Abstract
Enolase is a key enzyme in the glycolytic pathway; recent studies have also shown that enolase is found on the surface of several parasites, where it acts as a plasminogen-binding protein. In the present study, the enolase of Schistosoma japonicum has been cloned and expressed. In western blot analysis, the recombinant enolase from S. japonicum ( rSjENO) was recognized by rabbit sera directed against an antigen preparation from adult worms. Kinetic measurement revealed that rSjENO possesses good enzymatic activity. The real-time PCR showed that the enolase gene was highly expressed at 18–28 days of the life cycle. Immunofluorescence testing showed that SjENO was located mainly on the surface as well as in the inner tissues of the worms. Ligand-blotting analysis indicated that rSjENO could bind to human plasminogen as its receptor. In addition, a 24.28% reduction in the liver egg count and a reduction of 21.45% in the fecal egg count were observed in BALB/c mice vaccinated with rSjENO when compared with blank control mice. An ELISA assay suggested that high levels of specific IgG antibody could be induced by rSjENO in vaccinated mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schistosomiasis is one of the most serious parasitic zoonosis; it afflicts an estimated 200 million people from 76 countries and territories in South America, Africa, and Asia. The disease is caused by three major schistosome species: Schistosoma japonicum, Schistosoma mansoni, and Schistosoma haematobium. S. japonicum is prevalent in southern China, the eastern part of the Philippines, and other Asian countries. Approximately 4,130,000 people are currently infected with S. japonicum in China, and the disease remains a major public health problem in this country (Zheng 2009).
The schistosome has a complex life cycle, involving the adult worm, egg, miracidium, mother sporocyst, daughter sporocyst, cercariae, and schistosomulum; there are seven different developmental stages. The cercariae penetrate the skin of the mammalian host and transform into schistosomula; the latter travel through the blood to the liver via the lungs, then mature and develop into male and female adult worms in the hepatic portal vein. Schistosomula are the early developmental stage of the worms in the final host; they undergo dramatic changes in their cellular composition and cellular specialization. Some proteins that are differentially expressed at this stage may contribute to the growth and sexual differentiation of the worm and to its interaction with the host. Studies of the proteins that are differentially expressed in the schistosomula may reveal the growth and migration mechanism of the schistosome, and may be used further to identify targets for the development of new drugs and vaccines against schistosomiasis.
Enolase, a glycolytic and gluconeogenic enzyme, occurs widely in bacteria (e.g., Escherichia coli), eumycetes (yeasts), and vertebrates (e.g., humans). It belongs to a novel class of surface proteins that do not possess the classical machinery for surface transport, yet through an unknown mechanism are transported to the cell surface. The sequence similarity of enolase between species is 40–90% (Pancholi 2001; Jolodara et al. 2003; Bernal et al. 2004; Ramajo-Hernández et al. 2007; Vanegas et al. 2007; Yang et al. 2009). Our previous work has revealed, by the use of 2-D electrophoresis and mass-spectrum analysis, that enolase is one of the proteins that are differentially expressed at the stage of the schistosomulum (Zhao et al. 2007). Enolase is considered to be a major immunostimulatory protein in visceral leishmaniasis (Gupta et al. 2007); it has been reported also that this molecule can induce protective efficacy against Candida albicans infection (Van Devente et al. 1996; Montagnoli et al. 2004; Pitarch et al. 2006). Like other parasites, schistosomes need to exchange required substances and energy during metabolism within their host. It is well known that energy acquisition by schistosomes relies mainly on the glycolytic pathway, and enolase is a key enzyme in this pathway. Many studies have shown that enolase can be found on the surface of several eukaryotes and prokaryotes, where it acts as a plasminogen-binding receptor (Nakajima et al. 1994; Redlitz et al. 1995; Pancholi and Fischetti 1998). It is reasonable to assume that S. japonicum, an intravascular parasite, should interact directly with the hemostatic system of the host. Therefore, enolase is an important protein in the energy metabolism and development of schistosomes, but relatively few studies of this molecule in S. japonicum have been reported.
In this study, we cloned and expressed the enolase gene of S. japonicum, analyzed the expression differences of this gene among different development stages of S. japonicum, detected the enzyme activity and plasminogen-binding activity of the recombinant protein rSjENO, and evaluated the protective efficacy induced by rSjENO in mice. The results will increase our understanding of the enolase from S. japonicum.
