Abstract
We investigated the occurrence of blood parasites of two lizard species: the common or viviparous lizard (Zootoca vivipara) and the sand lizard (Lacerta agilis) in western Poland. Selected traits of lizard body morphology were studied with respect to the presence and intensity of haematozoan infection in blood samples collected from 218 adult lizards; 88 of the common lizard and 130 of the sand lizard. Haemogregarinid blood parasites were found to be the common parasite of both lizard species in studied locality with prevalence 39.8 (95% CL, 29.5–50.8) for Z. vivipara and 22.3 (95% CL, 15.5–30.4) for L. agilis. Incidence of parasitemia did not differ between sexes and was not correlated with morphological traits or presence of ectoparasites—Ixodes ricinus ticks. However, a significant difference between the two species of lizards was a greater frequency of haemogregarinid parasitism in Z. vivipara.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasites play an important role in the evolution of host life-history traits because they often impose important selective pressures on them. Parasites remove resources from their hosts that could otherwise be used for host growth, maintenance, or reproduction (Price 1980). The prevalence and the intensity of infection provide basic information about status and the possible impact of parasites in the lizard population in natural conditions. Parasite interactions influence numerous aspects of the ecology of their hosts (Smallridge and Bull 2000). Haemogregarinid blood parasites (Apicomplexa, Adeleorina) are the most common parasites of reptiles with worldwide distribution. This group comprises the families Hepatozoonidae, Karyolysidae, and Haemogregarinidae. Species of Haemogregarina parasitize turtles and possibly other reptiles in contact with leeches which transmit these haemoparasites (Telford 2008). Hepatozoon species are transmitted by various haematophagous arthropods such as mosquitos, mites, and ticks. Karyolysus species are transmitted by mites. The precondition for the successful transmission is ingestion of the vector invertebrate host (Telford 2008). The pathogenicity of haemogregarines for their hosts is poorly known. Low levels of parasitism do not appear to affect health or blood chemistry of the host organism. Blood protozoa may affect reptiles by reducing their ability to transport oxygen (Caudell et al. 2002). However, parasites will inevitably compete for energy and nutrients with the host, which consequently must resolve trade-offs between the amount of energy invested in the reproductive effort and any immunological battle against parasites (see Møller 1997). Adaptive immune responses against parasites are costly, manifested by, e.g., reduced body condition and a compromised defense against secondary infections by haemoprotid parasites (Olsson et al. 2005). Co-occurrence of different species of parasites in the same host can alter the life-history traits in ways different from effects of a single species.
Host organisms adaptively respond to a parasitism by altering particular life-history traits (Minchella 1985). Reports have documented that haemogregarinid parasites influenced several life-history traits, such as body mass condition (Amo et al. 2004), age-dependant host mortality (Sorci 1996), colour, composition of femoral secretions (Martin et al. 2008), and reduction of tail regeneration after autotomy (Oppliger and Clobert 1997).
In the current study, we investigated simultaneously the relationship among morphology and prevalence and intensity of blood parasite infection in two co-existing lizard species.
Materials and methods
Study species
The sand lizard (Lacerta agilis) is a short-legged, rather robust, small- to medium-sized lizard (up to 90 mm snout to vent length (SVL)) from the family Lacertidae. The sand lizard is ground-dwelling and a strongly diurnal species with one of the widest distribution ranges of all reptiles (Bischoff 1984). Sand lizards are largely insectivorous, actively chasing and consuming a range of spiders and insects (Corbett and Tamarind 1979).
The common lizard (Zootoca vivipara formerly Lacerta vivipara) is a small lacertid (adult SVL 50–70 mm) with allopatric oviparous (egg-laying) and viviparous (live-bearing) populations. It inhabits fragmented habitats such as peat bogs and heath lands. Common lizards are widely distributed throughout Europe and Asia, and their distribution overlaps the polar circle. Z. vivipara has the most extensive range of all lacertids, significantly larger than L. agilis. They actively forage on invertebrates, especially on insects.
Study area
The 70-km2 study area was located in southern Wielkopolska near the town Odolanów (51° 34′ N, 17° 40′ E). The mean density, established by 84 transect lines in 200-m lengths randomly covered the study area, was 0.85 ± 1.35/200 m of transect line for the common lizard and 0.31 ± 0.71/200 m for the sand lizard (Ekner et al. 2008). For more details about the study area, see Antczak et al. (2004). Lizards were captured in April of three consecutive years (2006–2008).
