmaniasis/burden/en/. Accessed 11 Jan 2009
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duran, N., Muz, M., Culha, G. et al. GC-MS analysis and antileishmanial activities of two Turkish propolis types. Parasitol Res 108, 95–105 (2011). https://doi.org/10.1007/s00436-010-2039-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2039-z