Materials and methods
Parasite and animals
The life cycle of S. japonicum (Anhui isolate, Chinese mainland strain) was maintained in New Zealand rabbits and Oncomelania hupensis in the Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences. The rabbits were infected with cercariae, and parasites were aseptically perfused at 7, 18, 23, 28, 32, and 42 days post-infection. The worms were detached manually, snap frozen, and stored in liquid nitrogen until use. Female BALB/c mice, 7 weeks old, were purchased from Shanghai Experimental Animal Centre, Chinese Academy of Sciences. All procedures did as per principles of the National Institute of Health for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Research 1996).
Gene cloning, phylogenetic, and sequence analysis
To clone cDNAs encoding S. japonicum enolase, PCR amplification was carried out using the cDNAs from the S. japonicum adult worm cDNA library as a template (Yuan and Wu 2000). Sense and antisense primers were designed according to the multiple cloning sites present in the pET28a expression vector (Invitrogen, USA) and the published sequence of the S. japonicum enolase gene (Waine et al. 1993). The sequences of the two primers were: SjENO_F (29-mer, EcoRI) 5′-CGCGAATTCGCAATTATAGCGATTCACGC-3′ and SjENO_R (29-mer, XhoI) 5′-TATCTCGAGAGATTTGAGGATGGCGGAAG-3′, respectively. Amplification was carried out in the DNA Thermal Cycler480 machine (Perkin Elimer, USA) in a reaction consisting of 400 ng of each primer, 100 μM dNTP mix, pH 8.8 buffer, 2 mM MgCl2, 2 U Taq Hot Start DNA polymerase, and 2 μl of the template cDNA in a final volume of 20 μl. The amplification conditions were 35 cycles at 94°C for 50 s, 55°C for 50 s, and 72°C for 1 min, with an initial denaturation at 94°C for 5 min.
Blast and PSI-Blast searches against the NCBI non-redundant protein sequence database, using SjENO as a query, were used to identify orthologs of SjENO. For phylogenetic analysis, alignments of the protein sequences were performed using the neighbor-joining method and plotted with MEGA.
Expression and purification of rSjENO
The amplified PCR product and the pET28a plasmid vector DNA were digested with EcoRI and XhoI restriction enzymes, purified and ligated using T4 ligase and transformed into competent BL21 (DE3) E. coli to produce the recombinant plasmid. The plasmids were identified further by PCR, restriction enzyme digestion, and sequencing analysis using standard protocols (Sambrook et al. 1989).
E. coli transformed with the recombinant plasmid was grown in Luria–Bertani medium containing 100 µg ml−1 kanamycin. Cultures were grown at 37°C to an A600 of 0.6–0.8, then induced with 1 mM isopropylthio-β-d-galactoside. Cells were removed at different time intervals(0, 2, 4, 6, and 8 h) after the induction, pelleted by centrifugation at 5,000 g for 10 min, and sonicated on ice for 20–30 min with 15 s intervals in a sonics machine (Sonics&Materials Inc, USA) after three freeze-thaw cycles. The lysate was centrifuged at 45,000×g for 20 min in a Beckman Ultracentrifuge (model L-100 XP). The expression products were analyzed by SDS-PAGE; the protein of rSjENO carrying the His-tag was expressed as soluble and inclusion body forms, and the soluble rSjENO protein was purified by Ni2+ affinity chromatography.
Antigenicity analysis of rSjENO by Western blot
After SDS-PAGE electrophoresis, the gels were soaked in the transfer buffer and transferred electrophoretically onto a 0.45-μm pore size nitrocellulose membrane (Whatman, Germany). The nitrocellulose membrane with transferred SjENO proteins was then probed with sera from rabbits that was raised against an antigen preparation from adult S. japonicum worms (SWAP). Bound antibody was detected using an anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody (Bio-Rad), visualized using precipitation type TMB Substrate Solution (Tiangen, Beijing), and imaged with ImageQuant 300 (GE, USA).