Blood collection and parasite detection
Lizards were captured using landing fishnets, by hand or by noosing, where a loop made from fishing nylon was attached to the end of a wooden stick and dangled in front of a lizard, who would be captured upon walking through the loop. Animals were sexed, aged (adult, sub-adult, juvenile), and examined for the presence of ectoparasites, including ticks Ixodes ricinus. All ticks were counted on the particular part of the body where they were found, removed manually with forceps, and stored in 70% ethanol for further identification.
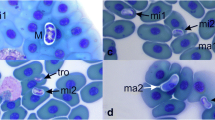
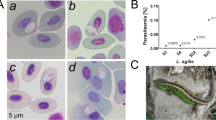
Blood samples from each adult lizard specimen were obtained by ventral puncture of the caudal vein with disposable sterile syringes. Blood smears were made and air-dried immediately in the field. Slides were stored and transported to the laboratory in plastic slide boxes. Slides were stained with Giemsa's solution (Sigma) for 30 min and examined with a light microscope at ×200 magnification. Approximately 50 microscopic fields on each smear were examined for the presence of blood parasites. When no parasites were detected by this method, the smear was considered negative. Intensity of infection (parasitemia) was estimated for each individual as the percentage of infected red blood cells found in an estimated number of 10,000 cells at ×1,000 magnification with oil immersion. Parasite scores were blind in the sense that the observer (BH) was given the ring number and no other information about the lizards from which samples were taken.
Measurement of morphometric traits
For each individual, we measured SVL with an accuracy of 0.5 mm, with digital callipers and body mass (± 0.1 g) with a digital scale. After measurements, the lizards were released in the same location they were captured. Collar scales, used for a different examination (Majláthová et al. 2008), were taken from each lizard. This avoided the possibility that the same lizards were considered twice in the analyses.
Statistical analyses
To improve sample size and show a more general pattern, data from three breeding seasons were pooled before analysis. Statistics were performed using SPSS for Windows (http://www.spss.com/), and tests are two-tailed. Confidential limits (95% CL) were for binary, absence–presence; data were calculated in Excel Macro (http://office.microsoft.com/pl-pl/excel). Data are presented mainly as means SD; however, abundant presence of haemogregarinid blood parasites allowed a rough quantification from microscope counts, with very few lizards falling into the last highest counting categories (Fig. 1). Hence, as in many other studies (see discussion in Votýpka et al. 2003; Olsson et al. 2005), we decided to divide the data into only two groups: infected and non-infected individuals. Because not all data were available for every captured individual, sample size differs slightly between analyses.
Results
Ectoparasites
Two species of ectoparasitic arthropods were found on both lizard species. Developmental stages of I. ricinus ticks as well as Ophionyssus saurarum mites were found.
Blood parasite prevalence
In the blood smears of 218 examined lizards, the haemogregarinid blood parasites were found in 138 individuals (63.3%, CL = 56.5–69.7; Fig. 2). The level of parasite prevalence differs significantly between the two studied species (logistic regression, Wald = 27.049, B ± SE = 1.57 ± 0.30, P < 0.0001) but not significantly between sexes in both species—compare 95% confidential limits in Table 1.
The mean number of haemogregarinid blood parasite count per 10,000 erythrocytes was 73.8 ± 110.4 for Z. vivipara (kurtosis = 2.85) and 85.7 ± 115.2 for L. agilis (kurtosis = 15.79).
Haemogregarinid infection vs. morphometric traits and number of ticks
No significant relationships between incidence of blood parasites and morphometric traits were found in both studied species and sex categories (Table 2).
Moreover, no significant relationship between the I. ricinus tick load on lizard and blood parasite occurrence was found in either lizard species (Table 2). Additional logistic regression analysis between the presence of blood parasites and number of ticks (log-transformed + 1 before analysis) gave insignificant results as well (P = 0.08 in sand lizard, and P = 0.67 in common lizard).