Analysis of enzyme activity
The protein concentration of purified rSjENO was determined using ultraviolet spectrometry (Eppendorf Biophotometer). All kinetic measurements were made at room temperature (20 ± 2°C). The enzyme activity of enolase was measured in the forward direction formation of phosphoenolpyruvate (PEP) from 2-phospho-d-glycerate (2-PGA) and the reverse direction (formation of 2-PGA from PEP) by monitoring the increase or decrease, respectively, in PEP absorbance at 240 nm in a continuous spectrophotometric assay on a UV-1800PC spectrophotometer (M. Wave Professional, Shanghai Mapada Instruments Co., Ltd). The concentration of PEP was determined using an absorption coefficient (ε 240nm = 1,400 L mol-1 cm-1). Enolase from rabbit muscle (Sigma) was applied as the standard control. Given that the absorption coefficient of PEP varies with pH and the concentration of Mg2+, an appropriate absorption coefficient was used (Wold and Ballous 1957). Typically, 2 ml of assay mixture was used, which contained 1 mM 2-PGA (for the forward reaction) or PEP (for the reverse reaction) and 1.5 mM MgCl2 in 50 mM Tris/HCl, pH 7.4. One unit of enzyme was defined as the amount of enzyme that converts 1 μMol substrate (2-PGA or PEP) into product (PEP or 2-PGA) in 1 min at 20 ± 2°C. The Michaelis constant for 2PGA and PEP was determined, and the influence on the activity of rSjENO of the pH, the temperature, and the concentration of metal ions was analyzed.
Analysis of plasminogen-binding activity by ligand blotting
For detection of plasminogen-binding activity, rSjENO proteins were electrotransferred from SDS-PAGE gels onto nitrocellulose membranes at 15 V for 45 min. The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline (TBS, 10 mM Tris–HCl, pH 7.4, 150 mM NaCl) overnight at 4°C, rinsed three times with washing buffer (TBS containing 0.05% Tween 20, TBST), and incubated for 2 h at 37°C with 35 μg/ml of human plasminogen (Acris, Germany) diluted in TBST, 25 mM EDTA, 1% BSA. After extensive washing in TBST, the blots were then incubated in TBST with 1% BSA containing 1:200 (v/v) rabbit anti-human plasminogen antibodies (Santa Cruz, USA) for 2 h at RT. The bound antibody was detected by incubating the blots for 1 h at RT with goat anti-rabbit IgG HRP conjugated (Bio-Rad) at 1:10,000 (v/v) in TBST with 1% BSA. Immune complexes were visualized using precipitation type TMB Substrate Solution (Tiangen, Beijing). Images were acquired with ImageQuant 300 (GE, USA). Negative controls were also included from which the plasminogen had been omitted. The assay was repeated three times.
Analysis of the expression from different developmental stages of S. japonicum and immunolocalization of worms
Expression analysis of worms from different developmental stages by real-time PCR
Worms were collected as described in “Parasite and animals” section, and the total RNAs were extracted separately using TRIZOL reagent (Invitrogen), quantified with ultraviolet spectrometry (Eppendorf Bio-photometer) and reverse transcribed in a final volume of 50 μL using a PrimeScriptTM RT reagent kit (TaKaRa) following the manufacturer's instructions. Real-time PCR was performed in a reaction mixture of 20 μl containing 1 ng reverse transcribed cDNA, 5 pmol of each enolase gene-specific primer (forward primer 5′ CAAATGGGTTCTGAGGTGTA, reverse primer 5′CGCAATCCATACCAATCTCT), and 10 μl of Power SYBR Green PCR Master Mix (TaKaRa). The reaction conditions were 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 60°C for 25 s, and 72°C for 40 s (fluorescence data collection). Data on the raw relative quantification were calculated using Bio-Rad iQ5 software. The 18S RNA gene was used as the internal standard.
Immunolocalization of enolase by indirect immunofluorescence assay
An indirect immunofluorescence assay was performed. Worms were collected as described in “Parasite and animals” section and were made into frozen sections. The sections were fixed with freezing acetone for 30 min at −20°C, washed three times in TBST, blocked in TBST with 5% BSA, and incubated at 4°C overnight with 1:200 diluted murine antiserum against rSjENO protein we got from mouse vaccination experiment as described in “Mouse vaccination experiment” section. CY3-conjugated anti-mouse IgG (Rockland, USA) was used as a secondary antibody at a dilution of 1:1,000 and incubated at RT for 1 h. All antibody dilutions were made in TBS with 1% BSA. Parasite nuclei were stained with 4′,6-diamidino-2-phenylindole (Rockland, USA) at a final concentration of 10 μg/ml.
Mouse vaccination experiment
The mice were divided randomly into three groups, with ten mice in each group. The animals to be vaccinated were first injected intraperitoneally with 20 μg of rSjENO emulsified with complete Freund's adjuvant, followed by two boosters with 20 μg recombinant antigen in incomplete Freund's adjuvant at 2-week intervals. Mice in the adjuvant control and blank control groups were injected in parallel with adjuvant or PBS only. Before vaccination and 10 days after each vaccination, the specific antibody titers against rSjENO were monitored. Two weeks after the third vaccination, each mouse was challenged with 30 ± 2 cercariae. The animals were perfused 6 weeks post challenge. The number of worms, fecal eggs, and liver eggs were counted. For counting of liver eggs, 0.5 mg samples of liver tissue from each infected mouse were homogenized in 10 ml 5% NaOH, respectively. The mixture was incubated at 56°C for 1 h. An average of three counts per 20 μl mixture was taken to estimate the number of eggs, and this was converted to eggs per gram (EPG). The worm and egg reduction rate were calculated as follows: percentage reduction in worm burden = (mean worm burden of control group − mean worm burden of vaccinated group)/mean worm burden of control group × 100%. Percentage reduction in liver/feces egg count = (mean EPG from control group − mean EPG from vaccinated group)/mean EPG from control group × 100%.
Results
Cloning and phylogenetic analysis of enolase from S. japonicum
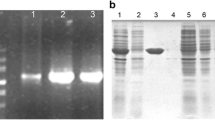
A cDNA of 1,305 bp was amplified by PCR and cloned into pMD18-T vector (Takara, Japan). Sequence analysis revealed that the gene encoded the enolase of S. japonicum. The gene was subcloned into the pET28a plasmid vector (Invitrogen, USA). The positive recombinants were screened further and identified by PCR (Fig. 1a), and restriction enzyme digestion was performed with EcoRI and XhoI (Fig. 1b).
The recombinant plasmid was identified by PCR and enzyme digestion. a Lane 1, the PCR product of the enolase gene amplified from the S. japonicum adult worm cDNA library. b Lane 1, the recombinant plasmid DNA pET28a(+)–SjEno digested with EcoRI and XhoI restriction enzymes. M, Marker DL2000 (Takara)
The full amino acid sequence of the recombinant protein was deduced (GenBank ID: GQ413934.1; Protein ID: ACV41761.1). The nucleotide sequence has three bases different to the published sequence, but the amino acid sequence is identical (Waine et al. 1993). Comparison of the amino acid sequence showed that the enolase of S. japonicum had 87% and 88% identity with those of Schistosoma manosi and Schistosoma bovis, respectively. The conserved sites were identical in these Schistosoma, including the substrate-binding sites and metal-binding sites. In addition, the deduced peptides had a similar amino acid composition to a putative plasminogen-binding motif reported for the Fasciola hepatica enolase (Bernal et al. 2004) (Fig. 2).
Amino acid sequence alignment of enolase from S. japonicum (NCBI:gb/ ACV41761.1) with S. mansoni (NCBI:gb/AAC46884.1) and S. bovis (NCBI:gb/ACC78611.1) using Clustal W. A putative plasminogen-binding motif (Bernal et al. 2004) is shadowed. The conserved sites: substrate binding, 371–374; metal binding, 245, 294, 319; binding site, 158, 167, 294, 319, 395
The result of the phylogenetic analysis is shown in Fig. 3. The enolase of S. japonicum is closest to those from S. mansoni and S. bovis, and the next closest were from the parasitic trematode F. hepatica and the parasitic nematode Trichinella spiralis; these formed a cluster with the human, bovine, and murine enolase.
Phylogenetic tree of 22 enolase proteins using the neighbor-joining method and plotted with MEGA .The GenBank accession number of each enolase is: Rattus norvegicus (P04764), Mus musculus (P17182), Bos taurus (Q9XSJ4), Homo sapiens (P06733), Trichinella spiralis (Q967U0), Fasciola hepatica (Q27655), Schistosoma japonicum (ACV41761), Schistosoma mansoni (Q27877), Schistosoma bovis (B2LXU1), Saccharomyces cerevisiae (P00924), Leishmania braziliensis (A4H7T6), Leishmania mexicana (Q3HL75), Leishmania infantum (A4HW62), Leishmania major (Q4QFL8), Cryptosporidium parvum Iowa II (Q5CRP8), Cryptosporidium muris (B6A9M6), Toxoplasma gondii (Q9UAE6), Plasmodium falciparum (Q27727), Babesia bovis (A7AP71), E. coli (strain K12) (P0A6P9), Streptococcus pneumoniae (Q97QS2), Bacillus subtilis (P37869)
Analysis of the expression, purification, and antigenicity of rSjENO
The rSjENO protein was expressed at the highest level 4 h after induction with IPTG (data not shown). For preparation of the rSjENO protein, the extract of the cell culture was separated into soluble supernatant and pellet fractions by centrifugation. Both fractions contained a reasonable amount of rSjENO (Fig. 4a, lanes 2 and 3). The soluble fraction was used for the purification of the recombinant protein using an agarose/Ni–nitrilotriacetic acid affinity chromatography column. The eluted protein showed a single band at the expected molecular mass (≈50 kDa) on SDS/PAGE (Fig. 4a, lane 1). The recombinant protein was identified further by Western blotting using anti-His serum (Fig. 4b) and serum raised in a rabbit against SWAP (Fig. 4c) as probes. A positive band of around 50 kDa was observed in both reactions, which revealed that the rSjENO had good antigenicity.