Discussion
In our study, blood from two co-existing lizard species, the viviparous lizard (Z. vivipara) and the sand lizard (L. agilis) in Poland was sampled. Observed parasites were termed as haemogregarinids because it is difficult to determine the parasites to species based only on the morphology of gametocytes in erythrocytes. The precise identification is possible only with combination of description of sporogonic stages (Olsen 1974). O. saurarum mites, found on lizards in studied locality, are known as vectors for Karyolysus sp. (Reichenow 1913). Based on the observed morphology of blood parasites, together with the finding of the O. saurarum mites, we assume that at least two different species, probably from the genus Karyolysus, were present in erythrocytes of lizards. Differences in prevalence of infection between the two species were found with 39.8% of Z. vivipara parasitized compared to 22.3% of L. agilis. The prevalence of blood parasites in comparison to other studies carried out all over the world was low. Amo et al. (2004) found almost 80% of Lacerta monticola in Spain infected with haemogregarines. The prevalence of blood parasite infections is often relatively stable for long periods (Bennett and Cameron 1974; Eisen 2000; Schall et al. 2000; Smallridge and Bull 2000) and independent of seasonal climatic changes in temperature (Schall 1986). In our studied lizard population, prevalence of haemogregarinid blood parasites did not change significantly over the period of our study.
Factors that can influence parasite abundance include host sex and age (Schall et al. 2000; Smallridge and Bull 2000), host reproductive effort (Norris et al. 1994; Nordling et al. 1998; Veiga et al. 1998), host condition and physiology (Salvador et al. 1997; Appleby et al. 1999; Dowell 2001), vector biology (Sol et al. 2000; Readon and Norbury 2004), and host density (Arneberg et al. 1998). Infection of Z. vivipara was found to be significantly higher than L. agilis. Differences between species may be either due to differences in life history, behaviour, immune system, changes in diet, or simply an increased chance of exposure to vector invertebrates in different habitats over time (Eisen and Wright 2001). For example, it was shown previously that high stress conditions such as predation pressure increased the load of haemogregarines in Z. vivipara (Oppliger et al. 1998; Martin and Lopez 1999). Our study was conducted in the farmland habitats in Western Poland where Z. vivipara is under high predation pressure by great grey shrike (Lanius excubitor) here (pers. observation).
Previous studies have demonstrated that the prevalence of blood parasites in lizards is frequently independent of environmental seasonal conditions (Schall 1986; Schall and Marghoob 1995). Geographical patterns in parasite infection prevalence may be more dependent on the specific host–parasite relationship. Parasite prevalence showed no geographical pattern in Australia relative to the parasite load which was higher in lizard populations in the tropics (Salkeld et al. 2008). Our study area was characterised by intensively farmed land that may influence blood parasite prevalence with anthropogenic factors. Insecticides may reduce vector abundance, and habitat fragmentation may impact parasite transmission dynamics (Allan et al. 2003).
Both genders were similarly susceptible to the infection, the prevalence was only slightly higher in males. Several authors similarly reported no intersexual differences in parasite prevalence (Smallridge and Bull 2000; Amo et al. 2005). On the other hand, significant differences were demonstrated between lizard gender, being higher in males which can be explained by the level of immunosuppressive testosterone in males (Belliure et al. 2004). Previous studies have demonstrated that an increase in host reproductive effort decreases parasite defense and, thus, increases parasite load (Gustafsson et al. 1994; Oppliger and Clobert 1997; Oppliger et al. 1998; Fargallo and Merino 2004).
Lack of correlation among blood parasites, tick load, and morphological traits was observed. Resistance to ectoparasites was found to be costly, as manifested by a reduced body condition and a compromised defence against secondary infection by blood parasites (Olsson et al. 2005). It was shown previously that in the population of the Swedish sand lizard, two distinct genotypes with respect to the major histocompatibility complex determined by restriction fragment length polymorphism differed in capacity to combat ticks. Males with genotype “O” were more resistant to ectoparasites but suffered from the higher haemoprotid parasite load (Olsson et al. 2005). However, we found no relationship between blood parasite prevalence and ectoparasite load or with body size. Thus far, I. ricinus was not proved to be the vector of any haemoparasites of lizards. Disproportion in results of different studies dealing with the relation of body size and blood parasite load exist.
Among the lizards, Tiliqua rugosa, there was a decline in the prevalence of Haemolivia mariae infection with increasing lizard size (Smallridge and Bull 2000), while the prevalence of infection was positively correlated with the adult size in Lacerta lepida (Amo et al. 2005).