Expression and purification analysis of the rSjENO protein. a Analysis of the expression using SDS/PAGE. Lane M, molecular mass markers; lane 1, elution of rSjENO; lanes 2 and 3, soluble and insoluble fraction of the E. coli extract, respectively. b Immunoblot of rSjENO probed with anti-His serum. Lane 1, pET28a–SjENO not induced with IPTG; lane 2, pET28a–SjENO induced with IPTG. c Western blotting analysis of the antigenicity of rSjEno using serum raised in rabbits against SWAP; lane 1, the purified rSjEno protein
Characterization of enzyme activity of purified rSjENO
Purified rSjENO was assayed for enolase activity by measurement of either the conversion of 2-PGA to PEP (forward reaction) or PEP to 2-PGA (reverse reaction). Kinetic measurements using 2-phosphoglycerate as the substrate gave a specific activity of ≈35.81 ± 2.02 U (mg protein)−1 and 15.87 ± 0.96 U (mg protein)−1 in the reverse direction. For the determination of K m, the initial reaction rates were measured at several different concentrations of 2-PGA (Fig. 5a) and PEP (Fig. 5b). The data were fitted to the Michaelis–Menten equation \( \left\{ {v = V\max \left[ S \right]/\left( {{K_{\rm{m}}} + \left[ S \right]} \right)} \right\} \) using SIGMAPLOT software. The Michaelis constant for the forward reaction K m2-PGA ≈ 1.99 mM and for the reverse reaction K mPEP ≈ 0.53 mM. Figure 5c, d shows that the maximal rSjENO activity was observed when the pH was around 6.5–7.0, irrespective of the substrate used, and most mammalian enolases have their activity maxima in the pH range 6.8–7.1 (Wold 1971???). The activity of the enzyme was inhibited by KCl, NaCl, MgCl2, and CaCl2 at the ion concentrations tested (Fig. 5e–h). A variety of temperatures from 15°C to 45°C in the reaction system had no significant influence on the activity of enolase (data not shown).
Characterization of enzyme activity of purified rSjENO. a Plot of [2-PGA] vs. activity. b Plot of [PEP] vs. activity for the determination of K m. A 40-μl sample of enzyme containing 6 and 3 μg of rSjENO, respectively, were used for the 2-PGA and PEP assay. The experimental data were fitted according to the Michaelis–Menten equation using SIGMAPLOT software. c pH was plotted against activity using 2-PGA and d PEP as substrates. e–h The variation in rSjENO activity with increasing concentrations of KCl, NaCl, MgCl2, and CaCl2, using 2-PGA as substrate. The data using PEP as substrate are not shown
Plasminogen binding to rSjENO protein by ligand blotting
As shown in Fig. 6, a positive band with a molecular weight of around 50 kDa was detected in the purified rSjENO lane, which suggests that the protein may be a specific binding protein for human plasminogen.
Vaccination studies with rSjENO in mice
To evaluate the protective efficacy induced by the rSjENO, BALB/c mice were vaccinated with purified rSjENO three times, challenged with S. japonicum cercariae, and then perfused 6 weeks post-infection. The results showed that a reduction of 24.28% in liver eggs and a reduction of 21.45% in the fecal egg count were obtained (P < 0.05) when compared with those of the blank control mice (Table 1), but no reduction in worm counts was observed in mice vaccinated with rSjENO (data not shown). The ELISA assay revealed that rSjENO induced a high level of specific antibodies in immunized mice, compared with the blank and adjuvant control mice (P < 0.01) (as shown in Fig. 7).
Detection of specific IgG antibodies by ELISA. A, B, C, D indicated that before immunization, 10 days after first immunization, 10 days after second immunization, and 10 days after third immunization. E and F indicated that 3 w and 6 w after challenged with cercariae. The mice were immunized with rSjENO protein, or adjuvant or PBS as control at 2-week intervals. Two weeks after the third vaccination, each mouse was challenged with 30 ± 2 cercariae. The mouse were perfused 6 weeks post challenge
Analysis of the expression of enolose in worms from different developmental stages of S. japonicum
Real-time PCR revealed that the S. japonicum enolase gene was expressed at a high level in worms at 18–28 days worms, followed by worms at 32, 42, and 7 days (Fig. 8).
Immunolocalization of enolose in worms of S. japonicum
The localization of SjENO was detected by immunofluorescence using murine serum raised against rSjENO. The result showed that the enzyme was presented mainly on the surface as well as in the inner tissues of the worms (Fig. 9).
Discussion
Enolase is considered a rather “dull” enzyme because it has been conserved through millions of years of evolution. The real-time PCR showed that the enolase gene was expressed in all developmental stages tested and was highly expressed at 18–28 days of the life cycle. This result was consistent with our previous studies, using 2-D electrophoresis and mass-spectrum analysis, on the differential expression of proteins in different development stages of S. japonicum (Zhao et al. 2007). From the time at which the cercariae penetrate the skin to maturation of the parasite and laying of eggs takes about 28 days for S. japonicum, about 35 days for S. mansoni, and 63 to 65 days for S. haematobium. Jiang et al. (1995) found that the development of eggs in S. japonicum can be identified initially at 19 days using a light microscope, and they are fully mature at 38 days. Therefore, days 18–28 correspond to the time that schistosomula develop into adult worms and begin to lay eggs, and the eggs begin to mature. The significantly increased expression of the gene during this time suggests that enolase is critical for the rapid growth, migration, and adaptation for survival of the schistosome in the host environment.
Pancholi compared the enolase enzymes from 39 representative species, the comparison showed 40–90% identity between the enolase from two different species (Pancholi 2001). Our phylogenetic analysis showed that the enolase of S. japonicum is most closely related to those from other schistosome species, such as S. mansoni and S. bovis and then to those of parasitic helminths such as F. hepatica and T. spiralis. Interestingly, SjENO is closer to the enzymes from schistosome-susceptible vertebrate hosts, such as humans, cattle, and mice than to those of some of the important parasitic protozoa, such as Plasmodium falciparum, Babesia bovis, Toxoplasma gondii, Cryptosporidium muris, Leishmania braziliensis, etc.
Enolase has been characterized as a plasminogen-binding protein on the surface of several other pathogens, and this interaction with plasminogen has been shown to induce fibrinolytic activity and to be involved in invasiveness within the host (Bergmann et al. 2001; Jong et al. 2003). In helminths, it was demonstrated that the enolase of S. bovis is one of the plasminogen-binding proteins in the tegument (Ramajo-Hernández et al. 2007). Enolase has also been identified as a plasminogen-binding protein in the excretion–secretion products of F. hepatica (Bernal et al. 2004) and in several tissues of Onchocerca volvulus (Jolodara et al. 2003). The plasminogen-binding property of pathogens in general is suggested to be one of the characteristics that contribute to tissue invasion and overall pathogenicity. Plasminogen activation is responsible for the degradation of intravascular clots and extracellular proteolysis in a wide variety of physiological and pathological process. In this study, we demonstrated using ligand blotting that rSjENO could bind human plasminogen in vitro. Since enolase is essentially a cytoplasmic enzyme (Pancholi 2001), enolase is widely distributed in worms and mainly located on the surface as well as in the inner tissues of the worms by an indirect immunofluorescence assay. Thus the enolase of S. japonicum may therefore be structurally related to the plasminogen-binding capacity of the worm.