In conclusion, haemogregarinid blood parasites are common parasites of the sand lizard (L. agilis) and common lizard (Z. vivipara) in habitats in Western Poland. Intensive farming and the high predation pressure in the studied localities may worsen impact of parasitism on the fitness of these protected animals.
References
Allan BF, Keesing F, Ostfeld RS (2003) Effect of forest fragmentation on lyme disease risk. Conserv Biol 17:267–272
Amo L, Fargallo JA, Martínez-Padilla J, Millán J, López P, Martin J (2005) Prevalence and intensity of blood and intestinal parasites in a field population of a Mediterranean lizard, Lacerta lepida. Parasitol Res 96:413–417
Amo L, Lopez P, Martin J (2004) Prevalence and intensity of haemogregarinid blood parasites in a population of the Iberian rock lizard, Lacerta monticola. Parasitol Res 94:290–293
Antczak M, Hromada M, Grzybek J, Tryjanowski P (2004) Breeding biology of the Great Grey Shrike Lanius excubitor in W Poland. Acta Ornithol 39:9–14
Appleby BM, Anwar MA, Petty SJ (1999) Short-term and long-term effects of food supply on parasite burdens in tawny owls, Strix aluco. Funct Ecol 13:315–321
Arneberg P, Skorping A, Grenfell BT, Read AF (1998) Host densities as determinants of abundance in parasite communities. Proc R Soc Lond B 265:1283–1289
Belliure J, Smith L, Sorci G (2004) Effect of testosterone on T cell mediated immunity in two species of Mediterranean Lacertid lizards. J Exp Zool 301A:411–418
Bennett GF, Cameron M (1974) Seasonal prevalence of avian hematozoa in passeriform birds of Atlantic Canada. Can J Zool 52:1259–1264
Bischoff W (1984) Lacerta agilis Linnaeus, 1758 Zauneidechse. In: Böhme W (ed) Handbuch der Reptilien und Amphibien Europas. Aula Verlag, Weisbaden, pp 23–68
Caudell JN, Whittier J, Conover MR (2002) The effects of haemogregarine-like parasites on brown tree snakes (Boiga irregularis) and slatey-grey snakes (Stegonotus cucullatus) in Queensland, Australia. Int Biodeterior Biodegrad 49:113–119
Corbett KF, Tamarind DL (1979) Conservation of the sand lizard, Lacerta agilis, by habitat management. Brit J Herp 5:799–823
Dowell SF (2001) Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis 7:369–374
Eisen RJ (2000) Variation in life-history traits of Plasmodium mexicanum, a malaria parasite infecting western fence lizards: a longitudinal study. Can J Zool 78:1230–1237
Eisen RJ, Wright NM (2001) Landscape features associated with infection by a malaria parasite (Plasmodium mexicanum) and the importance of multiple scale studies. Parasitology 122:507–513
Ekner A, Majláth I, Majláthová V, Hromada M, Bona M, Antczak M, Bogaczyk M, Tryjanowski P (2008) Densities and morphology of two co-existing lizard species (Lacerta agilis and Zootoca vivipara) in extensively used farmland in Poland. Folia Biol 56:165–171
Fargallo JA, Merino S (2004) Clutch size and haemoparasite species richness in adult and nestling blue tits. Ecoscience 11:168–174
Gustafsson L, Nordling D, Andersson MS, Sheldon BC, Qvarnstrom A (1994) Infectious diseases, reproductive effort and the cost of reproduction in birds. Philos Trans R Soc Lond B 346:323–331
Majláthová V, Majláth I, Hromada M, Tryjanowski P, Bona M, Antczak M, Víchova B, Dzimko S, Mihalca A, Petko B (2008) The role of the sand lizard (Lacerta agilis) in the transmission cycle of Borrelia burgdorferi sensu lato. Int J Med Microbiol 298:161–167
Martin J, Amo L, López P (2008) Parasites and health affect multiple sexual signals in male common wall lizards, Podarcis muralis. Naturwissenschaften 95:293–300
Martin J, Lopez P (1999) An experimental test of the costs of antipredatory refuge use in the wall lizard, Podarcis muralis. Oikos 84:499–505
Minchella DJ (1985) Host life-history variation in response to parasitism. Parasitology 90:205–216
Møller AP (1997) Parasitism and the evolution of host life history. In: Clayton DH, Moore J (eds) Host–parasite evolution: general principles and avian models. Oxford University Press, Oxford, pp 105–127