In this report, we clone and express enolase from S. japonicum in active form, and enzymatic activity was analyzed for the first time. Measurements of enzyme kinetics showed that the purified rSjENO in its active state may have similar substrate affinity to rabbit muscle enolase and those reported for mammalian, yeast, and other enolases (Hannaert et al. 2003). The value for K mPEP is similar to the standard control, rabbit muscle enolase, and those reported for mammalian, yeast, and other enolases (Hannaert et al. 2003). The K m value for 2-PGA is higher than that of the enolase enzymes from P. falciparum (K m = 0.041 mM) (Ipsita et al. 2004), yeast (K m = 0.057 mM) (Hannaert et al. 2003), and C. albicans (K m = 0.95 mM) (Kustrzeba-Wojcicka and Golczak 2000), but its value is lower than that of the enolase from Streptococcus rattus (K m = 4.35 mM) (Huther et al. 1990). The value of the Michaelis constant for a given enzyme can vary widely, depending on the isolation or purification conditions, the organism, and the assay system. Therefore, the fact that the K m value of the enolase from S. japonicum is different from the others is not surprising.
Considering the role of enolase in glycometabolism, the plasminogen-binding capacity of schistosomes, and the high level of expression at the stage of schistosomulum, as well as its exposure on the surface of worms, an experiment in mice was carried out to evaluate the protective efficacy induced by this recombinant protein. The preliminary vaccination experiment in mice revealed that the immunization of mice with the rSjENO protein induced a 24.28% reduction in the liver egg count and a 21.45% reduction in the fecal egg count (P < 0.05), compared with those of the blank control mice (Table 1). The ELISA assay suggested that high levels of specific IgG antibodies could be induced by the rSjENO protein in vaccinated mice. The result indicated that rSjENO may have the potential to inhibit egg production of schistosomes and may be a vaccine candidate. The lesions of schistosomiasis mainly consist of granulomas and fibrosis caused by the eggs. Eggs produced by adult female schistosomes are deposited in the liver, intestines, and other tissue sites and are key factors in the pathology and associated morbidity of schistosomiasis. The pathology caused by schistosomiasis is directly correlated with the number of schistosome eggs deposited in the host; therefore, more work is required to understand how the vaccine candidate interferes with the production and delivery of eggs.
We first identified by real-time PCR and 2-D electrophoresis that enolase was highly expressed at the stage of schistosomulum. The enolase, mainly located on the surface as well as in the inner tissues of the S. japonicum worms, may function for rapid growth as a glycolytic enzyme; it may be useful for migration within the host as a plasminogen-binding receptor; it may be related to egg production because of its high level of expression at 18–28 days of the life cycle; and it may be one reason for survival and escape from immunity in the host because of the close evolutionary relationship with the host. To elucidate the role of enolase throughout the life cycle of the different schistosome stages, especially at/before the stage of egg production, detailed functional experiments need to be conducted.
References
Bergmann S, Rohde M, Chhatwal GS, Hammerschmidt S (2001) Enolasa of Streptococcus pneumoniae is a plasminogen-binding protein displayed on the bacterial cell surface. Mol Microbio 40:1273–1287
Bernal D, de la Rubia JE, Carrasco-Abad AM, Toledo R, Mas-Comas MA (2004) Identification of enolase as a plasminogen-binding protein in excretory–secretory products of Fasciola hepatica. FEBS Lett 563:203–206
Gupta SK, Sisodia BS, Sinha S, Hajela K, Naik S, Shasany AK, Dube A (2007) Proteomic apporoach for identification and characterization of novel immunostimulatory proteins from soluble antigens of Leishmania donovani promastigotes. Proteomics 7:816–823
Hannaert V, Albert MA, Rigden DJ et al (2003) Kinetic characterization, structure modelling studies and crystallization of Trypanosoma brucei enolase. Eur J Biochem 270:3205–3213
Huther FJ, Psarros N, Duschner H (1990) Isolation, characterization and inhibition kinetics of enolase from Streptococcus rattus FA-1. Infect Immun 58:1043–1047
Institute of Laboratory Animal Research, Commission on Life Sciences, National Research Council (1996) Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C