Nordling D, Andersson M, Zohari S, Gustafsson L (1998) Reproductive effort reduces specific immune response and parasite resistance. Proc R Soc Lond 265:1291–1298
Norris K, Anwar M, Read AF (1994) Reproductive effort influences the prevalence of haematozoan parasites in great tits. J Anim Ecol 63:601–610
Olsen W (1974) Animal parasites. University Park Press, Toronto
Olsson M, Wapstra E, Madsen T, Ujvari B, Rugfelt C (2005) Costly parasite resistance: a genotype-dependent handicap in sand lizards? Biol Lett 1:375–377
Oppliger A, Clobert J (1997) Reduced tail regeneration in the common lizard, Lacerta vivipara, parasited by blood parasites. Funct Ecol 11:652–655
Oppliger A, Clobert J, Lecomte J, Lorenzon P, Boudjemadi K, John-Alder HB (1998) Environmental stress increases the prevalence and intensity of blood parasite infection in the common lizard Lacerta vivipara. Ecol Lett 1:129–138
Price PW (1980) Evolutionary biology of parasites. Princeton University Press, Princeton
Readon JT, Norbury G (2004) Ectoparasite and hemoparasite infections in a diverse temperate lizard assemblage at Macraes Flat, South Island, New Zealand. J Parasitol 90:1274–1278
Reichenow E (1913) Karyolysus lacertae, ein wirtswechselndes Coccidium an der Eidechse Lacerta muralis und der Milbe Liponyssus saurarum. Arb Kaiserlichen Gesundheitsamte 45:317–363
Salkeld DJ, Leonhard S, Girard YA, Hahn N, Mun J, Padgett KA, Lane RS (2008) Identifying the reservoir hosts of the Lyme disease spirochete Borrelia burgdorferi in California: the role of the western gray squirrel (Sciurus griseus). Am J Trop Med Hyg 79:535–540
Salvador A, Veiga JP, Martin J, Lopez P (1997) Testosterone supplementation in subordinate, small male lizards: consequences for aggressiveness, color development, and parasite load. Behav Ecol 8:135–139
Schall JJ (1986) Prevalence and virulence of a haemogregarine parasite of the Aruban whiptail lizard, Cnemidophorus arubensis. J Herpetol 20:318–324
Schall JJ, Marghoob AB (1995) Prevalence of a malarial parasite over time and space: Plasmodium mexicanum in its vertebrate host, the western fence lizard Sceloporus occidentalis. J Anim Ecol 64:177–185
Schall JJ, Pearson AR, Perkins SL (2000) Prevalence of malaria parasites (Plasmodium floridense and Plasmodium azurophilum) infecting a Puerto Rican lizard (Anolis gundlachi): a nine-year study. J Parasitol 86:511–515
Smallridge CJ, Bull CM (2000) Prevalence and intensity of the blood parasite Hemolivia mariae in a field population of the skink Tiquila rugosa. Parasitol Res 86:655–660
Sol D, Jovani R, Torres J (2000) Geographical variation in blood parasites in feral pigeons: the role of vectors. Ecography 23:307–314
Sorci G (1996) Patterns of haemogregarine load, aggregation and prevalence as a function of host age in the lizard Lacerta vivipara. J Parasitol 82:676–678
Telford SR (2008) Hemoparasites of the reptilia. Color atlas and text. CRC Press, Boca Raton
Veiga JP, Salvador A, Merino S, Puerta M (1998) Reproductive effort affects immune response and parasite infection in a lizard: a phenotypic manipulation using testosterone. Oikos 82:313–318
Votýpka J, Simek J, Tryjanowski P (2003) Blood parasites, reproduction and sexual selection in the red-backed shrike (Lanius collurio). Ann Zool Fenn 40:431–439
Acknowledgement
We are very grateful to M. Biskup and M. Bogaczyk for assistance in the field. This work was financially supported by the Scientific Grant Agency of the Ministry of Education of Slovak Republic and the Slovak Academy of Sciences No. 1/0139/08, MH was supported by grant MSM 6007665801, and NN 303 3174 33 by the Polish Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Majláthová, V., Majláth, I., Haklová, B. et al. Blood parasites in two co-existing species of lizards (Zootoca vivipara and Lacerta agilis). Parasitol Res 107, 1121–1127 (2010). https://doi.org/10.1007/s00436-010-1981-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-1981-0