Ipsita PB, Sadagopan K, Vora HK, Sehgal A, Sharma S, Jarori GK (2004) Cloning, over-expression, purification and characterization of Plasmodium falciparum enolase. Eur J Biochem 271:4845–4854
Jiang MS, Yang MY, Li Y et al (1995) Schistosoma japonicum eggs occurred in projection electron microscope. Conference proceedings of the 10th Anniversary for Parasite Professional Societies of China Zoological Society. China Science and Technology Press, Beijing, pp 134–138, in Chinese
Jolodara A, Fischerb P, Bergmann S, Buttner DW, Hammerschmidt S, Brattig NW (2003) Molecular cloning of an a-enolase from the human filarial parasite Onchocerca volvulus that binds human plasminogen. Biochimica et Biophysica Acta 1627:111–120
Jong AY, Chen SHM, Stins MF, Kim KS, Tuan TL, Huang SH (2003) Binding of Candida albicans enolase to plasminogen results in enhanced invasion of human brain microvascular endothelial cells. J Med Microbiol 52:615–622
Kustrzeba-Wojcicka I, Golczak M (2000) Enolase from Candida albicans purification and characterization. Comp Biochem Physio Part B 126:109–120
Montagnoli C, Sandini S, Bacci A, Ramani L, Valle RL (2004) Immunogenicity and protective effect of recombinant enolase of Candida albicans in a murine model of systemic candidiasis. Med Mycol 42:319–324
Nakajima K, Hamanoue M, Takemoto N, Hattori T, Kato K, Kohsaka S (1994) Plasminogen binds specially to a alpha-enolase on rat neuronal plasma membrane. J Neurochem 63:2048–2057
Pancholi V (2001) Multifunctional alpha-enolase: its role in disease. Cell Mol Life Sci 58:902–920
Pancholi V, Fischetti VA (1998) Alpha-enolase, a novel strong plasminogen binding protein of the surface of pathogenic streptococci. J Biol Chem 273:14503–14515
Pitarch A, Jimenez A, Nombela C, Gil C (2006) Decoding serological response to Candida cell wall immunome into novel diagnostic, prognostic, and therapeutic candidates for systemic candidiasis by proteomic and bioinformatic analyses. Mol Cell Proteomics 5:79–96
Ramajo-Hernández A, Pérez-Sánchez R, Ramajo-Martín V, Oleaga A (2007) Schistosoma bovis: plasminogen binding in adults and the identification of plasminogen-binding proteins from the worm tegument. Exp Parasitol 115:83–91
Redlitz A, Fowler BJ, Plow EF, Miles LA (1995) The role of an enolase-related molecule in plasminogen binding to cells. Eur J Biochem 227:407–415
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Vanegas G, Quiñones W, Carrasco-López C, Concepción JL, Albericio F, Avilán L (2007) Enolase as a plasminogen binding protein in Leishmania mexicana. Parasitol Res 101:1511–1516
Van Devente HJ, Goessens WH, van Vliet AJ, Verbrugh HA (1996) Anti-enolase antibodies partially protective against systemic candidiasis in mice. Clin Microbiol Infect 2:36–43
Waine GJ, Becker M, Kalinna B, Yang W, McManus DP (1993) Cloning and functional expression of a Schistosoma japonicum cDNA homologous to the enolase gene family. Biochem Biophys Res Commun 95:1211–1217
Wold F (1971) Enolase. In The Enzymes. New York :Academic Press 5: 499-538.
Wold F, Ballous CE (1957) Studies on the enzyme enolase I equilibrium. J Biol Chem 227:301–312
Yang JM, Cai YM, Feng XG, Lin JJ (2009) Research progress on the functions of enolase in parasites. Chin Parasitol Int 36:109–113 (In Chinese)
Yuan CX, Wu XF (2000) Construction of cDNA libraries from mature female and male worm of Schistosoma japonicum Chinese main land strain. Chin J Biotech 16:727–730 (In Chinese)
Zhao XY, Yao LX, Sun AG et al (2007) Analysis of differentially expressed partial protein of schistosomulum of Schistosoma japonicum using mass spectrometry. Vet Sci in Chin 37(1):1–6 (In Chinese)
Zheng J (2009) Achievements and challenges in schistosomiasis control in China. Chin J Parasit Parasitic Dis 27:398–401 (In Chinese)
Acknowledgement
The study was supported in part by grants from National Basic Research Program of China (No.2007CB513108 ), National Central Nonprofit Research Institutes for Basic R&D Project Special Funds (No.2007JB10), and National Nature Science Foundation of China (No.30671581). We thank Yaojun Shi for his excellent technical assistance. We thank Prof. Youming Cai and Prof. Jinming Liu for helpful discussions. We wish to thank Prof. Jingchu Luo (School of Life Science, Peking University) for phylogenetic analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, J., Qiu, C., Xia, Y. et al. Molecular cloning and functional characterization of Schistosoma japonicum enolase which is highly expressed at the schistosomulum stage. Parasitol Res 107, 667–677 (2010). https://doi.org/10.1007/s00436-010-1913-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-1913-z